Abstract
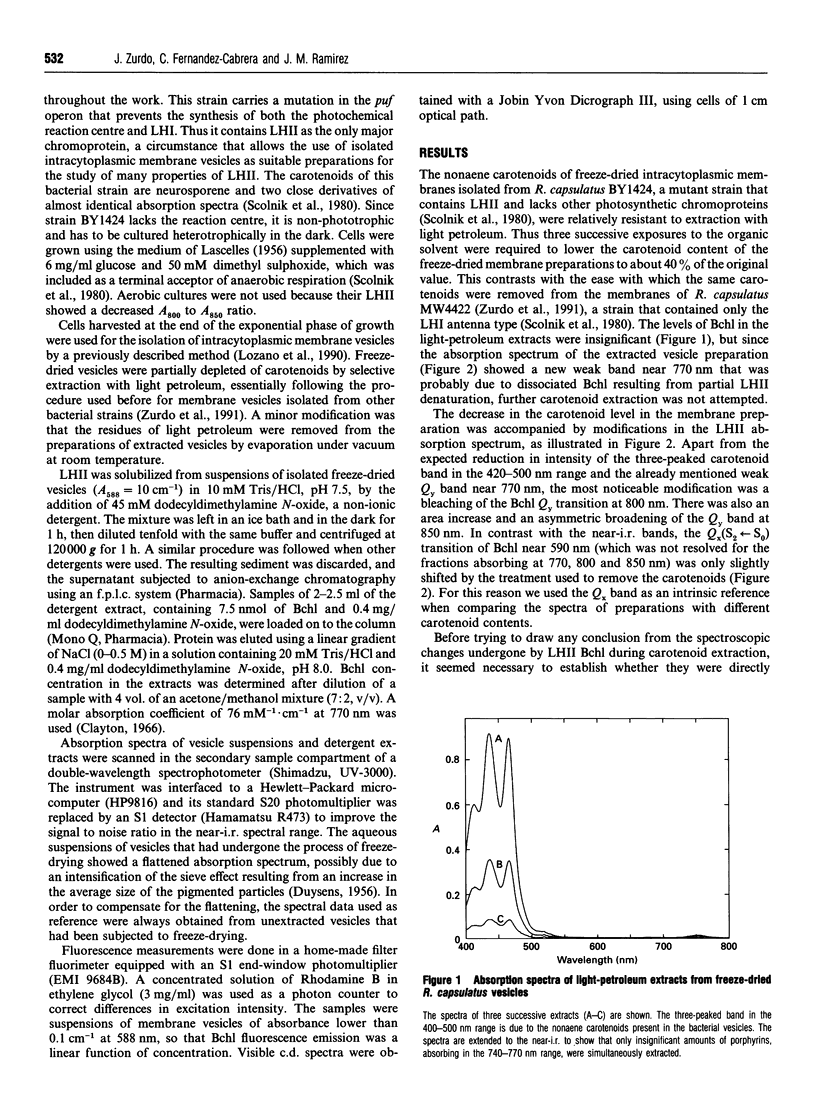
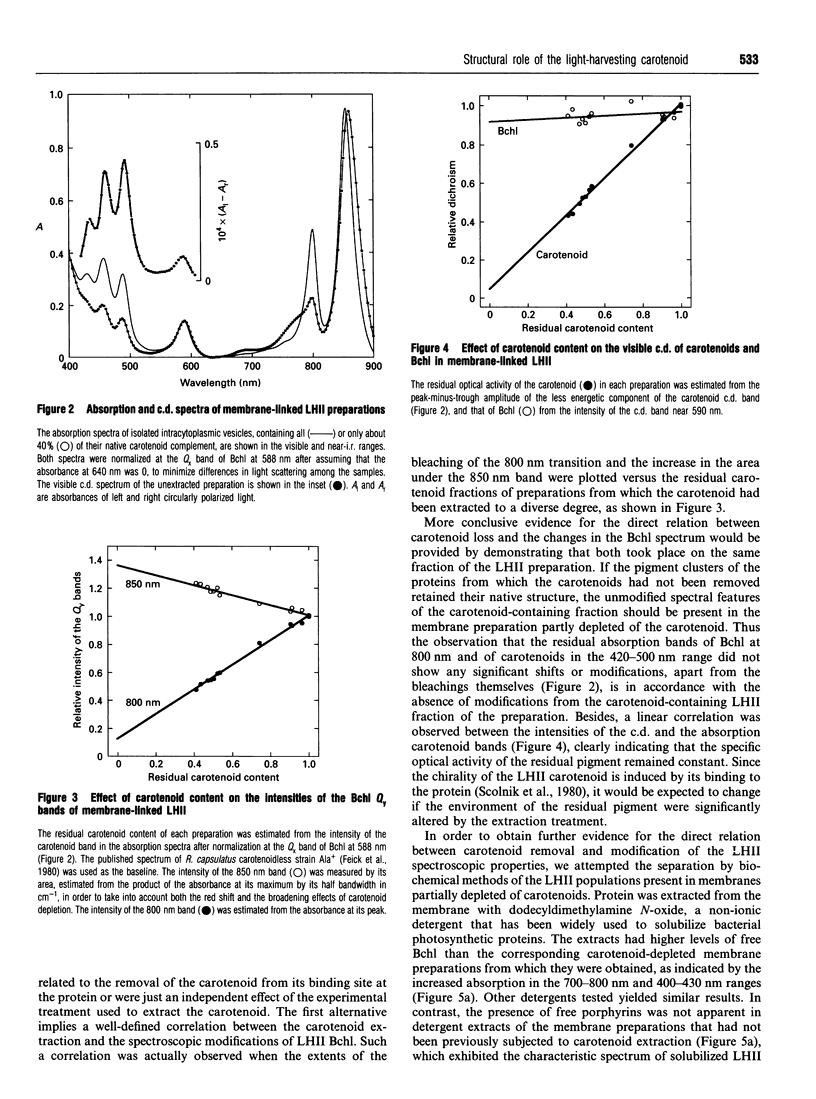
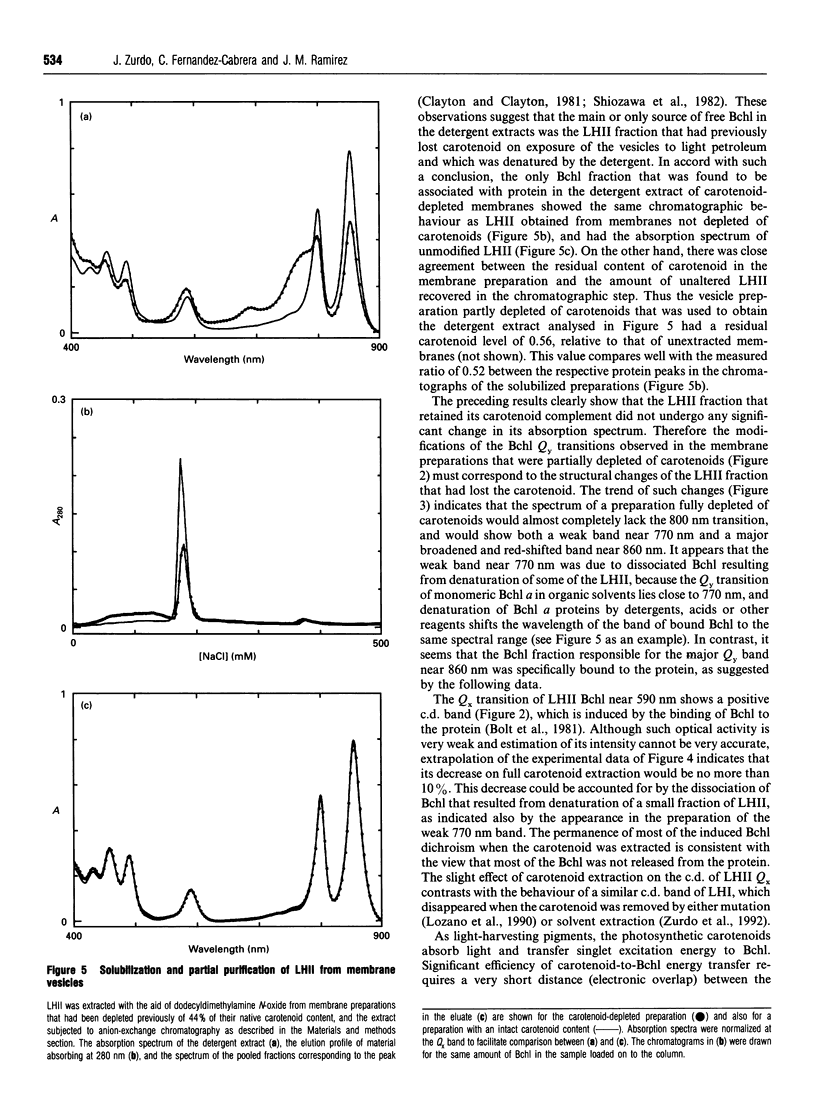
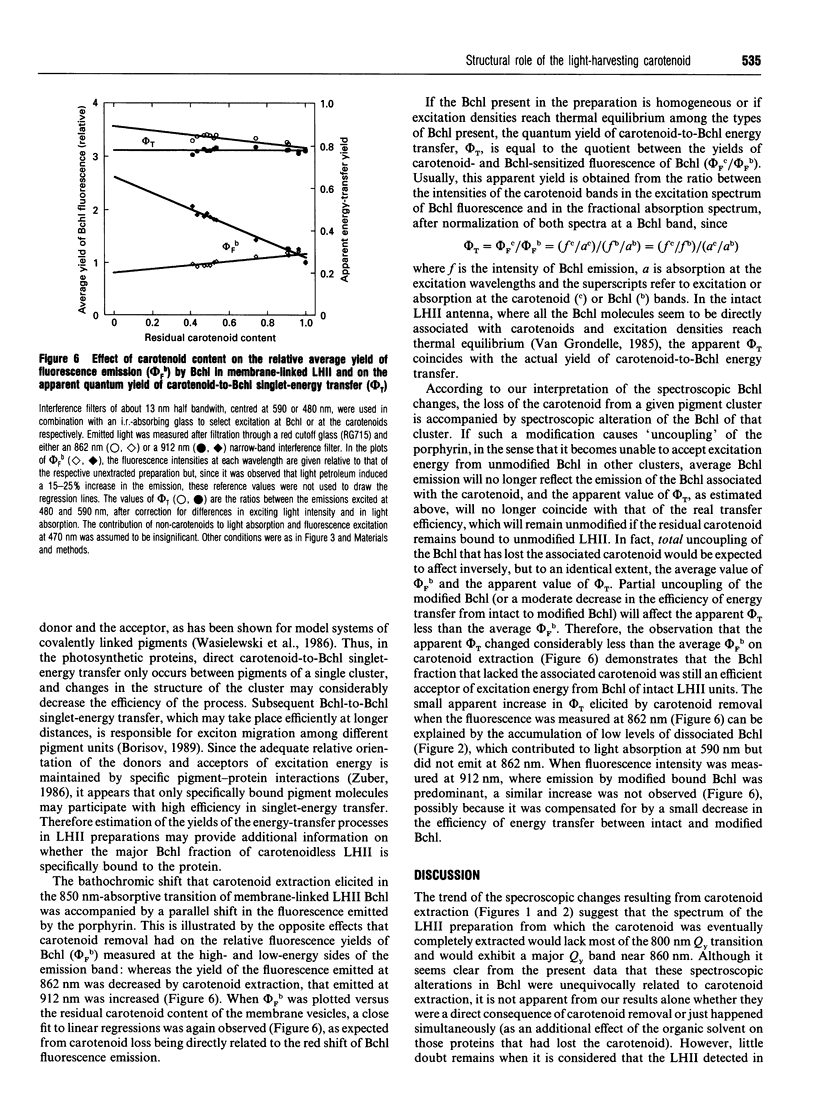
The membrane-linked light-harvesting II protein (LHII) of Rhodobacter capsulatus was partly depleted of carotenoids by selective extraction with light petroleum. Carotenoid removal was accompanied by bleaching of the Qy(S1<--S0) absorption band of bacteriochlorophyll (Bchl) a near 800 nm, by a bathochromic shift and a broadening of the other Bchl Qy band at 850 nm, and by the formation of a weak Qy band of dissociated Bchl near 770 nm. The changes in the 800 and 850 nm bands seemed to reflect alterations in only those Bchl molecules that had lost their associated carotenoids, firstly, because the extent of the changes was closely correlated to the degree of carotenoid extraction, and, secondly, because the residual fraction of carotenoid-containing LHII, which could be almost quantitatively recovered from the membrane after detergent solubilization and ion-exchange chromatography, showed an unmodified LHII absorption spectrum. The Bchl responsible for the shifted 850 nm band remained bound to protein, since its visible (Qx) transition seemed to retain the induced optical activity of the native bound pigment. Besides, the shifted Bchl could act as an efficient acceptor of singlet excitation energy from the pigments of the intact LHII fraction. The close similarity between the spectroscopic Bchl changes that accompany carotenoid extraction and the differential spectral features of carotenoidless LHII of Rhodobacter mutants, previously reported, strongly suggests that the direct cause of the spectral modifications is the absence of carotenoid and not any independent effect of the experimental manipulation of the membrane. Several interpretations of the structural changes that underlie the observed spectral changes are possible. The simplest one is to assume that carotenoid removal elicits an alteration in the angle between the Qy transition moments of two strongly interacting Bchl molecules.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolt J. D., Sauer K., Shiozawa J. A., Drews G. Linear and circular dichroism of membranes from Rhodopseudomonas capsulata. Biochim Biophys Acta. 1981 May 13;635(3):535–541. doi: 10.1016/0005-2728(81)90112-2. [DOI] [PubMed] [Google Scholar]
- Braun P., Scherz A. Polypeptides and bacteriochlorophyll organization in the light-harvesting complex B850 of Rhodobacter sphaeroides R-26.1. Biochemistry. 1991 May 28;30(21):5177–5184. doi: 10.1021/bi00235a010. [DOI] [PubMed] [Google Scholar]
- Clayton R. K., Clayton B. J. B850 pigment-protein complex of Rhodopseudomonas sphaeroides: Extinction coefficients, circular dichroism, and the reversible binding of bacteriochlorophyll. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5583–5587. doi: 10.1073/pnas.78.9.5583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogdell R. J., Frank H. A. How carotenoids function in photosynthetic bacteria. Biochim Biophys Acta. 1987;895(2):63–79. doi: 10.1016/s0304-4173(87)80008-3. [DOI] [PubMed] [Google Scholar]
- DUYSENS L. N. The flattening of the absorption spectrum of suspensions, as compared to that of solutions. Biochim Biophys Acta. 1956 Jan;19(1):1–12. doi: 10.1016/0006-3002(56)90380-8. [DOI] [PubMed] [Google Scholar]
- Davidson E., Cogdell R. J. Reconstitution of carotenoids into the light-harvesting pigment-protein complex from the carotenoidless mutant of Rhodopseudomonas as sphaeroides R26. Biochim Biophys Acta. 1981 Apr 13;635(2):295–303. doi: 10.1016/0005-2728(81)90028-1. [DOI] [PubMed] [Google Scholar]
- Feick R., Drews G. Isolation and characterization of light harvesting bacteriochlorophyll.protein complexes from Rhodopseudomonas capsulata. Biochim Biophys Acta. 1978 Mar 13;501(3):499–513. doi: 10.1016/0005-2728(78)90117-2. [DOI] [PubMed] [Google Scholar]
- Friesner R. A., Won Y. D. Spectroscopy and electron transfer dynamics of the bacterial photosynthetic reaction center. Biochim Biophys Acta. 1989 Nov 23;977(2):99–122. doi: 10.1016/s0005-2728(89)80062-3. [DOI] [PubMed] [Google Scholar]
- LASCELLES J. The synthesis of porphyrins and bacteriochlorophyll by cell suspensions of Rhodopseudomonas spheroides. Biochem J. 1956 Jan;62(1):78–93. doi: 10.1042/bj0620078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozano R. M., Fernández-Cabrera C., Ramírez J. M. The contribution of the carotenoid to the visible circular dichroism of the light-harvesting antenna of Rhodospirillum rubrum. Biochem J. 1990 Sep 1;270(2):469–472. doi: 10.1042/bj2700469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnik P. A., Zannoni D., Marrs B. L. Spectral and functional comparisons between the carotenoids of the two antenna complexes of Rhodopseudomonas capsulata. Biochim Biophys Acta. 1980 Dec 3;593(2):230–240. doi: 10.1016/0005-2728(80)90061-4. [DOI] [PubMed] [Google Scholar]
- Shiozawa J. A., Welte W., Hodapp N., Drews G. Studies on the size and composition of the isolated light-harvesting B800-850 pigment-protein complex of Rhodopseudomonas capsulata. Arch Biochem Biophys. 1982 Feb;213(2):473–485. doi: 10.1016/0003-9861(82)90573-2. [DOI] [PubMed] [Google Scholar]
- Zurdo J., Fernández-Cabrera C., Ramírez J. M. Enhancement of carotenoid-to-chlorophyll singlet energy transfer by carotenoid-carotenoid interaction. Biophys J. 1992 Jun;61(6):1462–1469. doi: 10.1016/S0006-3495(92)81952-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zurdo J., Lozano R. M., Fernandez-Cabrera C., Ramirez J. M. Dimeric carotenoid interaction in the light-harvesting antenna of purple phototrophic bacteria. Biochem J. 1991 Mar 15;274(Pt 3):881–884. doi: 10.1042/bj2740881. [DOI] [PMC free article] [PubMed] [Google Scholar]


