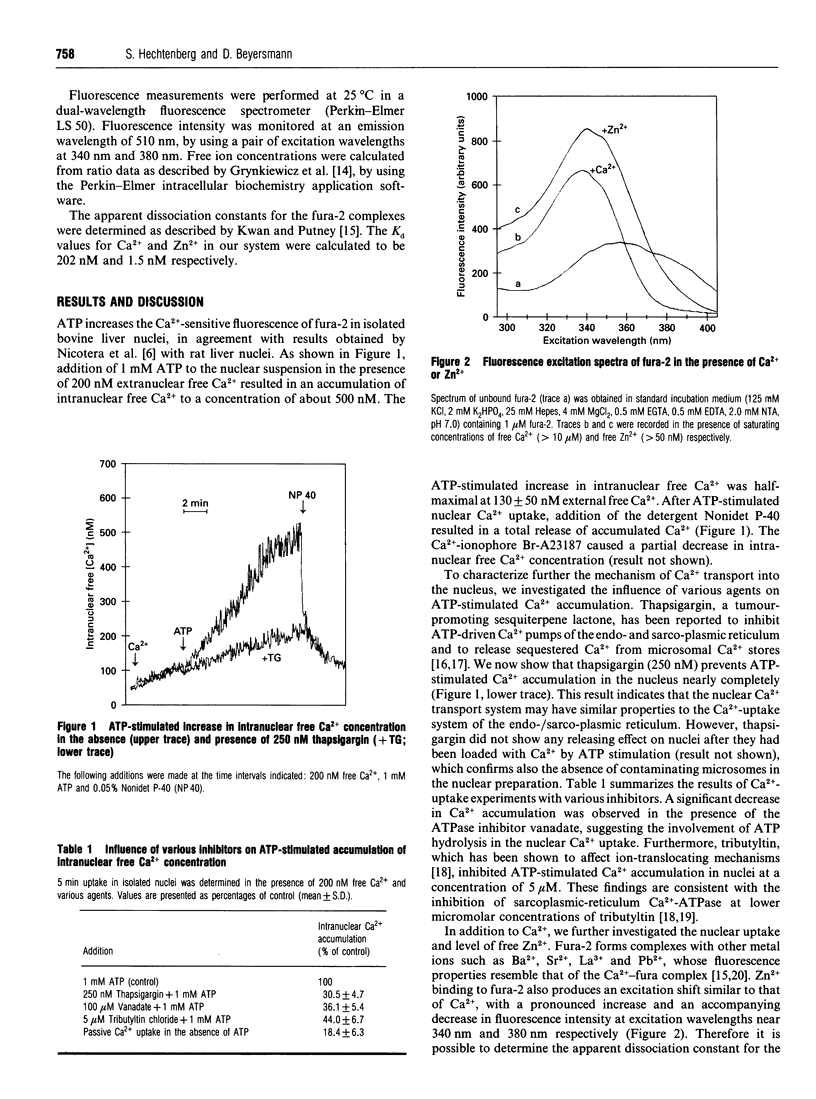
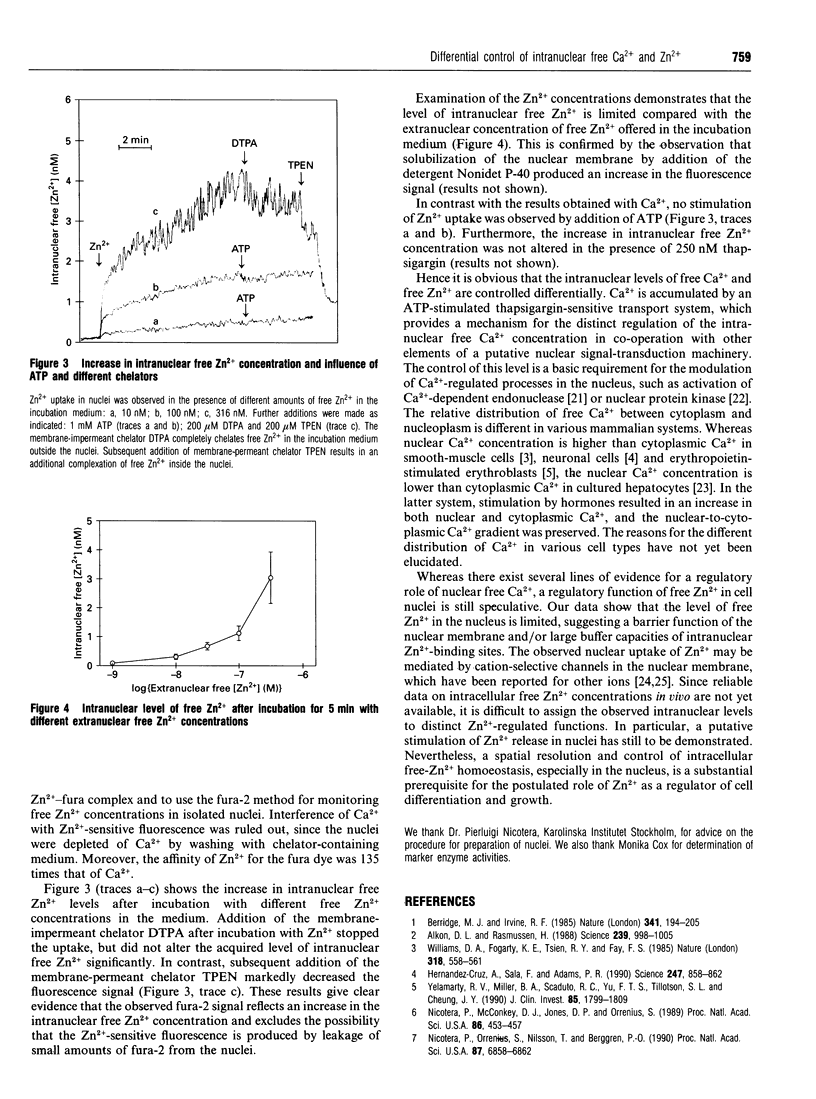
Abstract
Modulation of intracellular signal transduction by Ca2+ and possibly Zn2+ is based on an effective homoeostatic control of the corresponding free ion concentrations. We used the fluorescent indicator fura-2 to monitor concentrations of free Ca2+ and free Zn2+ in nuclei isolated from bovine liver. The nuclei showed an ATP-stimulated accumulation of intranuclear free Ca2+, which was inhibited in the presence of the Ca(2+)-pump inhibitor thapsigargin. Furthermore, uptake and intranuclear levels of free Zn2+ were measured after incubation with different extranuclear Zn2+ concentrations. There was no stimulating effect of ATP on Zn2+ uptake. Our data suggest that the levels of intranuclear free Ca2+ and free Zn2+ are controlled differentially. A distinct regulation of free ion levels in the nucleus may contribute to the specific control of nuclear events associated with gene transcription and cell differentiation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alkon D. L., Rasmussen H. A spatial-temporal model of cell activation. Science. 1988 Feb 26;239(4843):998–1005. doi: 10.1126/science.2830669. [DOI] [PubMed] [Google Scholar]
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Fabiato A. Computer programs for calculating total from specified free or free from specified total ionic concentrations in aqueous solutions containing multiple metals and ligands. Methods Enzymol. 1988;157:378–417. doi: 10.1016/0076-6879(88)57093-3. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hernández-Cruz A., Sala F., Adams P. R. Subcellular calcium transients visualized by confocal microscopy in a voltage-clamped vertebrate neuron. Science. 1990 Feb 16;247(4944):858–862. doi: 10.1126/science.2154851. [DOI] [PubMed] [Google Scholar]
- Jones D. P., McConkey D. J., Nicotera P., Orrenius S. Calcium-activated DNA fragmentation in rat liver nuclei. J Biol Chem. 1989 Apr 15;264(11):6398–6403. [PubMed] [Google Scholar]
- Kodavanti P. R., Cameron J. A., Yallapragada P. R., Vig P. J., Desaiah D. Inhibition of Ca2+ transport associated with cAMP-dependent protein phosphorylation in rat cardiac sarcoplasmic reticulum by triorganotins. Arch Toxicol. 1991;65(4):311–317. doi: 10.1007/BF01968965. [DOI] [PubMed] [Google Scholar]
- Kwan C. Y., Putney J. W., Jr Uptake and intracellular sequestration of divalent cations in resting and methacholine-stimulated mouse lacrimal acinar cells. Dissociation by Sr2+ and Ba2+ of agonist-stimulated divalent cation entry from the refilling of the agonist-sensitive intracellular pool. J Biol Chem. 1990 Jan 15;265(2):678–684. [PubMed] [Google Scholar]
- Malviya A. N., Rogue P., Vincendon G. Stereospecific inositol 1,4,5-[32P]trisphosphate binding to isolated rat liver nuclei: evidence for inositol trisphosphate receptor-mediated calcium release from the nucleus. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9270–9274. doi: 10.1073/pnas.87.23.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masmoudi A., Labourdette G., Mersel M., Huang F. L., Huang K. P., Vincendon G., Malviya A. N. Protein kinase C located in rat liver nuclei. Partial purification and biochemical and immunochemical characterization. J Biol Chem. 1989 Jan 15;264(2):1172–1179. [PubMed] [Google Scholar]
- Matzke A. J., Weiger T. M., Matzke M. A. Detection of a large cation-selective channel in nuclear envelopes of avian erythrocytes. FEBS Lett. 1990 Oct 1;271(1-2):161–164. doi: 10.1016/0014-5793(90)80397-2. [DOI] [PubMed] [Google Scholar]
- Mazzanti M., DeFelice L. J., Cohn J., Malter H. Ion channels in the nuclear envelope. Nature. 1990 Feb 22;343(6260):764–767. doi: 10.1038/343764a0. [DOI] [PubMed] [Google Scholar]
- Nicotera P., McConkey D. J., Jones D. P., Orrenius S. ATP stimulates Ca2+ uptake and increases the free Ca2+ concentration in isolated rat liver nuclei. Proc Natl Acad Sci U S A. 1989 Jan;86(2):453–457. doi: 10.1073/pnas.86.2.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicotera P., Orrenius S., Nilsson T., Berggren P. O. An inositol 1,4,5-trisphosphate-sensitive Ca2+ pool in liver nuclei. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6858–6862. doi: 10.1073/pnas.87.17.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrie L., Chesters J. K., Franklin M. Inhibition of myoblast differentiation by lack of zinc. Biochem J. 1991 May 15;276(Pt 1):109–111. doi: 10.1042/bj2760109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomsig J. L., Suszkiw J. B. Pb2(+)-induced secretion from bovine chromaffin cells: fura-2 as a probe for Pb2+. Am J Physiol. 1990 Nov;259(5 Pt 1):C762–C768. doi: 10.1152/ajpcell.1990.259.5.C762. [DOI] [PubMed] [Google Scholar]
- Waybill M. M., Yelamarty R. V., Zhang Y. L., Scaduto R. C., Jr, LaNoue K. F., Hsu C. J., Smith B. C., Tillotson D. L., Yu F. T., Cheung J. Y. Nuclear calcium gradients in cultured rat hepatocytes. Am J Physiol. 1991 Jul;261(1 Pt 1):E49–E57. doi: 10.1152/ajpendo.1991.261.1.E49. [DOI] [PubMed] [Google Scholar]
- Williams D. A., Fogarty K. E., Tsien R. Y., Fay F. S. Calcium gradients in single smooth muscle cells revealed by the digital imaging microscope using Fura-2. Nature. 1985 Dec 12;318(6046):558–561. doi: 10.1038/318558a0. [DOI] [PubMed] [Google Scholar]
- Yelamarty R. V., Miller B. A., Scaduto R. C., Jr, Yu F. T., Tillotson D. L., Cheung J. Y. Three-dimensional intracellular calcium gradients in single human burst-forming units-erythroid-derived erythroblasts induced by erythropoietin. J Clin Invest. 1990 Jun;85(6):1799–1809. doi: 10.1172/JCI114638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng J., Heuchel R., Schaffner W., Kägi J. H. Thionein (apometallothionein) can modulate DNA binding and transcription activation by zinc finger containing factor Sp1. FEBS Lett. 1991 Feb 25;279(2):310–312. doi: 10.1016/0014-5793(91)80175-3. [DOI] [PubMed] [Google Scholar]
- Zeng J., Vallee B. L., Kägi J. H. Zinc transfer from transcription factor IIIA fingers to thionein clusters. Proc Natl Acad Sci U S A. 1991 Nov 15;88(22):9984–9988. doi: 10.1073/pnas.88.22.9984. [DOI] [PMC free article] [PubMed] [Google Scholar]


