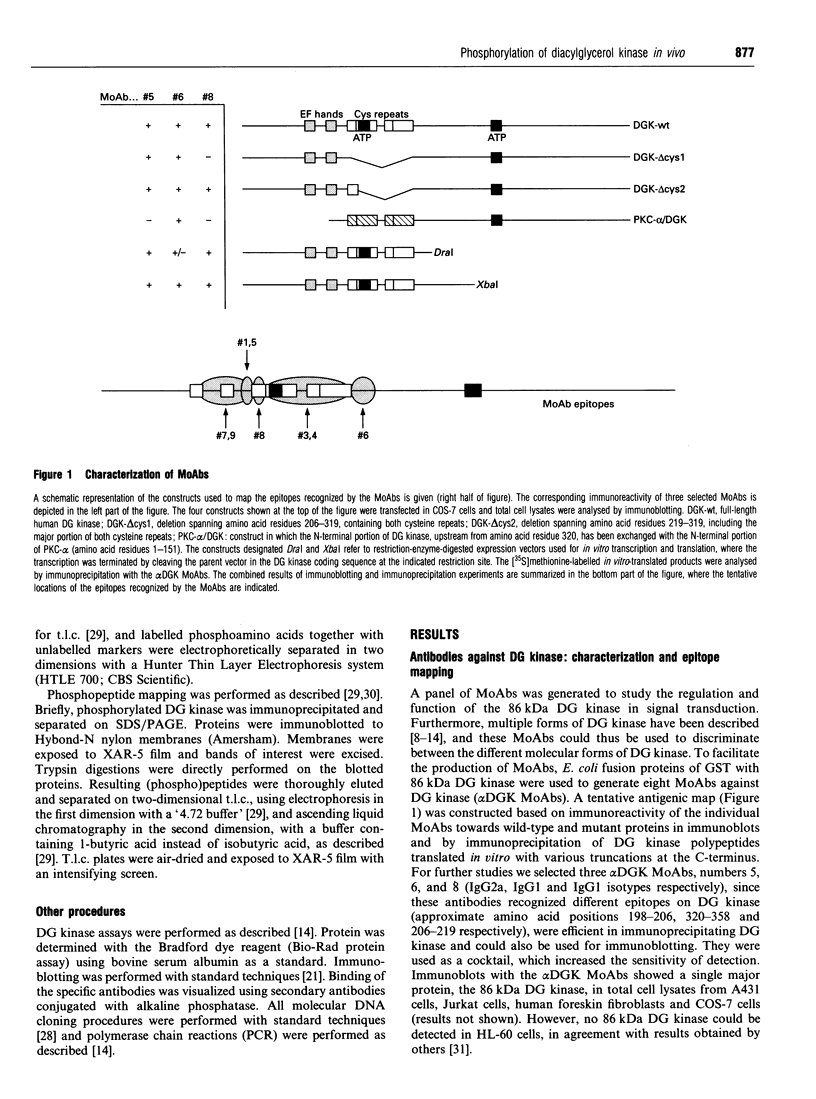
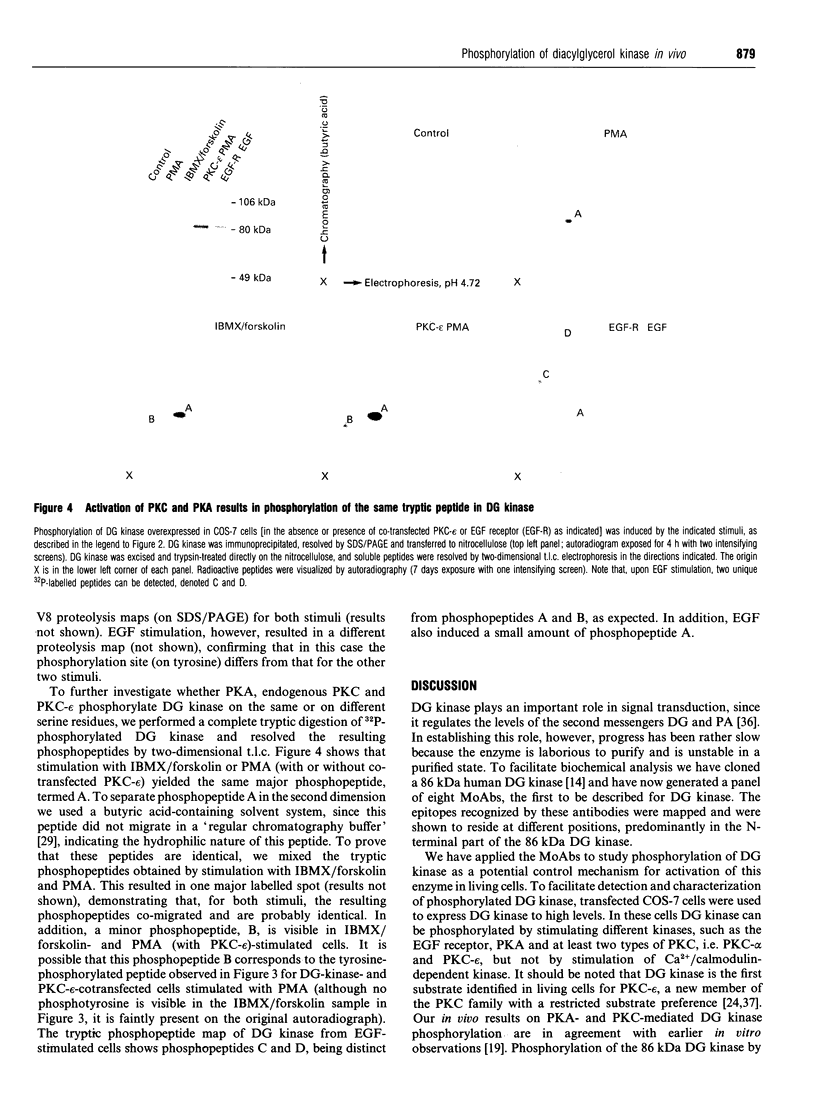
Abstract
In signal transduction, diacylglycerol (DG) kinase attenuates levels of the second messenger DG by converting it to phosphatidic acid. A previously cloned full-length human 86 kDa DG kinase cDNA was expressed as fusion protein in Escherichia coli, to aid in the generation of DG-kinase-specific monoclonal antibodies suitable for immunoprecipitation experiments. To investigate whether phosphorylation of DG kinase is a possible mechanism for its regulation, COS-7 cells were transiently transfected with the DG kinase cDNA and phosphorylation of the expressed DG kinase was induced by various stimuli. Activation of both cyclic AMP-dependent protein kinase and protein kinase C (PKC) resulted in phosphorylation of DG kinase on serine residues in vivo, and both kinases induced this phosphorylation within the same tryptic phosphopeptide, suggesting that they may exert similar control over DG kinase. No phosphorylation was observed upon ionomycin treatment, intended to activate Ca2+/calmodulin-dependent kinases. Co-transfections of DG kinase with either PKC-alpha or PKC-epsilon cDNA revealed that both protein kinases, when stimulated, are able to phosphorylate DG kinase. For PKC-epsilon, DG kinase is the first in vivo substrate identified. Stimulation with epidermal growth factor (EGF) of COS-7 cells transfected with both DG kinase and EGF-receptor cDNA results mainly in phosphorylation of DG kinase on tyrosine. Since the EGF receptor has an intrinsic tyrosine kinase activity, this finding implies that DG kinase may be a direct substrate for the activated EGF receptor.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J. Inositol trisphosphate and diacylglycerol: two interacting second messengers. Annu Rev Biochem. 1987;56:159–193. doi: 10.1146/annurev.bi.56.070187.001111. [DOI] [PubMed] [Google Scholar]
- Besterman J. M., Pollenz R. S., Booker E. L., Jr, Cuatrecasas P. Diacylglycerol-induced translocation of diacylglycerol kinase: use of affinity-purified enzyme in a reconstitution system. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9378–9382. doi: 10.1073/pnas.83.24.9378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocckino S. B., Wilson P. B., Exton J. H. Phosphatidate-dependent protein phosphorylation. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6210–6213. doi: 10.1073/pnas.88.14.6210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Cochet C., Filhol O., Payrastre B., Hunter T., Gill G. N. Interaction between the epidermal growth factor receptor and phosphoinositide kinases. J Biol Chem. 1991 Jan 5;266(1):637–644. [PubMed] [Google Scholar]
- De Boer M., Ossendorp F. A., Van Duijn G., Ten Voorde G. H., Tager J. M. Optimal conditions for the generation of monoclonal antibodies using primary immunisation of mouse splenocytes in vitro under serum-free conditions. J Immunol Methods. 1989 Jul 26;121(2):253–260. doi: 10.1016/0022-1759(89)90168-3. [DOI] [PubMed] [Google Scholar]
- Friedman B. A., van Amsterdam J., Fujiki H., Rosner M. R. Phosphorylation at threonine-654 is not required for negative regulation of the epidermal growth factor receptor by non-phorbol tumor promoters. Proc Natl Acad Sci U S A. 1989 Feb;86(3):812–816. doi: 10.1073/pnas.86.3.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebbink M. F., van Etten I., Hateboer G., Suijkerbuijk R., Beijersbergen R. L., Geurts van Kessel A., Moolenaar W. H. Cloning, expression and chromosomal localization of a new putative receptor-like protein tyrosine phosphatase. FEBS Lett. 1991 Sep 23;290(1-2):123–130. doi: 10.1016/0014-5793(91)81241-y. [DOI] [PubMed] [Google Scholar]
- Ishitoya J., Yamakawa A., Takenawa T. Translocation of diacylglycerol kinase in response to chemotactic peptide and phorbol ester in neutrophils. Biochem Biophys Res Commun. 1987 Apr 29;144(2):1025–1030. doi: 10.1016/s0006-291x(87)80066-9. [DOI] [PubMed] [Google Scholar]
- Iwashita S., Mitsui K., Shoji-Kasai Y., Senshu-Miyaike M. cAMP-mediated modulation of signal transduction of epidermal growth factor (EGF) receptor systems in human epidermoid carcinoma A431 cells. Depression of EGF-dependent diacylglycerol production and EGF receptor phosphorylation. J Biol Chem. 1990 Jun 25;265(18):10702–10708. [PubMed] [Google Scholar]
- Kanoh H., Kondoh H., Ono T. Diacylglycerol kinase from pig brain. Purification and phospholipid dependencies. J Biol Chem. 1983 Feb 10;258(3):1767–1774. [PubMed] [Google Scholar]
- Kanoh H., Yamada K., Sakane F. Diacylglycerol kinase: a key modulator of signal transduction? Trends Biochem Sci. 1990 Feb;15(2):47–50. doi: 10.1016/0968-0004(90)90172-8. [DOI] [PubMed] [Google Scholar]
- Kanoh H., Yamada K., Sakane F., Imaizumi T. Phosphorylation of diacylglycerol kinase in vitro by protein kinase C. Biochem J. 1989 Mar 1;258(2):455–462. doi: 10.1042/bj2580455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M., Homma Y., Nagai Y., Takenawa T. Epidermal growth factor stimulates diacylglycerol kinase in isolated plasma membrane vesicles from A431 cells. Biochem Biophys Res Commun. 1985 Jun 14;129(2):375–380. doi: 10.1016/0006-291x(85)90161-5. [DOI] [PubMed] [Google Scholar]
- Kato M., Takenawa T. Purification and characterization of membrane-bound and cytosolic forms of diacylglycerol kinase from rat brain. J Biol Chem. 1990 Jan 15;265(2):794–800. [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll M. H., Zavoico G. B., Schafer A. I. Second messenger function of phosphatidic acid in platelet activation. J Cell Physiol. 1989 Jun;139(3):558–564. doi: 10.1002/jcp.1041390315. [DOI] [PubMed] [Google Scholar]
- Lemaitre R. N., King W. C., MacDonald M. L., Glomset J. A. Distribution of distinct arachidonoyl-specific and non-specific isoenzymes of diacylglycerol kinase in baboon (Papio cynocephalus) tissues. Biochem J. 1990 Feb 15;266(1):291–299. doi: 10.1042/bj2660291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X. Q., Pawson T. The epidermal growth factor receptor phosphorylates GTPase-activating protein (GAP) at Tyr-460, adjacent to the GAP SH2 domains. Mol Cell Biol. 1991 May;11(5):2511–2516. doi: 10.1128/mcb.11.5.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo K. X., Hurley T. R., Sefton B. M. Cyanogen bromide cleavage and proteolytic peptide mapping of proteins immobilized to membranes. Methods Enzymol. 1991;201:149–152. doi: 10.1016/0076-6879(91)01014-s. [DOI] [PubMed] [Google Scholar]
- MacDonald M. L., Mack K. F., Richardson C. N., Glomset J. A. Regulation of diacylglycerol kinase reaction in Swiss 3T3 cells. Increased phosphorylation of endogenous diacylglycerol and decreased phosphorylation of didecanoylglycerol in response to platelet-derived growth factor. J Biol Chem. 1988 Jan 25;263(3):1575–1583. [PubMed] [Google Scholar]
- Margolis B., Rhee S. G., Felder S., Mervic M., Lyall R., Levitzki A., Ullrich A., Zilberstein A., Schlessinger J. EGF induces tyrosine phosphorylation of phospholipase C-II: a potential mechanism for EGF receptor signaling. Cell. 1989 Jun 30;57(7):1101–1107. doi: 10.1016/0092-8674(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Meisenhelder J., Suh P. G., Rhee S. G., Hunter T. Phospholipase C-gamma is a substrate for the PDGF and EGF receptor protein-tyrosine kinases in vivo and in vitro. Cell. 1989 Jun 30;57(7):1109–1122. doi: 10.1016/0092-8674(89)90048-2. [DOI] [PubMed] [Google Scholar]
- Park D. J., Min H. K., Rhee S. G. Inhibition of CD3-linked phospholipase C by phorbol ester and by cAMP is associated with decreased phosphotyrosine and increased phosphoserine contents of PLC-gamma 1. J Biol Chem. 1992 Jan 25;267(3):1496–1501. [PubMed] [Google Scholar]
- Parker P. J., Coussens L., Totty N., Rhee L., Young S., Chen E., Stabel S., Waterfield M. D., Ullrich A. The complete primary structure of protein kinase C--the major phorbol ester receptor. Science. 1986 Aug 22;233(4766):853–859. doi: 10.1126/science.3755547. [DOI] [PubMed] [Google Scholar]
- Payrastre B., van Bergen en Henegouwen P. M., Breton M., den Hartigh J. C., Plantavid M., Verkleij A. J., Boonstra J. Phosphoinositide kinase, diacylglycerol kinase, and phospholipase C activities associated to the cytoskeleton: effect of epidermal growth factor. J Cell Biol. 1991 Oct;115(1):121–128. doi: 10.1083/jcb.115.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson R. B., Kemp B. E. Protein kinase phosphorylation site sequences and consensus specificity motifs: tabulations. Methods Enzymol. 1991;200:62–81. doi: 10.1016/0076-6879(91)00127-i. [DOI] [PubMed] [Google Scholar]
- Pike L. J., Eakes A. T. Epidermal growth factor stimulates the production of phosphatidylinositol monophosphate and the breakdown of polyphosphoinositides in A431 cells. J Biol Chem. 1987 Feb 5;262(4):1644–1651. [PubMed] [Google Scholar]
- Sakane F., Yamada K., Kanoh H. Different effects of sphingosine, R59022 and anionic amphiphiles on two diacylglycerol kinase isozymes purified from porcine thymus cytosol. FEBS Lett. 1989 Sep 25;255(2):409–413. doi: 10.1016/0014-5793(89)81134-2. [DOI] [PubMed] [Google Scholar]
- Sakane F., Yamada K., Kanoh H., Yokoyama C., Tanabe T. Porcine diacylglycerol kinase sequence has zinc finger and E-F hand motifs. Nature. 1990 Mar 22;344(6264):345–348. doi: 10.1038/344345a0. [DOI] [PubMed] [Google Scholar]
- Schaap D., Parker P. J., Bristol A., Kriz R., Knopf J. Unique substrate specificity and regulatory properties of PKC-epsilon: a rationale for diversity. FEBS Lett. 1989 Jan 30;243(2):351–357. doi: 10.1016/0014-5793(89)80160-7. [DOI] [PubMed] [Google Scholar]
- Schaap D., Parker P. J. Expression, purification, and characterization of protein kinase C-epsilon. J Biol Chem. 1990 May 5;265(13):7301–7307. [PubMed] [Google Scholar]
- Schaap D., de Widt J., van der Wal J., Vandekerckhove J., van Damme J., Gussow D., Ploegh H. L., van Blitterswijk W. J., van der Bend R. L. Purification, cDNA-cloning and expression of human diacylglycerol kinase. FEBS Lett. 1990 Nov 26;275(1-2):151–158. doi: 10.1016/0014-5793(90)81461-v. [DOI] [PubMed] [Google Scholar]
- Stathopoulos V. M., Coco-Maroney A., Wei C. W., Goth M., Zaricznyj C., Macara I. G. Identification of two cytosolic diacylglycerol kinase isoforms in rat brain, and in NIH-3T3 and ras-transformed fibroblasts. Biochem J. 1990 Dec 15;272(3):569–575. doi: 10.1042/bj2720569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullrich A., Coussens L., Hayflick J. S., Dull T. J., Gray A., Tam A. W., Lee J., Yarden Y., Libermann T. A., Schlessinger J. Human epidermal growth factor receptor cDNA sequence and aberrant expression of the amplified gene in A431 epidermoid carcinoma cells. 1984 May 31-Jun 6Nature. 309(5967):418–425. doi: 10.1038/309418a0. [DOI] [PubMed] [Google Scholar]
- Yada Y., Ozeki T., Kanoh H., Nozawa Y. Purification and characterization of cytosolic diacylglycerol kinases of human platelets. J Biol Chem. 1990 Nov 5;265(31):19237–19243. [PubMed] [Google Scholar]
- Yamada K., Sakane F., Kanoh H. Immunoquantitation of 80 kDa diacylglycerol kinase in pig and human lymphocytes and several other cells. FEBS Lett. 1989 Feb 27;244(2):402–406. doi: 10.1016/0014-5793(89)80572-1. [DOI] [PubMed] [Google Scholar]
- van Blitterswijk W. J., Hilkmann H., de Widt J., van der Bend R. L. Phospholipid metabolism in bradykinin-stimulated human fibroblasts. I. Biphasic formation of diacylglycerol from phosphatidylinositol and phosphatidylcholine, controlled by protein kinase C. J Biol Chem. 1991 Jun 5;266(16):10337–10343. [PubMed] [Google Scholar]
- van Corven E. J., van Rijswijk A., Jalink K., van der Bend R. L., van Blitterswijk W. J., Moolenaar W. H. Mitogenic action of lysophosphatidic acid and phosphatidic acid on fibroblasts. Dependence on acyl-chain length and inhibition by suramin. Biochem J. 1992 Jan 1;281(Pt 1):163–169. doi: 10.1042/bj2810163. [DOI] [PMC free article] [PubMed] [Google Scholar]





