Abstract
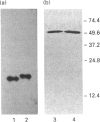
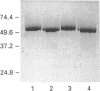
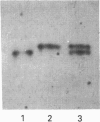
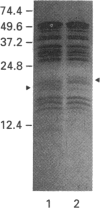
Two isoforms of acidic 1,2-alpha-D-mannosidases have been isolated from culture filtrate of Penicillium citrinum. The pI values of the two forms, designated 1,2-alpha-mannosidase Ia and Ib, were 4.6 and 4.7 respectively. Isoenzymes Ia and Ib exhibited the same molecular mass which was determined to be 53 kDa by SDS/PAGE and 54 kDa by gel-permeation chromatography. Enzymes Ia and Ib hydrolysed yeast mannan and 1,2-alpha-linked mannooligosaccharides, but did not hydrolyse p-nitrophenyl alpha-D-mannoside. The optimal pH for the hydrolysis of Man(alpha 1-->2)Man was 5.0 for both isoenzymes. Similar kinetic parameters were determined for the two forms. Activation energy was a little lower for Ia than Ib. There was little difference between the enzymes with regard to their performance at acidic or alkaline pH. The N-terminal amino acid sequences of the two enzymes were identical. Analysis of C-terminal peptides, which were prepared by tryptic digestion and anhydrotrypsin-agarose chromatography, showed that Ia and Ib had the same amino acid sequences in the C-terminal region. Tryptic digestion revealed a slight difference between the isoenzymes in the pattern of cleaved peptides on SDS/PAGE.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bischoff J., Kornfeld R. The soluble form of rat liver alpha-mannosidase is immunologically related to the endoplasmic reticulum membrane alpha-mannosidase. J Biol Chem. 1986 Apr 5;261(10):4758–4765. [PubMed] [Google Scholar]
- Bischoff J., Liscum L., Kornfeld R. The use of 1-deoxymannojirimycin to evaluate the role of various alpha-mannosidases in oligosaccharide processing in intact cells. J Biol Chem. 1986 Apr 5;261(10):4766–4774. [PubMed] [Google Scholar]
- Burditt L. J., Phillips N. C., Robinson D., Winchester B. G., Van-de-Water N. S., Jolly R. D. Characterization of the mutant alpha-mannosidase in bovine mannosidosis. Biochem J. 1978 Dec 1;175(3):1013–1022. doi: 10.1042/bj1751013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHAPPELL J. B., GREVILLE G. D. Effect of silver ions on mitochondrial adenosine triphosphatase. Nature. 1954 Nov 13;174(4437):930–931. doi: 10.1038/174930b0. [DOI] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Elbein A. D. Inhibitors of the biosynthesis and processing of N-linked oligosaccharide chains. Annu Rev Biochem. 1987;56:497–534. doi: 10.1146/annurev.bi.56.070187.002433. [DOI] [PubMed] [Google Scholar]
- Forsee W. T., Palmer C. F., Schutzbach J. S. Purification and characterization of an alpha-1,2-mannosidase involved in processing asparagine-linked oligosaccharides. J Biol Chem. 1989 Mar 5;264(7):3869–3876. [PubMed] [Google Scholar]
- Garfin D. E. Isoelectric focusing. Methods Enzymol. 1990;182:459–477. doi: 10.1016/0076-6879(90)82037-3. [DOI] [PubMed] [Google Scholar]
- Ichishima E., Arai M., Shigematsu Y., Kumagai H., Sumida-Tanaka R. Purification of an acidic alpha-D-mannosidase from Aspergillus saitoi and specific cleavage of 1,2-alpha-D-mannosidic linkage in yeast mannan. Biochim Biophys Acta. 1981 Mar 13;658(1):45–53. doi: 10.1016/0005-2744(81)90248-5. [DOI] [PubMed] [Google Scholar]
- Ishii S., Yokosawa H., Shiba S., Kasai K. Specific isolation of biologically-active peptides by means of immobilized anhydrotrypsin and anhydrochymotrypsin. Adv Exp Med Biol. 1979;120A:15–27. doi: 10.1007/978-1-4757-0926-1_3. [DOI] [PubMed] [Google Scholar]
- Kobayashi M., Hiura N., Matsuda K. Isolation of enzymes from polyacrylamide disk gels by a centrifugal homogenization method. Anal Biochem. 1985 Mar;145(2):351–353. doi: 10.1016/0003-2697(85)90373-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matta K. L., Bahl O. P. Glycosidases of Aspergillus niger. IV. Purification and characterization of -mannosidase. J Biol Chem. 1972 Mar 25;247(6):1780–1787. [PubMed] [Google Scholar]
- Nordén N. E., Lundblad A., Ockerman P. A., Jolly R. D. Mannosidosis in Angus cattle: partial characterization of two mannose containing oligosaccharides. FEBS Lett. 1973 Sep 15;35(2):209–212. doi: 10.1016/0014-5793(73)80286-8. [DOI] [PubMed] [Google Scholar]
- PARK J. T., JOHNSON M. J. A submicrodetermination of glucose. J Biol Chem. 1949 Nov;181(1):149–151. [PubMed] [Google Scholar]
- Phillips N. C., Robinson D., Winchester B. G., Jolly R. D. Mannosidosis in Angus cattle. The enzymic defect. Biochem J. 1974 Feb;137(2):363–371. doi: 10.1042/bj1370363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SMOGYI M. Notes on sugar determination. J Biol Chem. 1952 Mar;195(1):19–23. [PubMed] [Google Scholar]
- Schutzbach J. S., Forsee W. T. Calcium ion activation of rabbit liver alpha 1,2-mannosidase. J Biol Chem. 1990 Feb 15;265(5):2546–2549. [PubMed] [Google Scholar]
- Schweden J., Legler G., Bause E. Purification and characterization of a neutral processing mannosidase from calf liver acting on (Man)9(GlcNAc)2 oligosaccharides. Eur J Biochem. 1986 Jun 16;157(3):563–570. doi: 10.1111/j.1432-1033.1986.tb09703.x. [DOI] [PubMed] [Google Scholar]
- Stewart T. S., Mendershausen P. B., Ballou C. E. Preparation of a mannopentaose, mannohexaose, and mannoheptaose from Saccharomyces cerevisiae mannan. Biochemistry. 1968 May;7(5):1843–1854. doi: 10.1021/bi00845a032. [DOI] [PubMed] [Google Scholar]
- Takasaki S., Kobata A. Microdetermination of individual neutral and amino sugars and N-acetylneuraminic acid in complex saccharides. J Biochem. 1974 Oct;76(4):783–789. [PubMed] [Google Scholar]
- Tsukagoshi N., Furukawa M., Nagaba H., Kirita N., Tsuboi A., Udaka S. Isolation of a cDNA encoding Aspergillus oryzae Taka-amylase A: evidence for multiple related genes. Gene. 1989 Dec 14;84(2):319–327. doi: 10.1016/0378-1119(89)90506-4. [DOI] [PubMed] [Google Scholar]
- Tulsiani D. R., Harris T. M., Touster O. Swainsonine inhibits the biosynthesis of complex glycoproteins by inhibition of Golgi mannosidase II. J Biol Chem. 1982 Jul 25;257(14):7936–7939. [PubMed] [Google Scholar]
- Tulsiani D. R., Hubbard S. C., Robbins P. W., Touster O. alpha-D-Mannosidases of rat liver Golgi membranes. Mannosidase II is the GlcNAcMAN5-cleaving enzyme in glycoprotein biosynthesis and mannosidases Ia and IB are the enzymes converting Man9 precursors to Man5 intermediates. J Biol Chem. 1982 Apr 10;257(7):3660–3668. [PubMed] [Google Scholar]
- Tulsiani D. R., Touster O. The purification and characterization of mannosidase IA from rat liver Golgi membranes. J Biol Chem. 1988 Apr 15;263(11):5408–5417. [PubMed] [Google Scholar]
- Yokosawa H., Ishii S. Immobilized anhydrotrypsin as a biospecific affinity adsorbent for the peptides produced by trypsin-like proteases. Anal Biochem. 1979 Sep 15;98(1):198–203. doi: 10.1016/0003-2697(79)90726-7. [DOI] [PubMed] [Google Scholar]
- Yoshihisa T., Ohsumi Y., Anraku Y. Solubilization and purification of alpha-mannosidase, a marker enzyme of vacuolar membranes in Saccharomyces cerevisiae. J Biol Chem. 1988 Apr 15;263(11):5158–5163. [PubMed] [Google Scholar]