Abstract
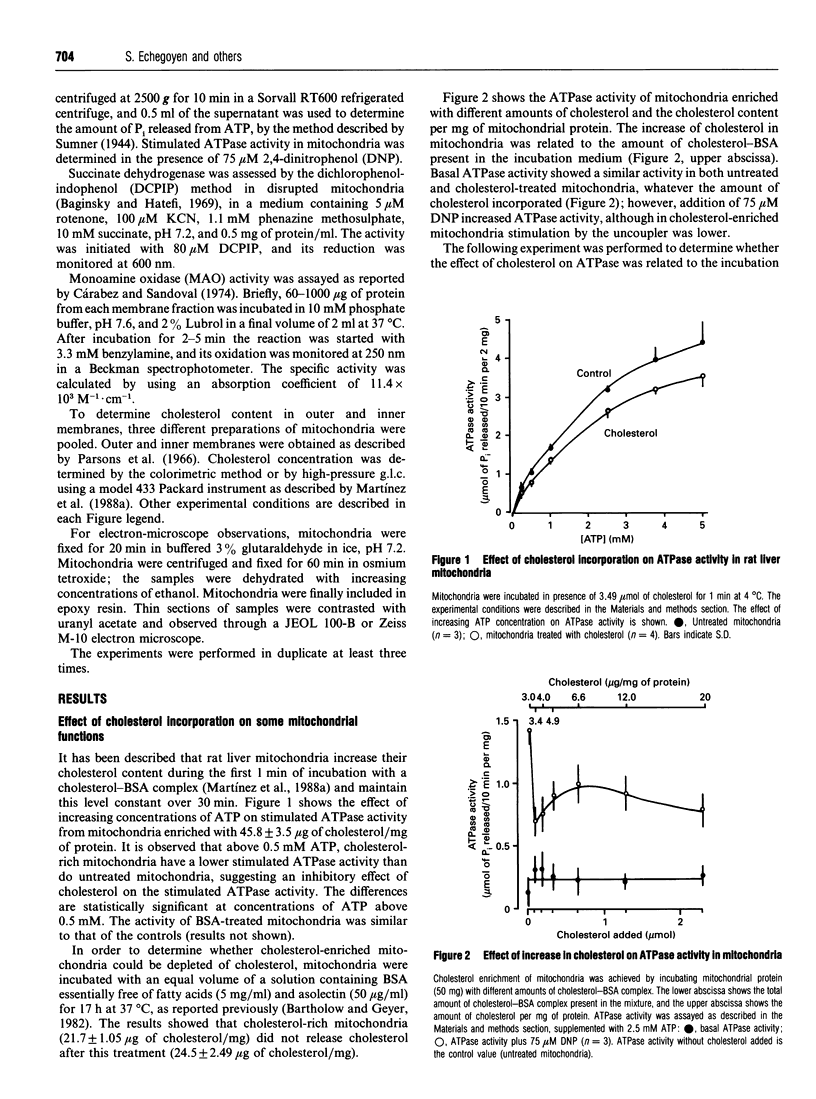
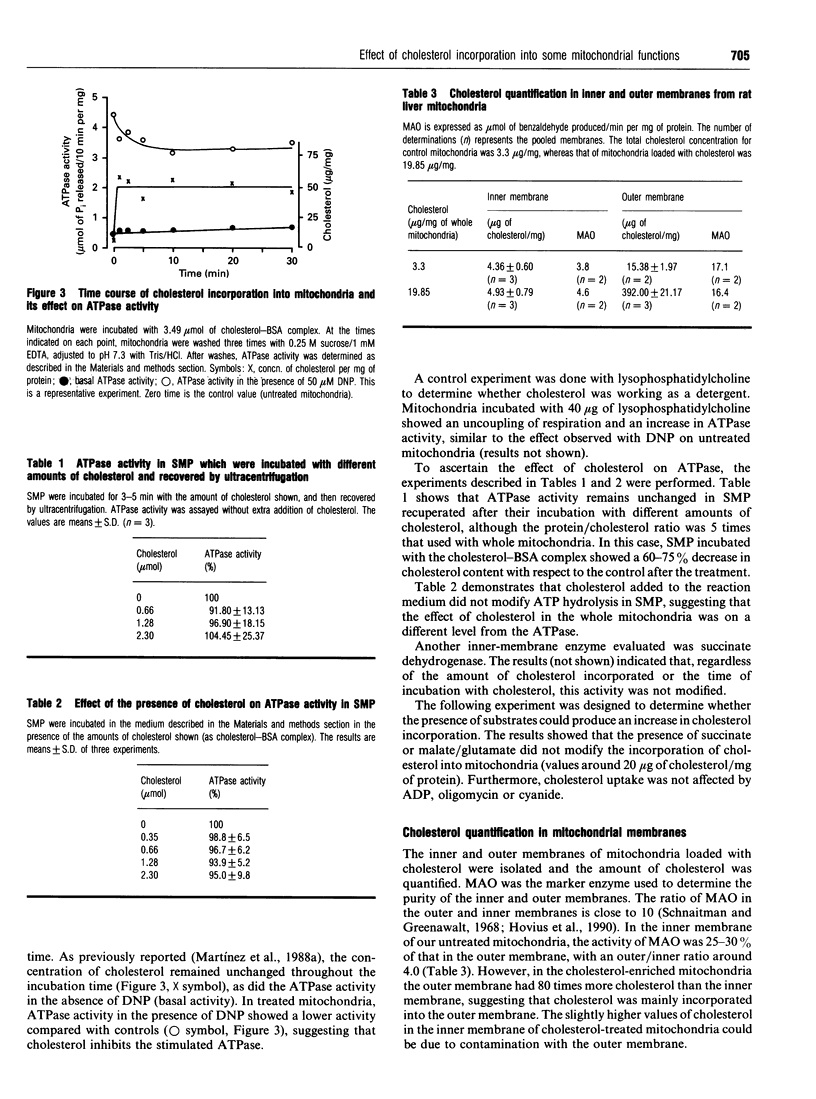
The effect of cholesterol incorporation on some functions of the mitochondrial inner membrane and on the morphology of rat liver mitochondria was studied. Basal ATPase and succinate dehydrogenase activities remained unchanged after cholesterol was incorporated into the mitochondria; however, uncoupled ATPase activity was partially inhibited. The presence of several substrates and inhibitors did not change the amount of cholesterol incorporated, which was localized mostly in the outer membrane. Electron-microscope observations revealed the presence of vesicles between the outer and inner membranes; these vesicles increased in number with the amount of cholesterol incorporated. The data suggest that cholesterol induces the formation of vesicles from the outer membrane, and modifies the activity of stimulated ATPase.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baginsky M. L., Hatefi Y. Reconstitution of succinate-coenzyme Q reductase (complex II) and succinate oxidase activities by a highly purified, reactivated succinate dehydrogenase. J Biol Chem. 1969 Oct 10;244(19):5313–5319. [PubMed] [Google Scholar]
- Bartholow L. C., Geyer R. P. Sterol efflux from mammalian cells induced by human serum albumin-phospholipid complexes. Dependence on phospholipid acyl chain length, degree of saturation, and net charge. J Biol Chem. 1982 Mar 25;257(6):3126–3130. [PubMed] [Google Scholar]
- Bellini F., Phillips M. C., Pickell C., Rothblat G. H. Role of the plasma membrane in the mechanism of cholesterol efflux from cells. Biochim Biophys Acta. 1984 Nov 7;777(2):209–215. doi: 10.1016/0005-2736(84)90422-x. [DOI] [PubMed] [Google Scholar]
- Bruckdorfer K. R., Sherry M. K. The solubility of cholesterol and its exchange between membranes. Biochim Biophys Acta. 1984 Jan 11;769(1):187–196. doi: 10.1016/0005-2736(84)90022-1. [DOI] [PubMed] [Google Scholar]
- Chanderbhan R., Noland B. J., Scallen T. J., Vahouny G. V. Sterol carrier protein2. Delivery of cholesterol from adrenal lipid droplets to mitochondria for pregnenolone synthesis. J Biol Chem. 1982 Aug 10;257(15):8928–8934. [PubMed] [Google Scholar]
- Chávez E., Bravo C. Anisotropic action of cetyl pyridinium chloride on rat heart mitochondria. Arch Biochem Biophys. 1982 Jan;213(1):81–86. doi: 10.1016/0003-9861(82)90442-8. [DOI] [PubMed] [Google Scholar]
- Colbeau A., Nachbaur J., Vignais P. M. Enzymic characterization and lipid composition of rat liver subcellular membranes. Biochim Biophys Acta. 1971 Dec 3;249(2):462–492. doi: 10.1016/0005-2736(71)90123-4. [DOI] [PubMed] [Google Scholar]
- Coleman P. S., Lavietes B., Born R., Weg A. Cholesterol enrichment of normal mitochondria in vitro: a model system with properties of hepatoma mitochondria. Biochem Biophys Res Commun. 1978 Sep 14;84(1):202–207. doi: 10.1016/0006-291x(78)90282-6. [DOI] [PubMed] [Google Scholar]
- Coleman P. S. Membrane cholesterol and tumor bioenergetics. Ann N Y Acad Sci. 1986;488:451–467. doi: 10.1111/j.1749-6632.1986.tb46578.x. [DOI] [PubMed] [Google Scholar]
- Cornelius F. Functional reconstitution of the sodium pump. Kinetics of exchange reactions performed by reconstituted Na/K-ATPase. Biochim Biophys Acta. 1991 Mar 7;1071(1):19–66. doi: 10.1016/0304-4157(91)90011-k. [DOI] [PubMed] [Google Scholar]
- Daum G. Lipids of mitochondria. Biochim Biophys Acta. 1985 Jun 12;822(1):1–42. doi: 10.1016/0304-4157(85)90002-4. [DOI] [PubMed] [Google Scholar]
- Dorbani L., Jancsik V., Linden M., Leterrier J. F., Nelson B. D., Rendon A. Subfractionation of the outer membrane of rat brain mitochondria: evidence for the existence of a domain containing the porin-hexokinase complex. Arch Biochem Biophys. 1987 Jan;252(1):188–196. doi: 10.1016/0003-9861(87)90023-3. [DOI] [PubMed] [Google Scholar]
- Feo F., Canuto R. A., Bertone G., Garcea R., Pani P. Cholesterol and phospholipid composition of mitochondria and microsomes isolated from morris hepatoma 5123 and rat liver. FEBS Lett. 1973 Jul 1;33(2):229–232. doi: 10.1016/0014-5793(73)80199-1. [DOI] [PubMed] [Google Scholar]
- Fugler L., Clejan S., Bittman R. Movement of cholesterol between vesicles prepared with different phospholipids or sizes. J Biol Chem. 1985 Apr 10;260(7):4098–4102. [PubMed] [Google Scholar]
- Graham J. M., Green C. The properties of mitochondria enriched in vitro with cholesterol. Eur J Biochem. 1970 Jan;12(1):58–66. doi: 10.1111/j.1432-1033.1970.tb00820.x. [DOI] [PubMed] [Google Scholar]
- Hartl F. U., Pfanner N., Nicholson D. W., Neupert W. Mitochondrial protein import. Biochim Biophys Acta. 1989 Jan 18;988(1):1–45. doi: 10.1016/0304-4157(89)90002-6. [DOI] [PubMed] [Google Scholar]
- Hovius R., Lambrechts H., Nicolay K., de Kruijff B. Improved methods to isolate and subfractionate rat liver mitochondria. Lipid composition of the inner and outer membrane. Biochim Biophys Acta. 1990 Jan 29;1021(2):217–226. doi: 10.1016/0005-2736(90)90036-n. [DOI] [PubMed] [Google Scholar]
- Kimura T. Transduction of ACTH signal from plasma membrane to mitochondria in adrenocortical steroidogenesis. Effects of peptide, phospholipid, and calcium. J Steroid Biochem. 1986 Nov;25(5B):711–716. doi: 10.1016/0022-4731(86)90299-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lange Y., Matthies H. J. Transfer of cholesterol from its site of synthesis to the plasma membrane. J Biol Chem. 1984 Dec 10;259(23):14624–14630. [PubMed] [Google Scholar]
- Lange Y., Ramos B. V. Analysis of the distribution of cholesterol in the intact cell. J Biol Chem. 1983 Dec 25;258(24):15130–15134. [PubMed] [Google Scholar]
- Levrat C., Louisot P. Dual localization of the mitochondrial phospholipase A2: outer membrane contact sites and inner membrane. Biochem Biophys Res Commun. 1992 Mar 16;183(2):719–724. doi: 10.1016/0006-291x(92)90542-s. [DOI] [PubMed] [Google Scholar]
- Madden T. D., Vigo C., Bruckdorfer K. R., Chapman D. The incorporation of cholesterol into inner mitochondrial membranes and its effect on lipid phase transition. Biochim Biophys Acta. 1980 Jul;599(2):528–537. doi: 10.1016/0005-2736(80)90197-2. [DOI] [PubMed] [Google Scholar]
- Martínez F., Eschegoyen S., Briones R., Cuellar A. Cholesterol increase in mitochondria: a new method of cholesterol incorporation. J Lipid Res. 1988 Aug;29(8):1005–1011. [PubMed] [Google Scholar]
- Martínez F., Gamboa S., Díaz-Sánchez V. Biochemical effects of gossypol in isolated mitochondria: monovalent cations and ATPase activity. Int J Biochem. 1988;20(2):189–192. doi: 10.1016/0020-711x(88)90485-5. [DOI] [PubMed] [Google Scholar]
- McLean L. R., Phillips M. C. Cholesterol desorption from clusters of phosphatidylcholine and cholesterol in unilamellar vesicle bilayers during lipid transfer or exchange. Biochemistry. 1982 Aug 17;21(17):4053–4059. doi: 10.1021/bi00260a022. [DOI] [PubMed] [Google Scholar]
- McLean L. R., Phillips M. C. Mechanism of cholesterol and phosphatidylcholine exchange or transfer between unilamellar vesicles. Biochemistry. 1981 May 12;20(10):2893–2900. doi: 10.1021/bi00513a028. [DOI] [PubMed] [Google Scholar]
- Ohki S., Leonards K. S. A possible role of cholesterol in membrane adhesion. Biochemistry. 1984 Nov 6;23(23):5578–5581. doi: 10.1021/bi00318a030. [DOI] [PubMed] [Google Scholar]
- Ohlendieck K., Riesinger I., Adams V., Krause J., Brdiczka D. Enrichment and biochemical characterization of boundary membrane contact sites from rat-liver mitochondria. Biochim Biophys Acta. 1986 Sep 11;860(3):672–689. doi: 10.1016/0005-2736(86)90567-5. [DOI] [PubMed] [Google Scholar]
- Parsons D. F., Williams G. R., Chance B. Characteristics of isolated and purified preparations of the outer and inner membranes of mitochondria. Ann N Y Acad Sci. 1966 Jul 14;137(2):643–666. doi: 10.1111/j.1749-6632.1966.tb50188.x. [DOI] [PubMed] [Google Scholar]
- Rouslin W., MacGee J., Gupte S., Wesselman A., Epps D. E. Mitochondrial cholesterol content and membrane properties in porcine myocardial ischemia. Am J Physiol. 1982 Feb;242(2):H254–H259. doi: 10.1152/ajpheart.1982.242.2.H254. [DOI] [PubMed] [Google Scholar]
- Sabine J. R. Defective control of lipid biosynthesis in cancerous and precancerous liver. Prog Biochem Pharmacol. 1975;10:269–307. [PubMed] [Google Scholar]
- Scallen T. J., Pastuszyn A., Noland B. J., Chanderbhan R., Kharroubi A., Vahouny G. V. Sterol carrier and lipid transfer proteins. Chem Phys Lipids. 1985 Sep;38(3):239–261. doi: 10.1016/0009-3084(85)90019-2. [DOI] [PubMed] [Google Scholar]
- Schnaitman C., Greenawalt J. W. Enzymatic properties of the inner and outer membranes of rat liver mitochondria. J Cell Biol. 1968 Jul;38(1):158–175. doi: 10.1083/jcb.38.1.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider H., Höchli M., Hackenbrock C. R. Relationship between the density distribution of intramembrane particles and electron transfer in the mitochondrial inner membrane as revealed by cholesterol incorporation. J Cell Biol. 1982 Aug;94(2):387–393. doi: 10.1083/jcb.94.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector A. A., Yorek M. A. Membrane lipid composition and cellular function. J Lipid Res. 1985 Sep;26(9):1015–1035. [PubMed] [Google Scholar]
- Steck T. L., Kezdy F. J., Lange Y. An activation-collision mechanism for cholesterol transfer between membranes. J Biol Chem. 1988 Sep 15;263(26):13023–13031. [PubMed] [Google Scholar]
- Stevens V. L., Tribble D. L., Lambeth J. D. Regulation of mitochondrial compartment volumes in rat adrenal cortex by ether stress. Arch Biochem Biophys. 1985 Oct;242(1):324–327. doi: 10.1016/0003-9861(85)90508-9. [DOI] [PubMed] [Google Scholar]
- Sumner J. B. A METHOD FOR THE COLORIMETRIC DETERMINATION OF PHOSPHORUS. Science. 1944 Nov 3;100(2601):413–414. doi: 10.1126/science.100.2601.413. [DOI] [PubMed] [Google Scholar]
- Vahouny G. V., Chanderbhan R., Noland B. J., Irwin D., Dennis P., Lambeth J. D., Scallen T. J. Sterol carrier protein2. Identification of adrenal sterol carrier protein2 and site of action for mitochondrial cholesterol utilization. J Biol Chem. 1983 Oct 10;258(19):11731–11737. [PubMed] [Google Scholar]
- Wattenberg B. W., Silbert D. F. Sterol partitioning among intracellular membranes. Testing a model for cellular sterol distribution. J Biol Chem. 1983 Feb 25;258(4):2284–2289. [PubMed] [Google Scholar]
- Werner S., Neupert W. Functional and biogenetical heterogeneity of the inner membrane of rat-liver mitochondria. Eur J Biochem. 1972 Feb 15;25(2):379–396. doi: 10.1111/j.1432-1033.1972.tb01707.x. [DOI] [PubMed] [Google Scholar]
- Yeagle P. L. Cholesterol and the cell membrane. Biochim Biophys Acta. 1985 Dec 9;822(3-4):267–287. doi: 10.1016/0304-4157(85)90011-5. [DOI] [PubMed] [Google Scholar]



