Abstract
Study Objectives
We examined growth trajectories of four actigraphy-derived sleep parameters (sleep minutes, sleep efficiency, and variability in sleep minutes and efficiency across a week of assessments) across childhood and adolescence and examined individual differences in trajectories according to participants’ race/ethnicity and sex. We also assessed the predictive effect of growth trajectories of sleep parameters on growth trajectories of mental health outcomes and moderation by race and sex.
Method
Youth (N = 199, 49% female, 65% white, 32% black, 3% biracial) and their parents participated in five waves of data (M ages were 9, 10, 11, 17, and 18 across waves). Participants were from a diverse range of socioeconomic backgrounds.
Results
Across participants, sleep minutes, sleep efficiency, and variability in sleep minutes and efficiency demonstrated significant linear change across childhood and adolescence. Whereas sleep duration shortened over time, sleep efficiency improved. Youth exhibited increases in night-to-night variability in sleep minutes and reductions in night-to-night variability in sleep efficiency. Highlighting the importance of individual differences, some race- and sex-related effects emerged. Black youth and male youth experienced steeper declines in their sleep duration across development relative to their respective counterparts. Black youth also demonstrated smaller improvements in sleep efficiency and greater variability in sleep efficiency compared to white youth. Finally, trajectories of sleep efficiency and variability in sleep minutes predicted trajectories of internalizing symptoms and externalizing behaviors.
Conclusions
Findings showed significant changes in developmental trajectories of four sleep parameters across childhood and adolescence. We discuss the empirical and translational implications of the findings.
Keywords: sleep minutes, sleep efficiency, sleep variability, internalizing symptoms, externalizing symptoms, children and adolescents, longitudinal, growth modeling
Graphical Abstract
Graphical Abstract.
Statement of Significance.
Youth sleep exhibited significant changes from childhood to adolescence that varied by race and sex. Findings support long-term associations between sleep and mental health and indicate that black youth and female youth may be more vulnerable to sleep health disparities relative to their counterparts. At a translational level, findings underscore the value of testing the long-term efficacy of therapeutic treatment. Given developmental changes in sleep patterns during emerging adulthood, it will be necessary to extend assessments of sleep trajectories beyond late adolescence.
Sleep exhibits considerable changes across childhood and adolescence [1, 2], and sleep problems are related to internalizing and externalizing symptoms in youth [3–7]. These findings are well-established by cross-sectional and short-term longitudinal studies. Longer-term studies may enhance our understanding of developmental changes in sleep and the potential role of childhood sleep as a predictor of mental health (used to refer to overall internalizing and externalizing symptoms) in subsequent developmental periods. A few such longitudinal investigations of sleep and mental health over longer developmental periods have been conducted [8, 9]. Building on this literature, the primary aim of this study was to examine growth trajectories of four sleep parameters across ages 9 to 18. Specifically, we assessed trajectories of actigraphically based sleep duration (minutes, total number of minutes scored as sleep between sleep onset and wake time) and quality (efficiency, percentage of time between sleep onset and wake time scored as sleep) because they are well-established sleep parameters [10]. Though not as commonly studied, emerging work underscores the value of examining variability in sleep as an important aspect of sleep–wake patterns, highlighting that night-to-night fluctuations in sleep regularity occur and may be robustly associated with mental health [11, 12]. Thus, we additionally assessed trajectories of actigraphically based variability in sleep duration and variability in sleep quality (intraindividual variability in sleep minutes and efficiency, night-to-night fluctuations in sleep over a 1-week period). Additionally, in follow-up analyses, we assessed whether growth trajectories of sleep parameters varied by participants’ race/ethnicity and sex. As a secondary aim, we tested growth trajectories of sleep parameters as predictors of growth trajectories of internalizing (depression and anxiety) and externalizing (aggression and delinquency) symptoms.
Developmental trajectories of sleep
During the transition from childhood to adolescence, significant changes in the sleep–wake cycle occur because of psychosocial (e.g. later bedtimes, increased homework, and extracurriculars) and biological (e.g. slowing of homeostatic pressure and pubertal maturation) factors [13, 14]. Despite evidence for reductions in sleep duration from childhood to adolescence [1, 15–18], limited research examines developmental trajectories of sleep duration. Even fewer studies have examined developmental changes in sleep quality, and the existing results are inconsistent. For instance, based on mean comparisons, objective indicators of sleep quality (e.g. efficiency) improved from age 8 to 10, but worsened from age 10 to 13 [19]. A similar pattern has been observed for subjective assessments of sleep quality: sleep problems declined across ages 9, 10, and 11, but increased from age 11 to 18 [20]. However, others have found mean-level declines in subjective sleep problems across ages 5 to 14 [21] or comparable mean levels of objective sleep quality across late adolescence [3]. Research has yet to examine trajectories of objective assessments of sleep quality from childhood to late adolescence.
Developmental trajectories of change in variability in sleep are also under-studied. The only study, to the best of our knowledge, to examine trajectories of variability in sleep, found that night-to-night fluctuations in sleep schedule and sleep duration increased from age 8 to 12 [1]. Another study measured variability in sleep minutes and found a nonlinear pattern where night-to-night fluctuations decreased from age 16 to 17 but increased from 17 to 18 [3]. Conversely, comparable rates of variability in sleep across pre-adolescents and adolescents have also been found [22]. Although developmental trajectories of variability in sleep efficiency have not been examined, one study assessed mean differences in night-to-night fluctuations in efficiency and found declines from age 16 to ages 17 and 18 [3].
Numerous reports suggest racial disparities in sleep health [23], suggesting possible variations in trajectories of sleep parameters by race. Prior work has consistently found that black youth exhibit shorter objectively measured and self-reported sleep duration relative to white youth [17, 18, 24]. Black youth also self-report lower sleep quality in comparison to white youth [24]. Racial differences in sleep are hypothesized to stem from several factors including psychosocial stressors such as discrimination [25, 26], poor sleep environment [27, 28], and bicultural stress (i.e. stress arising from the navigation of multiple cultural contexts) [29].
Additionally, some research reveals sex differences in sleep. Though prior work mostly suggests that male youth have shorter sleep than females [1, 3], some studies find that female adolescents have shorter sleep than their male counterparts [18]. Likewise, some studies have found better sleep efficiency among female youth [30]. Sex differences across the transition from childhood to adolescence may be attributed in part to differences in pubertal timing and socialization practices [31, 32].
Although prior work provides an initial foundation for understanding developmental trajectories in sleep over time, there are significant gaps in knowledge. First, most studies did not utilize statistical approaches that isolate between- and within-person variability in change in sleep over development, which may result in either inaccurate or misleading estimates [33]. Second, the few studies that distinguished between- and within-person variance used subjective measures of sleep. To the best of our knowledge, only one study assessed objective measures of sleep and partitioned between- and within-person variance in growth in sleep from age 8 to 12 [1]. This study revealed notable individual differences in sleep trajectories according to participants’ sex. Extending this work, the first aim of our study was to utilize latent growth modeling to assess trajectories of objective measures of sleep duration, quality, and variability across five waves of data spanning late childhood to late adolescence. Furthermore, we examined variations in trajectories across participants’ race and sex. By utilizing latent growth modeling, we were able to isolate the between-person variability in trajectories of sleep parameters and shed light on the rank-order stability in individual differences over time. Examining between-person stability may be particularly advantageous at a translational level by identifying youth who most consistently experience less-optimal sleep and, therefore, may be at increased risk for mental health difficulties.
Prospective associations between sleep and mental health
Conceptual models hypothesize that sleep problems specifically in late childhood may play a key role in amplifying the risk for mental health symptoms in adolescence [34]. Suggesting downstream effects of sleep–wake difficulties on mental health outcomes, one study found that objectively measured short sleep duration at age 10 predicted greater internalizing symptoms at age 13 [19]. Other studies provide support for links between sleep problems and mental health symptoms over longer periods of time [20, 21, 35]. Most of these studies utilized subjective assessments of sleep; still, their findings demonstrate long-term effects of sleep–wake difficulties across multiple developmental periods and reveal considerable between-person differences in growth in sleep and mental health symptoms over development. Examining between-person differences in developmental trajectories of sleep and mental health may identify youth who are most vulnerable to future sleep problems and mental health symptoms.
Youth exhibiting increases in sleep problems over time (across ages 1.5 to 7) displayed higher initial levels of internalizing and externalizing symptoms at age 11 [35]. Likewise, youth reporting higher initial levels of sleep problems and higher levels of sleep problems over time had greater internalizing and externalizing symptoms in late adolescence [20, 21]. Collectively, these findings underscore the importance of examining between-person differences in growth trajectories of sleep across childhood and adolescence as a risk factor for poor mental health in adolescence. However, the aforementioned studies did not assess trajectories of sleep regularity, nor did they simultaneously measure sleep and mental health trajectories. Building on this literature, the second goal of our study was to examine the predictive effect of sleep trajectories on the trajectories of two indicators of mental health (internalizing and externalizing symptoms).
Questions also remain regarding the moderating role of race and sex in associations between sleep and mental health, as few studies have addressed these issues. Better quality sleep may operate as a protective factor and offset mental health disparities among minority youth [23]. Specifically, black youth with better actigraphy-derived sleep quality (more efficient, fewer sleep–wake problems) reported fewer internalizing and externalizing symptoms [36]. Regarding the moderating role of sex, some studies suggest that female youth are more vulnerable to the negative effects of poor sleep [9, 37–41]. However, other research indicates that male youth experiencing poor sleep are more affected [9, 42]. Although these studies provide some evidence for moderating effects of race and sex, prior work has yet to test these individual difference variables in associations between trajectories of sleep and mental health across childhood and adolescence.
Current study
Regarding the first study aim, previous literature guided our hypothesis that, in models examining trajectories of sleep, sleep duration would decrease over time and variability in sleep minutes would increase over time. We also expected that internalizing symptoms and externalizing behaviors would increase over development. There is not sufficient literature to formulate a directional hypothesis regarding trajectories of sleep efficiency and variability in sleep efficiency. Although we expected worse sleep overall among black youth, our assessment of race- and sex-related effects in trajectories of sleep were considered exploratory. Regarding the second study aim, we expected that less-optimal sleep (fewer sleep minutes, lower sleep efficiency, greater variability in sleep minutes and efficiency) at age 9 (intercepts) and worsening of sleep over time (slopes) would predict higher levels of mental health problems at age 18 (intercepts) and increases in mental health problems across childhood and adolescence (slopes). Moreover, regarding moderation effects (aim 2), we hypothesized that links between sleep and mental health outcomes would be stronger among black youth, and that black youth obtaining longer and more efficient sleep would report fewer symptoms. Due to the inconclusive nature of the moderating role of sex in the pertinent literature, we do not provide a formal hypothesis about sex differences and analyses are exploratory.
Materials and Methods
Participants
The current study is part of an ongoing longitudinal study focused on sleep and health disparities among youth living in small towns and semirural communities in the southeastern United States (Auburn University Sleep Study). The current study utilizes five waves of data that span 9 years of development. Approximately 1 year (M = 1.07 years, SD = 0.26 years) lapsed between each time point, except between time 3 (T3) and time 4 (T4) where a 6-year gap in data collection took place. An advantage of using latent growth modeling in the current study is the ability to incorporate unequal time lags between measurement occasions [43].
Families were recruited at time 1 (T1) via letters distributed and sent home with children attending public elementary schools. Exclusion criteria at the time of recruitment included parental report of children’s diagnosis with a sleep disorder (e.g. sleep apnea) or an intellectual disability. T1 data collection took place between 2009 and 2010. Participants included 282 families, and the youth were 9.44 years old (SD = 0.71), 48% female (sex assigned at birth) 52% male, 65% white, and 35% black. Time 2 (T2) data collection occurred in 2010 and 2011. In total, 281 families participated including 227 (81%) families who participated at T1 and an additional 54 families who were recruited to enhance power associated with attrition1. Youth were 10.41 years old (SD = 0.67), 45% female and 55% male, and 63% white and 37% black. The majority of families participating at T2 returned for T3 (N = 275, 98%). T3 data were collected from 2011 to 2012, and youth were 11.35 years old (SD = 0.68), 46% female and 54% male, and 62% white and 38% black. T4 included 199 (59%) families who participated at least once at T1, T2, or T3. Data collection occurred between 2017 and 2018, and youth were 17.65 years old (SD = 0.78), 48% female and 52% male, and 66% white and 34% black. Finally, Time 5 (T5) consisted of 179 (90%) families who participated at least once at T1, T2, or T3 and participated at T4. Data collection took place between 2018 and 2019. Youth were 18.36 years old (SD = 0.96), 49% female and 51% male, and 65% white and 35% black. For clarity, we refer to mean participant ages (9, 10, 11, 17, and 18 years old, respectively) rather than study time points.
The final analytic sample consisted of 199 youth who participated at least once at ages 9, 10, or 11, and at age 17. The analytic sample primarily included families who had participated since the beginning of the study at age 9 (n = 170; 85%), and additionally included 29 families who began the study at age 10. Youth were 49% female and 51% male. The sample was representative of the broader community with 65% of youth identifying as white, followed by about a third who identified as black (32%), and 3% who identified as biracial (coded as black in analyses). The sample included families from a diverse range of socioeconomic backgrounds. Across the first three years of the study (ages 9–11), 26%–32% of families lived in poverty (income-to-needs ratio ≤ 1), 31%–37% lived near the poverty line (ratio > 1 and ≤ 2), 24%–31% were considered lower middle-class (ratio > 2 and < 3), and 11%–13% were middle-class status or higher (ratio ≥ 3). During the last 2 years of the study (ages 17 and 18), 14%–19% of families lived below the poverty line, 21%–26% lived near the poverty line, 17%–20% were lower middle-class, and 40%–43% were middle-class or higher.
Procedures and measures
Research procedures were approved by the Institutional Review Board at Auburn University prior to conducting the study. At each wave, parents consented and youth assented (if younger than 18) and consented (if 18 or older) to participation. Across time points, sleep data collection took place during the regular school year, excluding holidays. The study team delivered actigraphs to the families’ homes, and the youth were instructed to wear the actigraph on their nondominant wrist for seven consecutive nights. Participants also completed a nightly sleep diary that was used to corroborate actigraphy data [44]. Nights during which medication for acute illnesses was used were excluded from analyses. Shortly after the actigraph assessments, parents and youth visited the research laboratory. To compute youths’ body mass index (BMI), their height and weight were measured using a Tanita digital weight scale and wall-mounted stadiometer (Arlington Heights, IL, USA). At T1 and T2, parents completed questionnaires at our research laboratory. At T3, T4, and T5, parents had the option to complete questionnaires remotely.
Sleep.
Objective assessments of youth sleep were obtained via actigraphy recordings. Participants wore Octagonal Basic Motionloggers (Ambulatory Monitoring, Ardsley, NY, USA), which measured motion in 1-minute epochs using the zero-crossing mode. Analyses utilized the Octagonal Motionlogger Interface with Actme software and the analysis software package (Action W2, 2000 Ambulatory Monitoring). To derive youth sleep variables, the analysis package utilized the Sadeh scoring algorithm [45, 46]. Sleep duration was indexed by sleep minutes, the total number of minutes scored as sleep during the sleep period (i.e. actigraphy-based sleep onset time to wake time). To assess sleep quality, we measured sleep efficiency, the percentage of time between sleep onset and wake time scored as sleep (note that this sometimes is referred to as sleep percent in the literature) [47]. Finally, to capture variability in sleep duration and sleep quality over the week of actigraphic assessment, we calculated the variance of sleep minutes and variance of sleep efficiency using the coefficient of variance, which represents intraindividual SD across nights of assessment divided by intraindividual M [48]. Sleep definitions are consistent with the manual that accompanied the actigraphs and software (Ambulatory Monitoring Inc., Ardsley, NY, USA).
At age 9, youth had on average 5.89 (SD = 1.95) nights of actigraphy data. Fifty-nine percent of youth had the mode of seven nights of data, and sleep data were treated as missing for 8% of youth (i.e. had fewer than three nights of data). Missing data were due to forgetting to wear the device or actigraph malfunctions (the latter was rare). At age 10, the average number of nights of actigraphy data for youth was 5.90 (SD = 1.37). The mode of seven nights of data was observed in 42% of youth, and sleep data were considered missing for 3% of youth. At age 11, youth had an average of 5.46 (SD = 1.89) nights of actigraphy data. The mode of seven nights of data was observed in 40% of youth, and sleep data were treated as missing for 8% of youth. At age 17, the average number of nights of actigraphy data for youth was 5.72 (SD = 1.58). Forty-one percent of youth had the mode of seven nights of data, and sleep data were considered missing for 4% of youth. At age 18, the average number of nights of actigraphy data for youth was 4.96 (SD = 2.24). The mode of seven nights of data was observed in 30% of youth, and sleep data were treated as missing for 17% of youth. Actigraphy variables used in analyses were based on youth who had at least three nights of data, in line with recommendations [49].
Externalizing behaviors.
At each time point, mothers and fathers completed the 280-item Personality Inventory for Children-2 (PIC-2) [50] to assess youth externalizing behaviors. The PIC-2 is a well-established measure that has demonstrated good psychometric properties [50, 51]. The externalizing composite assessed symptoms of impulsivity, noncompliance, disruptive behavior, delinquent behavior, and aggression. Parents rated each item as true or false. Missing data rates varied across assessment time points and informants. For the mother report, missing data rates were 22%, 15%, 12%, 8%, and 30% across T1 to T5, respectively. For the father report, missing data rates were much higher with 54%, 43%, 42%, 32%, and 47% of data missing across T1 to T5, respectively. Importantly, some households only had data available from one parent, typically the mother, (e.g. in the case of single-parent households); in these instances, only data from the available parent were used and, accordingly, data were treated as missing only for 18%, 12%, 11%, 5%, and 26% of youth across T1 to T5, respectively. Rates of missing data are within the acceptable range for the use of full information maximum likelihood (FIML) estimation [52]. Because mothers’ and fathers’ reports of youth externalizing behaviors were highly correlated (rs ranged from .65 to .81, ps all < .001, across all waves of data), these were averaged at each time point. Internal consistency for the externalizing scale was good across the five time points (αs ranged from 0.78 to 0.87). Across each time point, 5.2% to 13.0% of children met the criteria for borderline or clinical levels of externalizing behaviors (T ≥ 60) [53]. Because the PIC-2 T scores were age and gender corrected, we utilized the raw PIC-2 externalizing scores to assess growth [54].
Internalizing symptoms.
At each wave, mothers and fathers also reported on youth internalizing symptoms using the PIC-2, which has well-established psychometric properties [50, 51]. The internalizing composite assessed symptoms of depression, anxiety, worry, fear, and psychosomatic problems. Rates of missing data were the same as rates for externalizing symptoms. In the instance of single-parent status, only data from the available parent were used. Mothers’ and fathers’ reports of youth internalizing symptoms were correlated (rs ranged from .44 to .80, ps all < .001, across all waves of data) and, consequently, averaged at each time point. Parents rated each item as true or false. Internal consistency for the Internalizing scale was good across the five time points (αs ranged from .79 to .87). Across the five waves, T scores indicated that 6.0% to 20.9% of children met criteria for borderline or clinical levels of internalizing symptoms (T ≥ 60) [53]. To assess growth, we used raw PIC-2 internalizing scores because PIC-2 T scores were age and gender corrected [54].
Covariates.
In conditional growth models, analyses included four covariates: (1) parent report of youth race (0 = white, 1 = back), (2) parent report of youth sex assigned at birth (0 = female, 1 = male), (3) parent report of family income, indexed by the family’s income-to-needs ratio (family income divided by the federal poverty threshold for household size; US Department of Commerce, 2009–2019), and (4) youth’s standardized BMI computed from height and weight. Measures of each family income (rs ranged from .59 to .85, ps < .001) and youth BMI (rs ranged from .63 to .97, ps < .001) were highly correlated across the five time points. Due to limited within-person variability and to improve model fit, we focused on between-person differences in family income scores and BMI scores by averaging across the five waves and used the variables as time-invariant controls.
Statistical analysis
Latent growth modeling analyses were utilized to assess: (1) developmental trajectories of sleep parameters across ages 9 to 18 and variations in trajectories by participants’ race and sex, and (2) prospective associations between trajectories of youth sleep and trajectories of youth mental health across ages 9 to 18 and the moderating role of participants’ race and sex. Following established procedures, growth models were fit in a step-by-step fashion [54]. First, unconditional univariate growth models were used to determine the trajectory of change and whether there was significant between-person variance in the latent growth parameters. As illustrated in the conceptual analytic model presented in Supplementary Figure S1, factor loadings for sleep and mental health variables were fixed at 0, 0.1, 0.2, 0.8, 0.9 and −0.9, −0.8, −0.7, −0.1, and 0, respectively. Thus, sleep intercepts were set to age 9 and mental health intercepts to age 18 [55]. Chi-square difference tests were utilized to determine the best-fitting growth trajectory [56, 57]. Between-person differences in growth trajectories were assessed by evaluating variances of latent growth parameters. Significant variance in the intercept and linear slope indicates between-person variability at a specific time point and between-person variability in the rate of linear change from ages 9 to 18, respectively. In contrast, nonsignificant variance in the intercept and linear slope indicates youth exhibit similar starting values or similar rates of linear change over time, respectively.
Second, best-fitting unconditional univariate growth models were used in subsequent analyses for aims 1 and 2. For aim 1, we examined whether unconditional growth trajectories of sleep varied by race (black vs. white) and sex (male vs. female). We estimated one sleep parameter and one moderator at a time for a total of eight consecutive models (4 sleep parameters X 2 moderators). We applied the multi-group function in Mplus to constrain each sleep trajectory to be equal across groups and compared the model fit to a model that allowed each sleep trajectory to vary freely across groups [58]. In the result of a significant chi-square difference test, the free-to-vary model is better fitting indicating that growth trajectories vary across groups [59].
For aim 2, the best fitting unconditional univariate growth models of sleep and both externalizing and internalizing problems were used to estimate unconditional parallel growth models. Specifically, we simultaneously entered one sleep parameter and one mental health variable into the model for a total of eight consecutive models (4 sleep X 2 mental health variables). Per established guidelines [54], the best-fitting unconditional parallel growth models were selected for conditional growth model analyses examining moderation by race and sex. To increase methodological rigor, we included the effects of several covariates (i.e. family income, youth race, youth sex, and BMI) as predictors of the latent growth factors of youth sleep and mental health. Covariates were allowed to covary.
Analyses were conducted in Mplus Version 8.4 [60]. We used FIML estimation to handle missing data and retain the full sample for analyses. Data in our sample were missing 13% of values, which is within the acceptable range for use of FIML [52]. A higher rate of missingness was associated with lower family income (r = −.25, p < .001), greater variability in sleep minutes at age 10 (r = .16, p = .03), greater variability in sleep efficiency at age 18 (r = .16, p = .05), shorter sleep at age 18 (r = −.16, p = .05), and greater externalizing symptoms at ages 9 (r = .17, p = .03) and 11 (r = .23, p = .003). Study variables were assessed for normality. Skewed variables (i.e. skewness value > 2.0) were winsorized and data that exceeded three SD were recoded as the score corresponding to three SD. Acceptable model fit included meeting at least two of the following three criteria: χ2/df < 3, comparative fit index > 0.90, and root mean square error of approximation < 0.08 [61].
Results
Preliminary analyses
Descriptive statistics and correlations among study variables are provided in Tables 1 and 2. Whereas moderate to large correlations emerged for externalizing behaviors and internalizing symptoms consistently across ages 9 to 18, correlations across time for sleep parameters were more inconsistent. Sleep minutes and sleep efficiency each exhibited modest to moderate correlations across ages 9 to 17 and 17 to 18. Variability in sleep minutes demonstrated moderate correlations across ages 9 to 11 and 17 to 18. Finally, correlations for variability in sleep efficiency across ages 9 to 17 and 17 to 18 were largely modest, but significant with the exception of age 10. Age 9 sleep minutes was negatively associated with internalizing symptoms at ages 9, 10, and 11. Lower sleep efficiency at age 10 was associated with externalizing behaviors across ages 9 to 18. Prospective correlations between sleep efficiency and internalizing symptoms emerged between ages 9 and 11 and 17 and 18, and a significant concurrent correlation emerged at age 18. Variability in sleep minutes at ages 10 and 17 was also positively associated with externalizing behaviors at ages 17 and 18. Variability in sleep minutes at ages 9 and 10 were positively associated with internalizing symptoms across ages 9 to 18, except between ages 9 and 18. Greater variability in sleep efficiency at age 10 was associated with externalizing behaviors across ages 9 to 18. Concurrent correlations between variability in sleep efficiency and internalizing symptoms emerged at age 9 and prospective correlations emerged between ages 9 and 11, 10 and 18, and 17 and 18.
Table 1.
Correlations Among Study Variables
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Race | — | ||||||||||||||||
| 2. Sex | 0.05 | — | |||||||||||||||
| 3. Income | −0.37* | 0.17* | — | ||||||||||||||
| 4. Body mass index | 0.12 | −0.07 | −0.13 | — | |||||||||||||
| 5. Sleep minutes 9 | −0.13 | −0.13 | 0.22* | −0.13 | — | ||||||||||||
| 6. Sleep minutes 10 | −0.05 | −0.07 | 0.13 | −0.14 | 0.35* | — | |||||||||||
| 7. Sleep minutes 11 | −0.26* | −0.11 | 0.22* | −0.22* | 0.47* | 0.35* | — | ||||||||||
| 8. Sleep minutes 17 | −0.26* | −0.20* | 0.14 | −0.00 | 0.24* | 0.23* | 0.31* | — | |||||||||
| 9. Sleep minutes 18 | −0.21* | −0.21* | 0.02 | −0.01 | 0.12 | 0.15 | 0.17 | 0.38* | — | ||||||||
| 10. Sleep efficiency 9 | −0.02 | −0.13 | 0.19* | 0.00 | 0.79* | 0.30* | 0.36* | 0.11 | −0.11 | — | |||||||
| 11. Sleep efficiency 10 | 0.11 | −0.09 | 0.02 | −0.10 | 0.26* | 0.73* | 0.26* | 0.09 | 0.10 | 0.42* | — | ||||||
| 12. Sleep efficiency 11 | −0.13 | −0.16* | 0.15* | −0.15* | 0.37* | 0.25* | 0.83* | 0.22* | 0.06 | 0.40* | 0.34* | — | |||||
| 13. Sleep efficiency 17 | −0.22* | −0.10 | 0.23* | −0.12 | 0.32 | 0.17* | 0.41* | 0.53* | 0.21* | 0.27* | 0.17* | 0.43* | — | ||||
| 14. Sleep efficiency 18 | −0.17* | −0.05 | 0.13 | −0.05 | 0.10 | 0.09 | 0.08 | 0.20* | 0.51* | 0.15 | 0.05 | 0.11 | 0.32* | — | |||
| 15. V sleep minutes 9 | 0.11 | 0.03 | −0.22* | −0.01 | −0.60* | −0.19* | −0.27* | −0.10 | −0.12 | −0.56* | −0.14 | −0.23* | −0.20* | −0.18 | — | ||
| 16. V sleep minutes 10 | 0.04 | −0.05 | −0.12 | 0.05 | −0.24* | −0.47* | −0.17* | −0.11 | −0.10 | −0.21* | −0.38* | −0.11 | −0.16* | −0.09 | 0.27* | — | |
| 17. V sleep minutes 11 | 0.17* | 0.05 | −0.13 | 0.03 | −0.29* | −0.12 | −0.61* | −0.17* | −0.12 | −0.21* | −0.10 | −0.58* | −0.24* | 0.00 | 0.28* | 0.30* | — |
| 18. V sleep minutes 17 | −0.01 | −0.11 | −0.07 | −0.08 | 0.01 | 0.03 | −0.09 | −0.22* | −0.15 | 0.01 | 0.05 | −0.03 | −0.28* | −0.09 | −0.01 | 0.10 | 0.13 |
| 19. V sleep minutes 18 | −0.03 | −0.23* | −0.07 | −0.03 | −0.04 | 0.02 | 0.09 | 0.07 | −0.19* | 0.03 | 0.06 | 0.05 | 0.12 | −0.19* | 0.12 | 0.04 | 0.08 |
| 20. V sleep efficiency 9 | 0.09 | 0.04 | −0.20* | −0.01 | −0.68* | −0.24* | −0.31* | −0.10 | 0.01 | −0.80* | −0.34* | −0.32* | −0.30* | −0.21* | 0.74* | 0.18* | 0.22* |
| 21. V sleep efficiency 10 | −0.07 | 0.00 | −0.07 | 0.15* | −0.14 | −0.59* | −0.16* | −0.02 | −0.14 | −0.23* | −0.80* | −0.19* | −0.09 | −0.05 | 0.14 | 0.48* | 0.15 |
| 22. V sleep efficiency 11 | 0.16* | 0.08 | −0.15 | 0.05 | −0.28* | −0.17* | −0.75* | −0.19* | −0.09 | −0.25* | −0.16* | −0.83* | −0.31* | 0.00 | 0.18* | 0.15 | 0.71* |
| 23. V sleep efficiency 17 | 0.13 | 0.04 | −0.16* | 0.09 | −0.25* | −0.16* | −0.29* | −0.41* | −0.24* | 0.20* | −0.19* | −0.30* | −0.81* | −0.35* | 0.15 | 0.22* | 0.11 |
| 24. V sleep efficiency 18 | 0.03 | −0.04 | −0.04 | 0.06 | 0.01 | −0.01 | 0.00 | −0.04 | −0.42* | −0.01 | −0.03 | −0.04 | −0.20* | −0.78* | 0.14 | 0.03 | 0.04 |
| 25. Externalizing 9 | 0.13 | 0.08 | −0.26* | 0.08 | −0.14 | −0.04 | −0.03 | −0.06 | −0.17 | −0.16 | 0.02 | −0.06 | −0.31* | −0.21* | 0.14 | 0.12 | −0.02 |
| 26. Externalizing 10 | 0.10 | 0.07 | −0.26* | 0.15* | −0.09 | −0.04 | −0.01 | −0.09 | −0.16 | −0.11 | 0.04 | −0.06 | −0.28* | −0.12 | 0.13 | 0.11 | 0.02 |
| 27. Externalizing 11 | 0.01 | 0.11 | −0.26* | 0.06 | −0.06 | −0.02 | −0.03 | −0.04 | −0.10 | −0.14 | −0.02 | −0.07 | −0.26* | −0.13 | 0.14 | 0.07 | −0.00 |
| 28. Externalizing 17 | −0.04 | −0.04 | −0.17* | 0.09 | −0.07 | −0.11 | −0.04 | −0.06 | 0.02 | −0.13 | −0.07 | −0.09 | −0.25* | −0.10 | 0.13 | 0.23* | 0.03 |
| 29. Externalizing 18 | −0.03 | 0.04 | −0.06 | 0.11 | −0.07 | −0.11 | −0.09 | −0.10 | −0.07 | −0.08 | −0.03 | −0.08 | −0.32* | −0.16 | 0.07 | 0.28* | 0.12 |
| 30. Internalizing 9 | 0.09 | 0.02 | −0.26* | 0.03 | −0.21* | −0.15 | −0.10 | −0.01 | −0.10 | −0.13 | −0.09 | −0.11 | −0.27* | −0.20* | 0.28* | 0.31* | 0.06 |
| 31. Internalizing 10 | 0.03 | −0.01 | −0.24* | 0.12 | −0.18* | −0.11 | −0.03 | −0.03 | −0.13 | −0.12 | −0.04 | −0.07 | −0.17* | −0.16 | 0.28* | 0.24* | 0.02 |
| 32. Internalizing 11 | −0.06 | 0.08 | −0.23* | 0.02 | −0.19* | −0.09 | −0.02 | 0.01 | −0.01 | −0.22* | −0.11 | −0.07 | −0.26* | −0.09 | 0.26* | 0.20* | 0.06 |
| 33, Internalizing 17 | −0.11 | −0.17* | −0.10 | −0.04 | 0.10 | −0.05 | 0.03 | 0.06 | 0.12 | −0.09 | 0.02 | 0.02 | −0.13 | −0.07 | 0.17* | 0.22* | 0.02 |
| 34. Internalizing 18 | −0.17* | −0.11 | 0.01 | 0.03 | −0.17 | −0.13 | 0.04 | 0.03 | −0.05 | −0.11 | −0.05 | 0.07 | −0.19* | −0.20* | 0.18 | 0.33* | 0.00 |
| 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | |
| 18. V sleep minutes 17 | — | ||||||||||||||||
| 19. V sleep minutes 18 | 0.17* | — | |||||||||||||||
| 20. V sleep efficiency 9 | 0.05 | 0.08 | — | ||||||||||||||
| 21. V sleep efficiency 10 | 0.00 | 0.01 | 0.20* | — | |||||||||||||
| 22. V sleep efficiency 11 | 0.10 | 0.03 | 0.21* | 0.13 | — | ||||||||||||
| 23. V sleep efficiency 17 | 0.47* | −0.09 | 0.30* | 0.14 | 0.20* | — | |||||||||||
| 24. V sleep efficiency 18 | 0.16 | 0.31* | 0.10 | 0.08 | 0.06 | 0.29* | — | ||||||||||
| 25. Externalizing 9 | 0.14 | 0.04 | 0.17* | 0.11 | −0.02 | 0.33* | 0.08 | — | |||||||||
| 26. Externalizing 10 | 0.07 | 0.08 | 0.09 | 0.05 | −0.02 | 0.20* | 0.04 | 0.73* | — | ||||||||
| 27. Externalizing 11 | 0.09 | 0.04 | 0.16 | 0.03 | 0.00 | 0.21* | 0.02 | 0.66* | 0.79* | — | |||||||
| 28. Externalizing 17 | 0.17* | 0.01 | 0.15 | 0.09 | 0.03 | 0.26* | 0.05 | 0.55* | 0.57* | 0.56* | — | ||||||
| 29. Externalizing 18 | 0.25* | 0.05 | 0.12 | 0.16* | 0.05 | 0.33* | 0.06 | 0.59* | 0.59* | 0.55* | 0.80* | — | |||||
| 30. Internalizing 9 | 0.08 | −0.09 | 0.21* | 0.23* | 0.03 | 0.26* | 0.14 | 0.56* | 0.34* | 0.30* | 0.34* | 0.25* | — | ||||
| 31. Internalizing 10 | 0.02 | 0.11 | 0.12 | 0.11 | −0.03 | 0.08 | 0.12 | 0.36* | 0.57* | 0.44* | 0.33* | 0.36* | 0.61* | — | |||
| 32. Internalizing 11 | 0.06 | 0.05 | 0.17* | 0.14 | 0.03 | 0.20* | 0.08 | 0.46* | 0.53* | 0.63* | 0.44* | 0.36* | 0.63* | 0.74* | — | ||
| 33. Internalizing 17 | 0.11 | −0.01 | 0.07 | 0.07 | −0.04 | 0.11 | 0.06 | 0.38* | 0.38* | 0.36* | 0.60* | 0.44* | 0.56* | 0.45* | 0.54* | — | |
| 34. Internalizing 18 | 0.15 | 0.05 | 0.15 | 0.19* | −0.05 | 0.20* | 0.12 | 0.43* | 0.36* | 0.32* | 0.56* | 0.65* | 0.54* | 0.52* | 0.55 | 0.79* | — |
Race was coded as 0 = white, 1 = black; sex was coded as 0 = female, 1 = male. V = variability in sleep. *p < .05.
Table 2.
Means and Standard Deviations for Study Variables
| M | SD | |
|---|---|---|
| Income | −0.02 | 0.87 |
| Body mass index | 0.69 | 1.14 |
| Sleep minutes age 9 | 460.12 | 56.14 |
| Sleep minutes age 10 | 445.65 | 52.72 |
| Sleep minutes age 11 | 435.73 | 62.65 |
| Sleep minutes age 17 | 408.80 | 63.59 |
| Sleep minutes age 18 | 392.68 | 63.31 |
| Sleep efficiency age 9 | 88.67 | 7.20 |
| Sleep efficiency age 10 | 88.77 | 7.06 |
| Sleep efficiency age 11 | 88.39 | 8.61 |
| Sleep efficiency age 17 | 93.72 | 6.68 |
| Sleep efficiency age 18 | 92.46 | 7.03 |
| Variability sleep minutes age 9 | 10.93 | 5.58 |
| Variability sleep minutes age 10 | 11.60 | 5.97 |
| Variability sleep minutes age 11 | 11.91 | 7.57 |
| Variability sleep minutes age 17 | 16.82 | 8.33 |
| Variability sleep minutes age 18 | 17.89 | 8.61 |
| Variability sleep efficiency age 9 | 5.79 | 4.56 |
| Variability sleep efficiency age 10 | 5.89 | 4.26 |
| Variability sleep efficiency age 11 | 6.05 | 5.45 |
| Variability sleep efficiency age 17 | 4.83 | 4.47 |
| Variability sleep efficiency age 18 | 5.22 | 4.54 |
| Externalizing age 9 | 5.47 | 4.43 |
| Externalizing age 10 | 4.98 | 4.19 |
| Externalizing age 11 | 4.50 | 4.11 |
| Externalizing age 17 | 4.00 | 4.28 |
| Externalizing age 18 | 3.78 | 4.28 |
| Internalizing age 9 | 4.76 | 3.95 |
| Internalizing age 10 | 4.37 | 3.87 |
| Internalizing age 11 | 3.96 | 3.81 |
| Internalizing age 17 | 5.12 | 4.84 |
| Internalizing age 18 | 5.17 | 5.30 |
Aim 1: developmental trajectories of sleep parameters
To determine the trajectory of growth for each sleep parameter, we fit a series of unconditional univariate growth models for each of the four sleep variables. Details regarding model building are provided in Appendix A. We accepted a linear growth model for variability in sleep minutes and variability in sleep efficiency that allowed observed residual variances to freely vary over time (Models 3d and 4d in Table A1), a linear growth model for sleep minutes that constrained observed residual variances to be equal over time (Model 1c in Table A1), and a linear growth model for sleep efficiency that (a) allowed observed residuals to vary freely and (b) specified a covariance between age 11 and age 17 residual variances (Model 2e in Table A1). Table 3 provides model fit statistics. Given limited research on trajectories of sleep quality, we also considered growth trajectories of wake after sleep onset (WASO) and number of long-wake episodes (LWE). Given high correlations with sleep efficiency and generally consistent growth trajectories to sleep efficiency, we present these trajectories in Appendix A for interested readers.
Table 3.
Model Fit and Unstandardized Parameter Estimates for Final Unconditional Univariate Growth Models of Sleep Parameters and Mental Health
| Model Fit | Unstandardized Parameter Estimated | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| χ2 | df | χ2/df | RMSEA | CFI | Intercept mean (µ) | Intercept variance (σ2) | Slope mean (µ) | Slope variance (σ2) | |
| Sleep minutes | 17.51 | 14 | 1.25 | 0.04 | 0.95 | 453.72*** | 1272.05*** | −64.15*** | 2278.33** |
| Sleep efficiency | 22.53 | 9 | 2.50 | 0.09 | 0.82 | 88.17*** | 24.68*** | 5.99*** | 35.34** |
| Variability in sleep minutes | 6.65 | 10 | 0.67 | 0.00 | 1.00 | 10.75*** | 12.13*** | 7.76*** | 29.50* |
| Variability in sleep efficiency | 9.56 | 10 | 1.15 | 0.00 | 1.00 | 5.98*** | 4.34*** | −1.06* | 7.10 |
| Internalizing symptoms | 31.29** | 14 | 2.24 | 0.08 | 0.94 | 5.13*** | 19.75*** | .83* | 14.89*** |
| Externalizing symptoms | 25.52* | 14 | 1.82 | 0.06 | 0.96 | 3.84*** | 14.90*** | −1.58*** | 12.10*** |
Intercepts centered at age 9. * = p ≤ .05. ** = p < .01. *** = p < .001.
We examined whether the intercepts and linear slopes of each variable exhibited significant between-person variability (Table 3). Across unconditional univariate growth models, the intercept of sleep minutes, sleep efficiency, variability in sleep minutes, and variability in sleep efficiency were each significantly different from zero and evidenced significant between-person variability. Likewise, the linear slope of each variable was significantly different from zero and, also, exhibited significant between-person variability, except for variability in sleep efficiency. On average, sleep minutes decreased, sleep efficiency increased, variability in sleep minutes increased, and variability in sleep efficiency decreased across ages 9 to 18 (Supplementary Figures S2–S5).
Variations in trajectories by race.
Building on the univariate models described above, we examined whether trajectories of sleep parameters differed for black youth and white youth. Details about model building and testing are provided in Appendix B. We accepted (1) the constrained model for variability in sleep minutes (Model 7a in Supplementary Table B1) and (2) the free-to-vary model with constrained observed residuals for white youth for sleep minutes, sleep efficiency, and variability in sleep efficiency (Models 5c, 6c, and 8c in Supplementary Table B1). A significantly better fit for the free-to-vary model for sleep minutes, sleep efficiency, and variability in sleep efficiency indicated that trajectories for these sleep variables significantly varied by race (Table 4).
Table 4.
Unconditional Univariate Growth Models of Sleep Parameters Moderated by Race
| Black | White | |||||||
|---|---|---|---|---|---|---|---|---|
| Intercept Mean (µ) |
Intercept Variance (σ2) |
Slope Mean (µ) |
Slope Variance (σ2) |
Intercept Mean (µ) |
Intercept Variance (σ2) |
Slope Mean (µ) |
Slope Variance (σ2) |
|
| Sleep Minutesa,b | 447.14*** | 1133.54*** | −81.40*** | 2024.94* | 459.44*** | 1293.11*** | −56.98*** | 2412.14** |
| Sleep efficiencyb | 88.71*** | 23.63*** | 3.28** | 26.88 | 87.99*** | 25.92*** | 7.18*** | 27.23* |
| Variability in sleep minutes | 10.75*** | 12.13*** | 7.76*** | 29.50* | 10.75*** | 12.13*** | 7.76*** | 29.50* |
| Variability in sleep efficiencyb | 6.21*** | 6.12* | −.75 | 13.38 | 5.78*** | 3.91* | −1.20* | .99 |
Intercepts centered at age 9. * = p ≤ .05. ** = p < .01. *** = p < .001.
aIntercepts significantly vary by race.
bSlopes significantly vary by race.
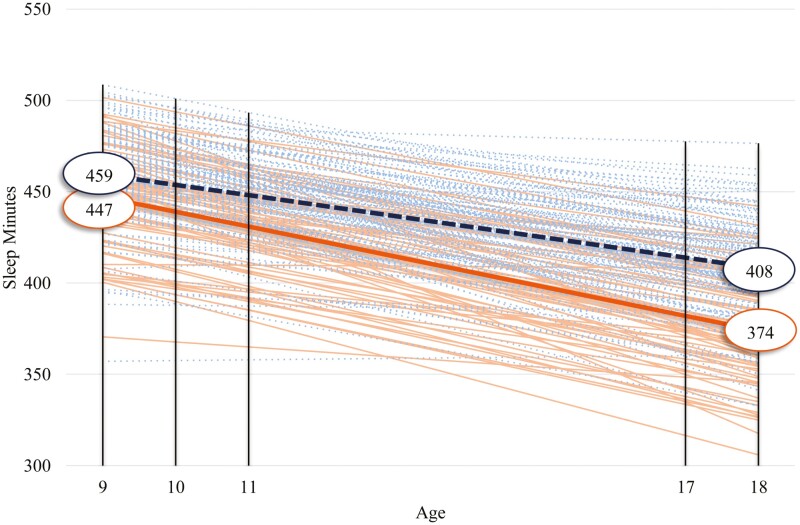
We conducted two follow-up tests. First, utilizing a series of chi-square difference tests we consecutively constrained the intercept mean, slope mean, intercept variance, and slope variance to be equal across groups for each sleep variable. If the constrained-to-equality model is worse fitting, this would indicate that the parameter in question significantly varies across groups (i.e. the model where the parameter is allowed to vary freely across groups is better fitting). Chi-square difference tests revealed that (1) the slope mean for sleep minutes significantly differed for black youth (slope µ = −81.40) and white youth (slope µ = −56.98), ∆χ2(1) = 4.81, p = .03; and (2) the slope mean for sleep efficiency significantly differed for black youth (slope µ = 3.28) and white youth (slope µ = 7.18), ∆χ2(1) = 7.72, p = .01. Chi-square difference tests results indicated that black youth exhibited steeper declines in sleep minutes and less steep increases in sleep efficiency.
Second, we utilized a series of t-tests to determine whether there were significant absolute differences in intercept and slope means across groups for each sleep variable. Regarding sleep minutes, follow-up t-tests revealed that the intercepts (t (197) = −2.36, p = .02) and slopes (t (197) = −3.45, p = .001) for black and white youth significantly differed. Black youth had shorter sleep than their white counterparts by approximately 12 minutes at age 9 (Figure 1). Moreover, black youth exhibited a steeper decline in sleep minutes losing about 81 minutes of sleep across ages 9 to 18 relative to white youth losing roughly 57 minutes of sleep, thus resulting in a 32-minute discrepancy across groups by age 18. Turning to sleep efficiency, a follow-up t-test revealed that black youth and white youth exhibited generally comparable sleep quality at age 9 (approximately within 1% of each other; t (197) = 0.97, p = .33; Figure 2). Although black and white youth experienced an improvement in sleep efficiency over time, white youth experienced a significantly steeper improvement resulting in approximately a 3% discrepancy at age 18 (t (197) = −5.04, p < .001). Finally, follow-up t-tests did not reveal significant differences in either the intercept (t (197) = 1.35, p = .18) or slope (t (197) = −1.30, p = .19) of variability in sleep efficiency based on race (Figure 3).
Figure 1.
Estimated sample mean and individual growth in sleep minutes for black and white youth. Dotted lines represent the sample mean (bolded) and individual participants’ growth for white youth; solid lines represent the sample mean (bolded) and individual participants’ growth for black youth.
Figure 2.
Estimated sample mean and individual growth in sleep efficiency for black and white youth. Dotted lines represent the sample mean (bolded) and individual participants’ growth for white youth; solid lines represent the sample mean (bolded) and individual participants’ growth for black youth.
Figure 3.
Estimated sample mean and individual growth in variability in sleep efficiency for black and white youth. Dotted lines represent the sample mean (bolded) and individual participants’ growth for white youth; solid lines represent the sample mean (bolded) and individual participants’ growth for black youth.
Variations in trajectories by sex.
Building on the univariate models described above, we examined whether trajectories of sleep parameters differed for female and male youth. Details about model building and testing are provided in Appendix C. We accepted the constrained model for sleep efficiency, variability in sleep minutes, and variability in sleep efficiency (Models 10a, 11a, and 12a in Supplementary Table C1), and the free-to-vary model with constrained observed residuals for female youth for sleep minutes (Model 9c in Supplementary Table C1), indicating that the trajectory for sleep minutes significantly differed across female and male youth (Table 5). Subsequently, we conducted two follow-up tests. First, we conducted a series of chi-square difference tests where we consecutively constrained the intercept mean, slope mean, intercept variance, and slope variance to be equal across groups for each sleep variable. No significant differences emerged.
Table 5.
Unconditional Univariate Growth Models of Sleep Parameters Moderated by Sex
| Male | Female | |||||||
|---|---|---|---|---|---|---|---|---|
| Intercept mean (µ) |
Intercept variance (σ2) |
Slope mean (µ) |
Slope variance (σ2) |
Intercept mean (µ) |
Intercept variance (σ2) |
Slope mean (µ) |
Slope variance (σ2) |
|
| Sleep minutesa,b | 448.97*** | 1419.02*** | −72.30*** | 2142.59* | 459.56*** | 1194.38*** | −55.94*** | 2269.68** |
| Sleep efficiency | 88.16*** | 24.68*** | 5.99** | 35.35** | 88.16*** | 24.68*** | 5.99** | 35.35** |
| Variability in sleep minutes | 10.75*** | 12.13*** | 7.76*** | 29.50* | 10.75*** | 12.13*** | 7.76*** | 29.50* |
| Variability in sleep efficiency | 5.98*** | 4.34** | −1.06* | 7.10 | 5.98*** | 4.34** | −1.06* | 7.10 |
Intercepts centered at age 9. * = p ≤ .05. ** = p < .01. *** = p < .001.
aIntercepts significantly vary by race.
bSlopes significantly vary by race.
Second, we conducted a series of t-tests to determine whether there were significant absolute differences in intercept and slope means across groups for each sleep variable. Follow-up t-tests revealed that intercepts (t (197) = −2.06, p = .04) and slopes (t (197) = −2.46, p = .02) significantly differed for male youth and female youth. Specifically, male youth had significantly less sleep than female youth by approximately 11 minutes at age 9 (Figure 4). Male youth also exhibited a steeper decline in sleep minutes losing about 72 minutes of sleep across ages 9 to 18 relative to female youth losing roughly 56 minutes, thus resulting in a 26-minute discrepancy across groups by age 18.
Figure 4.
Estimated sample mean and individual growth in sleep minutes for female and male youth. Dotted lines represent the sample mean (bolded) and individual participants’ growth for male youth; solid lines represent the sample mean (bolded) and individual participants’ growth for female youth.
Aim 2: prospective associations between sleep trajectories and mental health trajectories
Prior to fitting unconditional parallel growth models, we fit unconditional univariate growth models for externalizing and internalizing symptoms. Details regarding model building are provided in Appendix D. We accepted linear growth models that constrained observed residual variances to be equal over time for internalizing and externalizing symptoms (Models 13c and 14c in Supplementary Table D1). We examined whether the intercepts and linear slopes of each variable exhibited significant between-person variability (Table 3). Across internalizing and externalizing models, the intercepts of internalizing and externalizing were each significantly different from zero and evidenced significant between-person variability at each measurement occasion. Likewise, the linear slope of each variable was significantly different from zero and exhibited significant between-person variability. Whereas externalizing symptoms decreased over time (Supplementary Figure S6), internalizing symptoms increased from age 9 to 18 (Supplementary Figure S7).
Building on final unconditional univariate models of sleep and mental health (Models 1c, 2e, 3d, 4d, 13c, and 14c described in Appendix A and D), we examined unconditional parallel growth models that simultaneously estimated one sleep parameter and one mental health outcome at a time (Supplementary Table S1). Model fit across the eight unconditional growth models (i.e. models 15–22), was good. Consequently, we accepted these models to use in conditional main effects growth models. To examine prospective associations between trajectories of sleep and trajectories of mental health, we modeled a series of conditional main effect growth models. Building on models 15–22, we: (1) respecified covariances between the intercept and slope of sleep and the intercept and slope of mental health to be a direct effect, and (2) controlled for age, sex, BMI, and family income. Accordingly, in eight consecutive models, we examined the intercept and slope of each sleep parameter as predictors of the intercept and slope of both externalizing and internalizing symptoms (Supplementary Figure S1).
As shown in Table 6, a significant association emerged between the intercept of sleep efficiency and the intercept of externalizing behaviors (Supplementary Figure S8). Better sleep at age 9 was associated with lower levels of externalizing symptoms at age 18. Additionally, the slope of sleep efficiency predicted the intercept of both externalizing (Supplementary Figure S8) and internalizing symptoms (Supplementary Figure S9). Improvements in sleep efficiency over time were associated with lower levels of externalizing and internalizing symptoms at age 18.
Table 6.
Standardized Parameter Estimates for Associations Between Sleep Parameters and Mental Health
| Externalizing | Internalizing | |||||||
|---|---|---|---|---|---|---|---|---|
| Sleep minutes | Sleep efficiency | Variability in sleep minutes | Variability in sleep efficiency | Sleep minutes | Sleep efficiency | Variability in sleep minutes | Variability in sleep efficiency | |
| i MH on | ||||||||
| iSLP | −0.19 | −0.57* | 0.51** | 0.31† | −0.16 | −0.49† | 0.40* | 0.22 |
| sSLP | −0.12 | −0.67* | 0.37* | 0.28 | 0.04 | −0.64* | 0.10 | 0.23 |
| Race | −0.18† | −0.26* | −0.13 | −0.15† | −0.19† | −0.31** | −0.20* | −0.21* |
| Sex | −0.03 | −0.05 | 0.09 | 0.01 | −0.17* | −0.21* | −0.15 | −0.15* |
| Income | −0.13† | −0.05 | −0.07 | −0.10 | −0.05 | −0.01 | 0.00 | −0.06 |
| BMI | 0.05 | 0.02 | 0.11 | 0.03 | −0.08 | −0.07 | −0.03 | −0.06 |
| s MH on | ||||||||
| iSLP | −0.27† | −0.19 | 0.47** | 0.28 | −0.10 | −0.23 | 0.22 | 0.04 |
| sSLP | 0.05 | 0.05 | 0.31 | 0.01 | 0.02 | −0.35 | 0.30 | 0.19 |
| Race | −0.10 | −0.08 | −0.09 | −0.08 | −0.14 | −0.21† | −0.12 | −0.16 |
| Sex | −0.18 | −0.17 | −0.08 | −0.18 | −0.28** | −0.29** | −0.18 | −0.25* |
| Income | 0.31* | 0.27** | 0.33** | 0.32* | 0.26* | 0.27* | 0.26* | 0.22† |
| BMI | −0.14 | −0.08 | −0.03 | −0.10 | −0.15 | −0.14† | −0.09 | −0.12 |
| Fit indices | ||||||||
| χ 2 | 89.36† | 94.77* | 83.32 | 91.09* | 103.05* | 112.79*** | 92.33* | 97.59* |
| df | 73 | 68 | 69 | 69 | 73 | 68 | 69 | 69 |
| χ2/df | 1.22 | 1.39 | 1.21 | 1.32 | 1.41 | 1.66 | 1.34 | 1.41 |
| RMSEA | 0.03 | 0.04 | 0.03 | 0.04 | 0.05 | 0.06 | 0.04 | 0.05 |
| CFI | 0.97 | 0.95 | 0.98 | 0.96 | 0.94 | 0.92 | 0.95 | 0.94 |
Sleep parameters were centered at age 9. Adjustment outcomes were centered at age 18. i, intercept; s, linear slope; SLP, sleep predictor; MH, mental health outcome; RMSEA, root mean square error of approximation; CFI, comparative fit index. † = p < .10. *p ≤ .05. **p ≤ .01. ***p ≤ .001.
Significant associations also emerged between latent growth parameters of variability in sleep minutes and latent growth parameters of internalizing and externalizing symptoms. Specifically, the intercept of variability in sleep minutes predicted the intercept of externalizing (Supplementary Figure S10) and internalizing symptoms (Supplementary Figure S11). Greater variability in sleep minutes at age 9 was associated with higher levels of externalizing and internalizing symptoms at age 18. Additionally, the intercept of variability in sleep minutes predicted the slope of externalizing symptoms (Supplementary Figure S10). Greater variability in sleep minutes at age 9 was associated with increases in externalizing symptoms across ages 9 to 18. Finally, the slope of variability in sleep minutes predicted the intercept of externalizing symptoms (Supplementary Figure S10). Increases in variability in sleep minutes over time were associated with higher levels of externalizing symptoms at age 18.
Variations in trajectories by race.
We conducted follow-up analyses to determine whether links between the intercept and slope of each sleep parameter and the intercept and slope of externalizing and internalizing symptoms varied by youth race. Across eight consecutive models, we mean-centered youth race and computed interaction terms by multiplying youth race with the intercept and slope of each sleep parameter. We subsequently regressed the intercept and slope of mental health on the intercept and slope of sleep, youth race, youth race X intercept, youth race X slope, and covariates. Youth race moderated the association between the slope of variability in sleep efficiency and the intercept of internalizing symptoms (β = −0.37, p = .04; Supplementary Table S2). Although neither simple slope was significantly different from zero (black youth: b = 2.60, p = .40; white youth: b = −1.40, p = .68), black youth experiencing increases in variability in sleep efficiency over time had more internalizing symptoms at age 18 (M = 8.26) than white youth with similar increases in the same sleep parameter M = −0.19), a difference of 2.43 SD (Figure 5). No other significant interactions emerged, and caution should be exercised in interpreting this effect given the possibility that it is a chance finding.
Figure 5.
Moderating effect of youth race in the pathway between the linear slope of variability in sleep efficiency across ages 9 to 18 and the intercept of internalizing symptoms at age 18.
Variations in trajectories by sex.
We also conducted follow-up analyses to determine whether youth sex moderated the pathway between the intercept and slope of each sleep parameter and the intercept and slope of externalizing and internalizing symptoms. Following steps to examine the moderating effects of youth race, we mean-centered youth sex and computed interaction terms by multiplying youth sex with the intercept and slope of each sleep parameter. Across eight consecutive models, we regressed the intercept and slope of mental health on the intercept and slope of sleep, youth sex, youth sex X intercept, youth sex X slope, and the covariates. Three significant interactions emerged (Supplementary Table S3). Youth sex moderated links between the slope of sleep minutes and: (1) the intercept of internalizing symptoms and (2) the slope of internalizing symptoms. Follow-up simple slope analyses revealed that simple slopes were not significant in the link between the slope of sleep minutes and the intercept of internalizing symptoms (male youth: b = 0.09, p = .15; female youth: b = −0.08, p = .16). However, female youth experiencing declines or less steep increases in sleep minutes over time had higher levels of internalizing symptoms at age 18 (M = 25.29) compared to male youth experiencing comparable changes in sleep duration (M = 11.35), a difference of 4.58 SD (Figure 6). Likewise, neither simple slope was significant in the link between the slope of sleep minutes and the slope of internalizing symptoms (male youth: b = 0.10, p = .21; female youth: b = −0.06, p = .28). Among youth experiencing declines or less steep increases in sleep duration over time, degree of change in internalizing symptoms differed by 14.70 SD with male youth exhibiting smaller increases in internalizing symptoms (M = 2.80) than female youth (M = 24.81; Figure 7).
Figure 6.
Moderating effect of youth sex in the pathway between the linear slope of sleep minutes across ages 9 to 18 and the intercept of internalizing symptoms at age 18.
Figure 7.
Moderating effect of youth sex in the pathway between the linear slope of sleep minutes across ages 9 to 18 and the linear slope of internalizing symptoms across ages 9 to 18.
Youth sex also moderated the pathway between the slope of sleep efficiency and the intercept of internalizing symptoms. Simple slope analyses revealed that the slope of sleep efficiency predicted the intercept of internalizing symptoms for male youth (b = −0.71, p = .02) but not female youth (b = 0.14, p = .74). More specifically, among male youth, increases in sleep efficiency across ages 9 to 18 were associated with lower levels of internalizing symptoms at age 18 (Figure 8). When examining the moderating effect of youth sex on the intercepts and slopes of variability in sleep minutes and variability in sleep efficiency, we received inadmissible solutions and thus did not further pursue moderation analyses.
Figure 8.
Moderating effect of youth sex in the pathway between the linear slope of sleep efficiency across ages 9 to 18 and the intercept of internalizing symptoms at age 18.
Alternate direction of effects.
While several studies found that sleep is a stronger predictor of mental health than vice versa [3], the other direction of effects has also been reported [62]. Thus, in addition to testing the predictive effect of trajectories of sleep on trajectories of mental health, we evaluated the alternate direction of effects. In doing so, we examined the intercepts and linear slopes of externalizing and internalizing symptoms as predictors of the intercepts and linear slopes of sleep parameters. These findings are presented in Appendix E.
Discussion
Along with many biological, psychological, and social developmental processes, sleep changes from childhood to adolescence. While some changes are well-documented, such as decreasing sleep minutes, changes in other sleep parameters have been less clear or received less consideration in longitudinal investigations. This study sought to fill a gap in the literature by examining trajectories of four sleep parameters from childhood to adolescence (sleep minutes, sleep efficiency, and regularity in sleep minutes and efficiency). Importantly, sleep occurs in the context of other developmental processes; therefore, the present study modeled sleep trajectories as related to mental health trajectories from childhood to adolescence. Analysis of developmental trajectories revealed a significant linear change in trajectories of sleep duration, quality, and variability in duration and efficiency, as well as considerable variation in trajectories by youths’ race and sex. Conditional effects models revealed significant associations among the latent growth parameters of sleep and mental health. Moreover, these links were qualified by three significant interactions with youth sex assigned at birth and one interaction with race.
Sleep minutes
Consistent with prior investigations [1, 15], and our hypotheses, we observed decreases in sleep minutes at a rate of approximately 6 minutes per year from ages 9 to 18. By age 18, this amounted to a decrease of almost one full hour of sleep relative to when adolescents were children at age 9. While such changes are consistent with the existing literature [1, 15–18] and often interpreted as a decreasing sleep need across development, the decline in sleep duration from childhood to adolescence takes place within the context of organic changes and social-environmental demands. As youth mature, they experience a shift in circadian timing toward a later sleep phase and a slower building of homeostatic pressure that can result in evening wakefulness [14, 63], both contributing to later bedtimes. Furthermore, youth typically gain increasing autonomy around sleep schedules as they advance into adolescence [64, 65]. Moreover, afterschool/evening activities (e.g. school responsibilities, extracurriculars, and socializing with friends) and morning demands (e.g. school start times) further compress the time window available for sleep as children transition to adolescence.
Sleep efficiency
Sleep efficiency improved from late childhood to adolescence, resulting in an average 5% improvement by age 18 compared to age 9. There are plausible explanations for this improvement. First, conditions that may contribute to sleep fragmentation in childhood (e.g. nightmares) tend to decrease from childhood to adolescence [66]. Second, adolescents may gain increasing control over their sleep environment and engage in behaviors that facilitate sleep [67, 68]. Third, as sleep duration decreases from childhood to adolescence, sleep quality may increase as a compensatory mechanism [16, 69, 70]. However, our findings contradict previous work that has demonstrated declines in sleep quality over time [19, 20]. Differences in findings may be explained at an analytic level. Similar to the current study, investigations that have found improvements in sleep quality over time have utilized latent growth modeling to isolate the between-person variance in sleep quality growth parameters [20, 21]. In contrast, evidence for declines in sleep quality over time has been based on mean values [19, 20], which do not account for how repeated measures may be influenced by differences in inter- and intra-individual variance.
Supporting our hypotheses, we found several effects of the intercept and slope of sleep efficiency on the intercepts of youth externalizing and internalizing symptoms. First, higher levels of sleep efficiency at age 9 (intercept) were associated with lower levels of externalizing symptoms at age 18 (intercept). Second, improvements in sleep efficiency from age 9 to 18 (slope) were associated with lower levels of externalizing and internalizing symptoms at age 18. Findings are consistent with previous research examining between-person variability in subjective sleep quality growth parameters as predictors of subsequent internalizing and externalizing symptoms in late childhood (age 11) [35] and late adolescence (ages 17 and 18) [20, 21]. Our findings have important empirical and translational implications. At an empirical level, extending previous research, we provided the first assessment of the developmental trajectory of an objective assessment of sleep quality across late childhood and adolescence that links between-person variability in both initial levels of sleep quality and change in sleep quality over time with youth mental health outcomes. Although we found a developmental trend for improvements in sleep efficiency in unconditional growth modeling (Table 3, Supplementary Figure S3), conditional growth modeling revealed that youth who consistently experienced low sleep efficiency or declines in efficiency over time were most at risk for mental health symptoms nine years later (Table 6, Supplementary Figures S8 and S9). Thus, at a translational level, these findings underscore the value of developing interventions that target sleep problems not only with the goal of influencing current well-being, but also determining influences over a long period of time.
Intraindividual variability in sleep minutes
Consistent with the only other study that we know of that has examined trajectories of intraindividual variability in sleep duration [1], we found that variability in sleep minutes increased over development. Supporting our hypothesis, variability increased by about 1 minute per year, resulting in average night-to-night fluctuations of approximately 18 minutes by age 18, compared to 10 minutes at age 9. Increases in variability in sleep minutes are likely influenced by social jetlag, which reflects the gap between socially determined sleep schedules (e.g. school–work days) and free sleep schedules (e.g. weekend sleep) [71, 72]. Randler and colleagues [73] found near-linear increases in social jetlag across infancy and late adolescence, paralleling the linear change in variability observed in our study. Beginning as early as preschool, it is common for youth to stay awake later on weekends than on school nights [71]. In addition to the widening difference in weekend-weekday sleep duration, Boatswain-Jacques and colleagues [1] further hypothesized, that increasing variability in sleep duration over time may alternatively be the result of overall day-to-day fluctuations. For instance, factors such as academic and discretionary activities may fluctuate on a daily basis, increasing variability in sleep schedule and duration [71].
As we expected, findings revealed several associations among growth parameters for variability in sleep minutes and youth externalizing and internalizing symptoms. First, greater variability in sleep duration at age 9 (intercept) was associated with higher levels of internalizing and externalizing symptoms at age 18 (intercept). Second, greater variability in sleep duration at age 9 was also associated with increases in externalizing symptoms from age 9 to 18. Our findings are consistent with previous research which demonstrated that variability in sleep duration predicted mental health symptoms up to two years later [3]. Extending this work, we demonstrate that variability in sleep minutes is a robust predictor of mental health nine years later. These findings suggest that clinical interventions aimed at increasing consistency in night-to-night sleep routines may well prevent and improve problems both concurrently and over development.
Intraindividual variability in sleep efficiency
Finally, while some prior studies have reported changes in sleep efficiency over time, trajectories of variability in efficiency have not been examined. Our findings show a pattern of decreasing variability in sleep efficiency over time. Each year, youth on average experienced a slight (.11%) reduction in variability in efficiency resulting in a very small (1%) reduction in variability in sleep efficiency at age 18 compared to age 9. Furthermore, variability in sleep efficiency did not exhibit significant interindividual differences in rates of change indicating that youth exhibited comparable declines in variability in sleep efficiency over time. In contrast to previous research with youth [3] and contrary to expectations, we did not find links between variability in sleep efficiency and mental health. Differences in analytic approaches may explain the null effects. We utilized latent growth modeling, which partitioned inter- and intra-individual variance. Studies utilizing alternative approaches may conflate both sources of variance, potentially leading to different results [33]. This is the first study to test between-person differences in trajectories of variability in sleep efficiency and additional research on downstream consequences is warranted.
Moderation by youth race
Addressing a significant gap in research, we examined how developmental trajectories of multiple sleep parameters vary by race. Several significant differences emerged. First, black youth experienced shorter sleep at age 9, having approximately 12 minutes less sleep than their counterparts. This gap almost tripled by age 18, with black youth experiencing significantly steeper declines in sleep duration, resulting in approximately 34 minutes less sleep than white youth. Second, both black youth and white youth had similar levels of sleep efficiency at age 9 (89% and 88%, respectively). However, by age 18, white youth exhibited improvements in sleep efficiency, showing approximately 3% more efficient sleep than black youth. The findings suggest that black youth are at comparatively greater risk for sleep problems over time, as defined by sleep duration and quality. Systemic problems including discrimination and racism are known correlates and predictors of poor sleep for African American children and adolescents [17, 23, 74]. Racial/ethnic discrimination is associated with more sleep disturbance, and—in analyses of daily associations—youth report lower sleep quality at night immediately after experiences of discrimination [25]. Discrimination-related sleep disparities are one proposed mechanism associated with subsequent health disparities [18, 23]. For instance, we found, in the current study, that race moderated the link between changes in variability in sleep efficiency and youth internalizing symptoms—specifically, among youth experiencing increases in variability in sleep efficiency over time, black youth exhibited significantly more internalizing symptoms at age 18 than white youth. Researchers should exercise caution in interpreting this one moderation effect given the possibility that it is a chance finding.
Moderation by youth sex
We compared male and female youths’ sleep trajectories from late childhood to late adolescence. Our analyses extend prior work which demonstrated that stability in between-person differences in sleep from late childhood to early adolescence varies by sex [1]. In our study, multi-group moderation analyses revealed that at age 9, males slept about 11 minutes less than females. By age 18, this difference more than doubled, with males experiencing a significantly steeper decline in sleep duration, resulting in approximately 26 minutes less sleep than females. Findings corroborate prior work [1, 15] and demonstrate that sex differences in sleep minutes persist and grow into late adolescence. In contrast, sex-related effects were not evident for changes in sleep efficiency or variability. Sex differences in sleep minutes may be the result of various biological and environmental factors. For example, pubertal timing and hormonal shifts may predispose boys to stay up later at night despite having to wake up at similar times [32, 75]. Further, environmental influences including socialization practices and higher screen time may explain sex differences [31, 75]. Some research suggests that girls require more sleep than boys [75]. However, explanations for sex-related effects are speculative and more research is needed to fully understand the role of such individual differences.
Few studies have examined the moderating role of sex in longitudinal associations between sleep and mental health. Consistent with previous research that has found worse mental health among female youth with shorter sleep [38, 41], we found that the effects of sleep duration were more pronounced for female youth. Among youth experiencing declines or less steep increases in sleep duration over time, female youth reported higher levels of internalizing symptoms at age 18 and greater increases in internalizing symptoms over time relative to males. Conversely, we found that the effect of sleep efficiency was greater among male youth. Specifically, males who had improved sleep efficiency across ages 9 to 18 had fewer internalizing symptoms at 18, compared to males whose sleep efficiency declined over time. Our findings mirror the mixed effects in the literature. Additionally, they extend them by considering longer-term developmental trajectories across late childhood and late adolescence, an issue that requires more exploration in the field.
Limitations
It is important to interpret findings in the context of several study limitations. First, although the current hypotheses were tested in a sociodemographically and racially diverse sample, the findings may not be generalizable to other samples (e.g. clinical samples, urban samples, affluent families, and individuals of other ethnicities). Second, a 6-year gap occurred in data collection between time 3 (i.e. age 11) and time 4 (i.e. age 17). Thus, we cannot account for developmental trajectories during this time period. Although linear growth models fit our data well, an alternative possibility is that growth may exhibit shifts, directional changes, or even periods of stability during unmeasured developmental stages of early and mid-adolescence, followed by the linear changes we observed in late adolescence. Relatedly, our analyses were restricted to ages 9 and 18, and we cannot account for changes in sleep prior to age 9 or after age 18. Third, we assessed variability in sleep over a 1-week period and longer time frames may further clarify the nature of associations with youth mental health. Fourth, we utilized parent-report of youth mental health. Finally, we examined trajectories of change in sleep and mental health. These models were designed to examine associations among rank order stability in individual differences in sleep and externalizing and internalizing symptoms over development. Although these models revealed important associations (e.g. youth consistently experiencing inadequate sleep were most at risk for subsequent mental health symptoms), an important remaining question is how individuals’ sleep and mental health change over time relative to their own averages or scores at a previous time point [76]. For instance, some youth may exhibit worse sleep efficiency in adolescence relative to their sleep efficiency in childhood; however, despite exhibiting declines in their sleep efficiency, they may still achieve better sleep efficiency relative to the sample mean and, consequently, maintain their rank ordering from childhood to adolescence. These youth would exhibit higher levels of sleep efficiency relative to the sample mean while displaying lower levels of sleep efficiency relative to their own mean.
Conclusions
Findings contribute to an emerging literature documenting developmental trajectories of sleep across childhood and adolescence. Expanding on previous studies examining trajectories of objective assessments of sleep, we provide the first test of developmental trajectories of objective measures of sleep efficiency and variability in sleep efficiency. We found significant linear growth in four sleep parameters (i.e. sleep minutes, sleep efficiency, and variability in sleep minutes and efficiency). Moreover, there was significant between-person variability in growth for sleep minutes, sleep efficiency, and variability in sleep minutes. There were notable variations in trajectories by race and sex, and significant associations emerged among growth parameters for sleep and mental health. Given the long-term effect of sleep on youth externalizing and internalizing outcomes, researchers should test the long-term effects of sleep interventions.
Supplementary Material
Acknowledgments
This research was supported by Grants R01-HL136752 and R01-HL093246 awarded to Mona El-Sheikh from the National Heart, Lung, and Blood Institute. The content is solely the responsibility of the authors and does not necessarily reflect the official views of the National Institutes of Health. We wish to thank our research laboratory staff, particularly the laboratory coordinator Bridget Wingo, for data collection and preparation, as well as the adolescents and parents who participated.
Footnotes
Follow-up independent samples t-Test revealed that the additional families recruited at T2 did not significantly differ from families recruited at T1 on demographic or primary study variables.
Contributor Information
Morgan J Thompson, Department of Human Development and Family Science, Auburn University, Auburn, AL, USA.
Brian T Gillis, Department of Human Development and Family Science, Auburn University, Auburn, AL, USA.
J Benjamin Hinnant, Department of Human Development and Family Science, Auburn University, Auburn, AL, USA.
Stephen A Erath, Department of Human Development and Family Science, Auburn University, Auburn, AL, USA.
Joseph A Buckhalt, Department of Human Development and Family Science, Auburn University, Auburn, AL, USA.
Mona El-Sheikh, Department of Human Development and Family Science, Auburn University, Auburn, AL, USA.
Data availability
Data are not available yet for sharing with others. Per National Institutes of Health data sharing guidelines, they will be available to other scholars at a later date after the completion of the ongoing longitudinal study.
Financial Disclosure
Financial disclosure: None except funding from the National Heart, Lung, and Blood Institute (Grants R01-HL136752 and R01-HL093246; PI M. El-Sheikh). Nonfinancial disclosures: The authors have declared no nonfinancial conflicts of interest.
References
- 1. Boatswain-Jacques A-F, Dusablon C, Cimon-Paquet C, et al. From early birds to night owls: a longitudinal study of actigraphy-assessed sleep trajectories during the transition from pre- to early adolescence. Sleep. 2023;46(11). doi: 10.1093/sleep/zsad127 [DOI] [PubMed] [Google Scholar]
- 2. Reynolds AM, Spaeth AM, Hale L, et al. Pediatric sleep: current knowledge, gaps, and opportunities for the future. Sleep. 2023;48(7). doi: 10.1093/sleep/zsad060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kelly RJ, Zeringue MM, El-Sheikh M.. Adolescents’ sleep and adjustment: reciprocal effects. Child Dev. 2022;93(2):540–555. doi: 10.1111/cdev.13703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Liu J, Magielski J, Glenn A, Raine A.. The bidirectional relationship between sleep and externalizing behavior: a systematic review. Sleep Epidemiol. 2022;2:100039. doi: 10.1016/j.sleepe.2022.100039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Short MA, Chee MWL.. Adolescent sleep restriction effects on cognition and mood. Prog Brain Res. 2019;246:55–71. doi: 10.1016/bs.pbr.2019.02.008 [DOI] [PubMed] [Google Scholar]
- 6. Smith VC, Leppert KA, Alfano CA, Dougherty LR.. Construct validity of the Parent–Child Sleep Interactions Scale (PSIS): associations with parenting, family stress, and maternal and child psychopathology. Sleep Med. 2014;15(8):942–951. doi: 10.1016/j.sleep.2014.04.002 [DOI] [PubMed] [Google Scholar]
- 7. Williamson AA, Zendarski N, Lange K, et al. Sleep problems, internalizing and externalizing symptoms, and domains of health-related quality of life: bidirectional associations from early childhood to early adolescence. Sleep. 2021;44(1). doi: 10.1093/sleep/zsaa139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jung E, Jin B.. Associations between sleep problems, cognitive, and socioemotional functioning from preschool to adolescence. Child Youth Care Forum. 2019;48(6):829–848. doi: 10.1007/s10566-019-09509-5 [DOI] [Google Scholar]
- 9. Shimizu M, Gillis BT, Buckhalt JA, El-Sheikh M.. Linear and nonlinear associations between sleep and adjustment in adolescence. Behav Sleep Med. 2020;18(5):690–704. doi: 10.1080/15402002.2019.1665049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. El-Sheikh M, Sadeh A.. Sleep and development: introduction to the monograph. Monogr Soc Res Child Dev. 2015;80(1):1–14. doi: 10.1111/mono.12141 [DOI] [PubMed] [Google Scholar]
- 11. Becker SP, Sidol CA, Van Dyk TR, Epstein JN, Beebe DW.. Intraindividual variability of sleep/wake patterns in relation to child and adolescent functioning: a systematic review. Sleep Med Rev. 2017;34:94–121. doi: 10.1016/j.smrv.2016.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bei B, Wiley JF, Trinder J, Manber R.. Beyond the mean: a systematic review on the correlates of daily intraindividual variability of sleep/wake patterns. Sleep Med Rev. 2016;28:108–124. doi: 10.1016/j.smrv.2015.06.003 [DOI] [PubMed] [Google Scholar]
- 13. Carskadon MA. Sleep in adolescents: the perfect storm. Pediatr Clin North Am. 2011;58(3):637–647. doi: 10.1016/j.pcl.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Crowley SJ, Wolfson AR, Tarokh L, Carskadon MA.. An update on adolescent sleep: new evidence informing the perfect storm model. J Adolesc. 2018;67:55–65. doi: 10.1016/j.adolescence.2018.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. El-Sheikh M, Bagley EJ, Keiley MK, Erath SA.. Growth in body mass index from childhood into adolescence: the role of sleep duration and quality. J Early Adolesc. 2014;34(8):1145–1166. doi: 10.1177/0272431613519499 [DOI] [Google Scholar]
- 16. Evans MA, Buysse DJ, Marsland AL, et al. Meta-analysis of age and actigraphy-assessed sleep characteristics across the lifespan. Sleep. 2021;44(9). doi: 10.1093/sleep/zsab088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hoyt LT, Deardorff J, Marceau K, et al. Girls’ sleep trajectories across the pubertal transition: emerging racial/ethnic differences. J Adolesc Health. 2018;62(4):496–503. doi: 10.1016/j.jadohealth.2017.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Maslowsky J, Ozer EJ.. Developmental trends in sleep duration in adolescence and young adulthood: evidence from a national United States sample. J Adolesc Health. 2014;54(6):691–697. doi: 10.1016/j.jadohealth.2013.10.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kelly RJ, El-Sheikh M.. Reciprocal relations between children’s sleep and their adjustment over time. Dev Psychol. 2014;50(4):1137–1147. doi: 10.1037/a0034501 [DOI] [PubMed] [Google Scholar]
- 20. Shimizu M, Zeringue MM, Erath SA, Hinnant JB, El-Sheikh M.. Trajectories of sleep problems in childhood: associations with mental health in adolescence. Sleep. 2021;44(3). doi: 10.1093/sleep/zsaa190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wang B, Isensee C, Becker A, et al. Developmental trajectories of sleep problems from childhood to adolescence both predict and are predicted by emotional and behavioral problems. Front Psychol. 2016;7:1874–1874. doi: 10.3389/fpsyg.2016.01874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goodhines P, Barker D, Gredvig-Ardito C, et al. (2022). Characterizing sleep regularity from actigraphy in younger and older adolescents. Sleep. 2022;45. doi: 10.1093/sleep/zsac079.179 [DOI] [Google Scholar]
- 23. El-Sheikh M, Gillis BT, Saini EK, Erath SA, Buckhalt JA.. Sleep and disparities in child and adolescent development. Child Dev Perspect. 2022;16(4):200–207. doi: 10.1111/cdep.12465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Guglielmo D, Gazmararian JA, Chung J, Rogers AE, Hale L.. Racial/ethnic sleep disparities in US school-aged children and adolescents: a review of the literature. Sleep Health. 2018;4(1):68–80. doi: 10.1016/j.sleh.2017.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yip T, Cheon YM, Wang Y, Cham H, Tryon W, El-Sheikh M.. Racial disparities in sleep: associations with discrimination among ethnic/racial minority adolescents. Child Dev. 2020;91(3):914–931. doi: 10.1111/cdev.13234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yip T, Xie M, Cham H, El-Sheikh M.. Linking ethnic/racial discrimination to adolescent mental health: sleep disturbances as an explanatory pathway. Child Dev. 2022;93(4):973–994. doi: 10.1111/cdev.13747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McWood LM, Zeringue MM, Martin-Piñón O, Buckhalt JA, El-Sheikh M.. Linear and nonlinear associations between the sleep environment, presleep conditions, and sleep in adolescence: moderation by race and socioeconomic status. Sleep Med. 2022;93:90–99. doi: 10.1016/j.sleep.2021.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spilsbury JC, Storfer-Isser A, Drotar D, Rosen CL, Kirchner HL, Redline S.. Effects of the home environment of school-aged children’s sleep. Sleep. 2005;28(11):1419–1427. doi: 10.1093/sleep/28.11.1419 [DOI] [PubMed] [Google Scholar]
- 29. Sladek MR, Doane LD, Park H.. Latino adolescents’ daily bicultural stress and sleep: gender and school context moderation. Health Psychol. 2020;39(3):179–189. doi: 10.1037/hea0000824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Martin-Piñón O, Erath SA, El SM.. Linking autonomic nervous system reactivity with sleep in adolescence: sex as a moderator. Dev Psychobiol. 2021;63(4):650–661. doi: 10.1002/dev.22041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Franco P, Putois B, Guyon A, et al. Sleep during development: sex and gender differences. Sleep Med Rev. 2020;51:101276. doi: 10.1016/j.smrv.2020.101276 [DOI] [PubMed] [Google Scholar]
- 32. Lucien JN, Ortega MT, Shaw ND.. Sleep and puberty. Curr Opin Endocr Metab Res. 2021;17:1–7. doi: 10.1016/j.coemr.2020.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hamaker EL, Kuiper RM, Grasman RPPP.. A critique of the cross-lagged panel model. Psychol Methods. 2015;20(1):102–116. doi: 10.1037/a0038889 [DOI] [PubMed] [Google Scholar]
- 34. Akbar SA, Mattfeld AT, Laird AR, McMakin DL.. Sleep to Internalizing Pathway in Young Adolescents (SIPYA): a proposed neurodevelopmental model. Neurosci Biobehav Rev. 2022;140:104780. doi: 10.1016/j.neubiorev.2022.104780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ksinan Jiskrova G, Vazsonyi AT, Klánová J, Dušek L.. Childhood sleep functioning as a developmental precursor of adolescent adjustment problems. Child Psychiatry Hum Dev. 2020;51(2):239–253. doi: 10.1007/s10578-019-00926-0 [DOI] [PubMed] [Google Scholar]
- 36. El-Sheikh M, Kelly RJ, Buckhalt JA, Hinnant JB.. Children’s sleep and adjustment over time: the role of socioeconomic context. Child Dev. 2010;81(3):870–883. doi: 10.1111/j.1467-8624.2010.01439.x [DOI] [PubMed] [Google Scholar]
- 37. El-Sheikh M, Bub KL, Kelly RJ, Buckhalt JA.. Children’s sleep and adjustment: a residualized change analysis. Dev Psychol. 2013;49(8):1591–1601. doi: 10.1037/a0030223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mathew GM, Hale L, Chang A-M.. Sex moderates relationships among school night sleep duration, social jetlag, and depressive symptoms in adolescents. J Biol Rhythms. 2019;34(2):205–217. doi: 10.1177/0748730419828102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nunes S, Campbell MK, Klar N, Reid GJ, Stranges S.. Relationships between sleep and internalizing problems in early adolescence: results from Canadian National Longitudinal Survey of Children and Youth. J Psychosom Res. 2020;139:110279. doi: 10.1016/j.jpsychores.2020.110279 [DOI] [PubMed] [Google Scholar]
- 40. van Zundert RMP, van Roekel E, Engels RCME, Scholte RHJ.. Reciprocal associations between adolescents’ night-time sleep and daytime affect and the role of gender and depressive symptoms. J Youth Adolesc. 2015;44(2):556–569. doi: 10.1007/s10964-013-0009-3 [DOI] [PubMed] [Google Scholar]
- 41. Zink J, O’Connor SG, Blachman-Demner DR, Wolff-Hughes DL, Berrigan D.. Examining the bidirectional associations between sleep duration, screen time, and internalizing symptoms in the ABCD study. J Adolesc Health. 2024;74(3):496–503. doi: 10.1016/j.jadohealth.2023.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rubens SL, Evans SC, Becker SP, Fite PJ, Tountas AM.. Self-reported time in bed and sleep quality in association with internalizing and externalizing symptoms in school-age youth. Child Psychiatry Hum Dev. 2017;48(3):455–467. doi: 10.1007/s10578-016-0672-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Voelkle MC. Latent growth curve modeling as an integrative approach to the analysis of change. Psychol Sci. 2007;49(4):375–414. [Google Scholar]
- 44. Acebo C, Carskadon M.. Scoring Actigraph Data using ACTION-W 2. Providence, RI: Brown University, Bradley Sleep Center; 2001. [Google Scholar]
- 45. Sadeh A, Acebo C, Seifer R, Aytur S, Carskadon MA.. Activity-based assessment of sleep-wake patterns during the 1st year of life. Infant Behav Dev. 1995;18(3):329–337. doi: 10.1016/0163-6383(95)90021-7 [DOI] [Google Scholar]
- 46. Sadeh A, Sharkey M, Carskadon MA.. (1994). Activity-based sleep-wake identification: an empirical test of methodological issues. Sleep. 1994;17(3):201–207. doi: 10.1093/sleep/17.3.201 [DOI] [PubMed] [Google Scholar]
- 47. Ancoli-Israel S, Martin JL, Blackwell T, et al. The SBSM guide to actigraphy monitoring: clinical and research applications. Behav Sleep Med. 2015;13:S4–S38. doi: 10.1080/15402002.2015.1046356 [DOI] [PubMed] [Google Scholar]
- 48. Snedecor GW, Cochran WG.. Statistical Methods. 6th ed. Ames, IA: The Iowa State College Press; 1967. [Google Scholar]
- 49. Acebo C, Sadeh A, Seifer R, et al. Estimating sleep patterns with activity monitoring in children and adolescents: how many nights are necessary for reliable measures? Sleep. 1999;22(1):95–103. doi: 10.1093/sleep/22.1.95 [DOI] [PubMed] [Google Scholar]
- 50. Lachar D, Gruber CP.. Personality Inventory for Children. 2nd ed. Los Angeles, CA: Western Psychological Services; 2001. [Google Scholar]
- 51. Wirt RD, Lachar D, Klinedinst JK, Seat PD.. Multidimensional Description of Child Personality: A Manual for the Personality Inventory of Children. Los Angeles, CA: Western Psychological Services; 1990. [Google Scholar]
- 52. Enders CK, Bandalos DL.. The relative performance of full information maximum likelihood estimation for missing data in structural equation models. Struct Equation Model. 2001;8(3):430–457. doi: 10.1207/s15328007sem0803_5 [DOI] [Google Scholar]
- 53. Lachar D, Gruber CP.. Personality Inventory for Youth (PIY). Los Angeles, CA: Western Psychological Services; 1995. [Google Scholar]
- 54. Singer JD, Willett JB.. Applied longitudinal Data Analysis: Modeling Change and Event Occurrence. London: Oxford University Press; 2003. [Google Scholar]
- 55. Biesanz JC, Deeb-Sossa N, Papadakis AA, Bollen KA, Curran PJ.. The role of coding time in estimating and Interpreting growth curve models. Psychol Methods. 2004;9(1):30–52. doi: 10.1037/1082-989X.9.1.30 [DOI] [PubMed] [Google Scholar]
- 56. Bollen KA. Structural Equations with Latent Variables. New York, NY: Wiley; 1989. [Google Scholar]
- 57. Kline RB. Principles and Practice of Structural Equation Modeling. New York, NY: Guilford; 2015. [Google Scholar]
- 58. Selig JP, Card NA, Little TD.. Latent variable structural equation modeling in cross-cultural research: multigroup and multilevel approaches. In: van de Vijver FJR, van Hemert DA, Poortinga Y, eds. Individuals and Cultures in Multi-Level Analysis. Mahwah, NJ: Erlbaum; 2015. [Google Scholar]
- 59. Werner C, Schermelleh-Engel K.. Deciding Between Competing Models: Chi-Square Difference Tests. Frankfurt: Goethe University; 2010. [Google Scholar]
- 60. Muthén LK, Muthén BO.. Mplus User’s Guide. 8th ed. Los Angeles, CA: Muthén & Muthén; 2019. [Google Scholar]
- 61. Browne MW, Cudeck R.. Alternative ways of assessing model fit. In: Bollen KA, Long JS, eds. Testing Structural Equation Models. Beverly Hills, CA: Sage; 1993: 111–135. doi: 10.1177/0049124192021002005 [DOI] [Google Scholar]
- 62. Tochigi M, Usami S, Matamura M, et al. Annual longitudinal survey at up to five time points reveals reciprocal effects of bedtime delay and depression/anxiety in adolescents. Sleep Med. 2016;17:81–86. doi: 10.1016/j.sleep.2015.08.024 [DOI] [PubMed] [Google Scholar]
- 63. Fontanellaz-Castiglione CE, Markovic A, Tarokh L.. Sleep and the adolescent brain. Curr Opin Physiol. 2020;15:167–171. doi: 10.1016/j.cophys.2020.01.008 [DOI] [Google Scholar]
- 64. Russo PM, Bruni O, Lucidi F, Ferri R, Violani C.. Sleep habits and circadian preference in Italian children and adolescents. J Sleep Res. 2007;16(2):163–169. doi: 10.1111/j.1365-2869.2007.00584.x [DOI] [PubMed] [Google Scholar]
- 65. Short MA, Gradisar M, Gill J, Camfferman D.. Identifying adolescent sleep problems. PLoS One. 2013;8(9):e75301–e75306. doi: 10.1371/journal.pone.0075301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Mindell JA. Sleep disorders in children. Health Psychol. 1993;12(2):151–162. doi: 10.1037//0278-6133.12.2.151 [DOI] [PubMed] [Google Scholar]
- 67. Godsell S, White J.. Adolescent perceptions of sleep and influences on sleep behaviour: a qualitative study. J Adolesc. 2019;73:18–25. doi: 10.1016/j.adolescence.2019.03.010 [DOI] [PubMed] [Google Scholar]
- 68. Kadzikowska-Wrzosek R. Insufficient sleep among adolescents: the role of bedtime procrastination, chronotype and autonomous vs. controlled motivational regulations. Curr Psychol. 2020;39(3):1031–1040. doi: 10.1007/s12144-018-9825-7 [DOI] [Google Scholar]
- 69. Kuula L, Pesonen A-K, Merikanto I, et al. Development of late circadian preference: sleep timing from childhood to late adolescence. J Pediatr. 2018;194:182–189.e1. doi: 10.1016/j.jpeds.2017.10.068 [DOI] [PubMed] [Google Scholar]
- 70. Pesonen A-K, Martikainen S, Heinonen K, et al. Continuity and change in poor sleep from childhood to early adolescence. Sleep. 2014;37(2):289–297. doi: 10.5665/sleep.3400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nicholson L, Bohnert AM, Crowley SJ.. A developmental perspective on sleep consistency: preschool age through emerging adulthood. Behav Sleep Med. 2023;21(1):97–116. doi: 10.1080/15402002.2021.2024192 [DOI] [PubMed] [Google Scholar]
- 72. Wittmann M, Dinich J, Merrow M, Roenneberg T.. Social jetlag: misalignment of biological and social time. Chronobiol Int. 2006;23(1-2):497–509. doi: 10.1080/07420520500545979 [DOI] [PubMed] [Google Scholar]
- 73. Randler C, Vollmer C, Kalb N, Itzek-Greulich H.. Breakpoints of time in bed, midpoint of sleep, and social jetlag from infancy to early adulthood. Sleep Med. 2019;57:80–86. doi: 10.1016/j.sleep.2019.01.023 [DOI] [PubMed] [Google Scholar]
- 74. Slopen N, Lewis TT, Williams DR.. Discrimination and sleep: a systematic review. Sleep Med. 2016;18:88–95. doi: 10.1016/j.sleep.2015.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Olds T, Blunden S, Petkov J, Forchino F.. The relationships between sex, age, geography and time in bed in adolescents: a meta-analysis of data from 23 countries. Sleep Med Rev. 2010; 14(6):371–378. doi: 10.1016/j.smrv.2009.12.002 [DOI] [PubMed] [Google Scholar]
- 76. Bainter SA, Howard AL.. Comparing within-person effects from multivariate longitudinal models. Dev Psychol. 2016;52(12):1955–1968. doi: 10.1037/dev0000215 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are not available yet for sharing with others. Per National Institutes of Health data sharing guidelines, they will be available to other scholars at a later date after the completion of the ongoing longitudinal study.