Abstract
Introduction:
Feedback is a fundamental aspect of aphasia treatments. However, learning from feedback is a cognitively demanding process. At the most basic level, individuals must detect feedback and extract outcome-related information (i.e., feedback processing). Neuroanatomical and neuropsychological differences associated with post-stroke aphasia may influence feedback processing and potentially how people with aphasia (PWA) respond to feedback-based treatments. To better understand how post-stroke aphasia affects feedback-based learning, the current study leverages event-related potentials (ERPs) to (1) characterize the relationship between feedback processing and learning, (2) identify cognitive skills that are associated with feedback processing, and (3) identify behavioural correlates of feedback-based learning in PWA.
Methods:
Seventeen PWA completed a feedback-based novel word learning task. Feedback processing was measured using the feedback-related negativity (FRN), an ERP hypothesized to reflect the detection and evaluation of outcomes communicated via feedback. Individuals also completed neuropsychological assessments of language (phonological processing, verbal short-term memory) and executive functioning.
Results:
PWA elicited an FRN that was sensitive to feedback valence. The magnitude of the FRN was not associated with novel word learning but was strongly correlated with performance on another feedback-based task, the Berg Card Sort. Cognitive variables (information updating, selective attention) but not language variables were associated with novel word learning.
Discussion & Conclusion:
For PWA, feedback processing may be associated with learning in some but not all feedback-based contexts. These findings may inform future research in determining which variables moderate the relationship between feedback processing and learning with the long-term goal of identifying how feedback can be modified to support successful learning during aphasia rehabilitation.
Keywords: aphasia, feedback, learning, event-related potentials, feedback-related negativity
Introduction
Aphasia affects two million Americans and may impact quality of life more than cancer and Alzheimer’s Disease (Lam & Wodchis, 2010; National Institute on Deafness and Other Communication Disorders, 2015) making rehabilitation aimed at improving communication of the utmost importance. Aphasia treatments are effective at the group level (Brady et al., 2016; Breitenstein et al., 2017; Robey, 1998); however, there is a substantial amount of unexplained individual-level variability in outcomes (Charidimou et al., 2014; Lazar et al., 2008). Heterogeneity in rehabilitation outcomes has driven researchers to identify variables that may affect how well one responds to behavioural interventions for aphasia with the goal of personalizing aphasia treatment (for discussion see Kristinsson et al., 2022).
Cognitive abilities, for example, are variable in people with aphasia (PWA) (Fonseca et al., 2019; Marinelli et al., 2017) and have been identified in several studies as predictive of aphasia treatment response (Dignam et al., 2016; El Hachioui et al., 2014; Gilmore et al., 2019; Lambon Ralph et al., 2010; Seniów et al., 2009). Cognitive abilities likely play a central role in supporting learning during rehabilitation. In rehabilitative contexts, learning occurs when repeated training experiences confer durable change to the neural systems being targeted by an intervention. Research in neurorehabilitation has found that variations in treatment administration can influence the learning mechanisms engaged and ultimately, the cognitive skills necessary for treatment success (Baddeley & Wilson, 1994; Kearney et al., 2019; Levin & Demers, 2021). Similarly, differences in how aphasia interventions are administered are thought to influence the mechanisms of learning engaged; however, how these differences in administration specifically influence the cognitive skills necessary for successful learning remains relatively unknown (Nunn et al., 2023).
Learning from feedback, for example, is fundamental to many aphasia treatments (Simmons-Mackie et al., 1999; Sze et al., 2021) and is hypothesized to place additional demands on learning systems. We define feedback as a signal from an external source on the accuracy of an action. During feedback-based learning, individuals perform an action (e.g., a naming attempt) and receive feedback from an external source on the accuracy of that action (e.g., “that’s not right”). To use this feedback, individuals must detect the feedback and its valence (positive or negative) and generate a corresponding neural signal that will influence future actions (henceforth called “feedback processing”) (Holroyd & Coles, 2002; Luft, 2014). This signal is hypothesized to play a critical role in error-driven Hebbian learning by altering the strength of connections between neurons (O’Reilly et al., 2017). In aphasia rehabilitation, this error-detection mechanism has been described as one of three cognitive skills that prevent learners from reinforcing erroneous responses (Fillingham et al., 2003; Lambon Ralph & Fillingham, 2007). Errors must first be detected (either via self-monitoring or feedback) (Ohlsson, 1996; Postma, 2000). Then, internal representations must be updated with the correct response (e.g., Exton-McGuinness et al., 2015; Metcalfe & Eich, 2019) and attentional-executive skills must support learners in temporarily pausing learning or reinforcing the correct response over the error (Fillingham et al., 2003; Lambon Ralph & Fillingham, 2007).
Critically, the ability to process feedback may be affected in some people with aphasia which in turn can affect their ability to learn successfully in feedback-based contexts. For example, Vallila-Rohter and Kiran (2013) found that not all individuals with post-stroke aphasia were able to learn in a feedback-based nonlinguistic task. Similarly, Peñaloza et al. (2016) found that during a feedback-based novel word learning task, PWA with lesions to frontal brain regions that are known to support executive skills, and potentially feedback-based learning, had difficulty learning novel words. Characterizing feedback processing ability in PWA may clarify how treatment ingredients such as feedback may place demands on cognitive systems and potentially contribute to variability in treatment response.
Electrophysiological measures can be used to measure feedback processing and determine the extent to which it affects feedback-based learning in PWA. Event-related potentials (ERPs) represent electrical brain activity that is collected from electroencephalographic (EEG) data through signal averaging (Sur & Sinha, 2009). Each ERP is associated with distinct cognitive or sensory processes (Sur & Sinha, 2009). The feedback-related negativity (FRN), for example, is a measure of feedback processing. Specifically, the FRN is thought to represent a prediction error elicited by feedback (Holroyd & Coles, 2002; Talmi et al., 2013) and be a product of the dorsal anterior cingulate cortex (Becker et al., 2014; Hauser et al., 2014; Holroyd & Coles, 2002; Nieuwenhuis et al., 2002). The FRN is associated with the extraction of outcome-related information and relatedly, is larger when elicited by negative relative to positive feedback (Miltner et al., 1997; Sambrook & Goslin, 2015; Williams et al., 2021). Critically, the magnitude of the FRN is strongly related to learning outcomes (Arbel et al., 2013, 2014, 2017; Arbel & Wu, 2016; Luft, 2014; van der Helden et al., 2010). In a review paper, Luft et al. (2014) found that the FRN magnitude consistently predicted learning outcomes in error-based contexts. The well-established relationship between the FRN and learning makes it an ideal measure to elucidate whether impairments in the processing of feedback affect feedback-based learning for PWA.
There are neuroanatomical and neuropsychological reasons to suspect feedback processing may be affected in PWA. Activation in the anterior cingulate cortex (hypothesized neural generator of the FRN) can be variable in individuals with post-stroke aphasia (Abel et al., 2015; Brownsett et al., 2014; Fridriksson et al., 2009). Fridriksson et al. (2009) found that individuals with greater anterior cingulate cortex activation had better treatment outcomes. Fridriksson et al. (2009) hypothesize that this association is not because the anterior cingulate cortex supports language but because it supports error detection. Individuals who can detect errors may have better outcomes because they can make behavioural adjustments to avoid repeating errors. This work provides evidence that neural systems that support learning from feedback can be affected in PWA and their functional integrity may influence treatment outcomes.
Neuropsychological variables, such as executive functioning skills, may also affect feedback processing and ultimately, learning. Executive functions such as information updating and mental set shifting may influence the stimulus-response associations held in working memory and predictions about the valence of upcoming feedback. The FRN is thought to be larger when an outcome differs from what is expected (Holroyd & Coles, 2002; Talmi et al., 2013) and thus, its magnitude may be sensitive to the accuracy of one’s predictions about feedback. Kóbor et al. (2015) found evidence to support this hypothesis. In their study, individuals with better executive skills (measured by computing the mean of the three standardized values of a verbal fluency task, listening span task, and Go/No-Go task) had larger FRN magnitudes following feedback during a decision-making task (Kobor et al., 2015). Attention may also influence the magnitude of the FRN even though the FRN does not specifically reflect attentional allocation. Selective attention is used to identify environmental cues relevant to learning and possible responses, both of which are thought to be used for computing prediction errors like the FRN (Rmus et al., 2021). Supporting this notion, individuals with reduced attention, such as individuals with attention deficit/hyperactivity disorder show smaller magnitude FRNs (Gong et al., 2014; Xiao et al., 2015). Importantly, selective attention, information updating, and mental set shifting are executive skills that can be impaired in individuals with post-stroke aphasia (Christensen & Wright, 2010; Fonseca et al., 2019; Fridriksson, Nettles, et al., 2006; Mohapatra & Marshall, 2020; Murray, 2012; Purdy, 2002; Simic et al., 2020) El Hachioui et al. (2014) found that 46.4% of PWA 3-months post-stroke and 35.5% of PWA 1-year post stroke had scores on at least one of the following assessments indicating executive dysfunction: Wisconsin Card-Sort Task (Lineweaver et al., 1999), Trail-Making Tests (Reitan, 1958), and Weigl Sorting Test (Laiacona et al., 2000). A better understanding of the relationship between feedback processing and executive skills in PWA would support clinicians in identifying which individuals may have difficulty processing feedback. Additionally, correlating ERPs with neuropsychological variables may improve the clinical utility of ERPs in aphasia research (Silkes & Anjum, 2021).
Despite the potential cognitive demands of feedback-based learning, there are benefits to feedback-based learning contexts. In naming treatment, for example, research has identified robust advantages in the retention of treatment gains when training requires effortful retrieval of targets from long-term memory (for review see Nunn et al., 2023). While effortful retrieval does not encourage error production, errors do occur, which benefit from feedback (see evidence in psychology: Butler & Roediger, 2008; Pashler et al., 2005). Furthermore, clinical interventions that intentionally reduce errors and thus, the need for feedback, have been criticized as potentially being ineffective at engaging clients with mild-moderate naming impairments (Conroy et al., 2009; Lacey et al., 2004, cf. Fillingham et al., 2006). Finally, it is difficult, if not impossible, to eliminate errors from aphasia rehabilitation due to intraindividual variability and concomitant conditions such as apraxia of speech. Thus, it may be more practical to aim to (1) identify individuals who do not learn efficiently from feedback and (2) determine how clinicians can modify feedback to support learning at the individual level. The benefits of feedback-based learning on long-term retention and client engagement, in addition to its ability to be modified within treatment contexts, make a closer examination of feedback-based learning in PWA highly relevant.
The current study aimed to (1) determine whether feedback processing measured via the FRN is associated with learning, (2) evaluate the relationship between feedback processing and executive functioning ability, and (3) identify behavioural cognitive and language variables that correlate with feedback-based learning. To achieve these objectives, PWA completed a feedback-based novel word learning task. Novel word learning tasks have previously been used in aphasia to gain insight into underlying mechanisms that support language rehabilitation (Breitenstein et al., 2004; Dignam et al., 2016; Freedman & Martin, 2001; Gupta et al., 2006; Kelly & Armstrong, 2009; Peñaloza et al., 2016, 2017; Tuomiranta et al., 2011). Importantly, novel word learning ability has been shown to predict treatment response in PWA, suggesting it has prognostic value (Dignam et al., 2016). Thus, insights into the relationship between feedback processing and novel word learning task may have direct implications for treatment and support the long-term goal of identifying ways that feedback administration can be modified to complement PWAs’ learning and language abilities.
Method
Participants
This study was approved by the Institutional Review Board (IRB) of Mass General Brigham. Twenty PWA consented to participate in this study. Three participants were deemed ineligible (two due to stroke location and one did not meet the criteria necessary to be considered aphasic) resulting in a sample size of 17. See Table 1 for the demographic variables of participants. All eligible participants had aphasia due to a left hemisphere stroke. Participants did not report concomitant neurologic or psychiatric conditions. Due to the linguistic and visual demands of the task, all participants were fluent English speakers and passed a pure-tone hearing screening unilaterally with or without hearing aids. All participants scored at least a 42/60 on the Western Aphasia Battery – Revised (WAB – R) Yes/No Questions sub-test (Kertesz, 2007), had vision that was normal or corrected-to-normal per self-report, and passed the line-bisection task in the Comprehensive Aphasia Test (Swinburn et al., 2004) to screen for visual inattention. One participant had a history of right inattention which had been remediated.1 Aphasia severity was characterized using the WAB – R. The WAB-R was double-scored by the first author (KN), the third author (SVR), and a graduate research assistant. Any disagreements were resolved between raters. All participants scored below the WAB-R aphasia quotient cut-off of 93.8.
Table 1.
Demographic characteristics of study sample
| Participant | Age | Sex | Race and Ethnicity | Education | Yrs Post-Stroke | WAB-R AQ |
|---|---|---|---|---|---|---|
| A1 | 62 | F | Non-Hispanic White | Masters | 3 | 82.5 |
| A2 | 67 | F | Non-Hispanic White | Bachelors | 7 | 64.0 |
| A4 | 61 | M | Non-Hispanic White | Doctorate | 11 | 93.5 |
| A5 | 68 | M | Non-Hispanic White | Bachelors | 4 | 91.7 |
| A8 | 81 | M | Non-Hispanic White | Associates | 2 | 85.1 |
| A9 | 69 | M | Non-Hispanic White | Masters | 7 | 89.5 |
| A10 | 57 | M | Non-Hispanic White | HS | 13 | 91.6 |
| A11 | 60 | M | Non-Hispanic White | Masters | 4 | 70.9 |
| A12 | 51 | M | Non-Hispanic White | Doctorate | 7 | 87.6 |
| A13 | 63 | M | Non-Hispanic Black or African American | HS | 33 | 60.8 |
| A14 | 60 | M | Non-Hispanic White | HS | 23 | 50.0 |
| A15 | 67 | M | Non-Hispanic White | Masters | 9 | 84.6 |
| A16 | 54 | M | Non-Hispanic White | Masters | 10 | 66.3 |
| A17 | 71 | M | Non-Hispanic White | Masters | 4 | 79.0 |
| A18 | 63 | F | Non-Hispanic Black or African American | Bachelors | 1 | 83.0 |
| A19 | 37 | M | Non-Hispanic White | Bachelors | 21 | 85.1 |
| A20 | 43 | F | Non-Hispanic White | Masters | 2 | 68.6 |
|
Summary
M (SD) |
60.8 (10.4) |
F: 4
M:13 |
Non-Hispanic Black or African American: 2
Non-Hispanic White: 15 |
HS: 3
Associates: 1 Bachelors: 4 Masters: 7 Doctorate: 2 |
9.5 (8.7) | 78.5 (12.7) |
Cognitive and Linguistic Measures
Participants completed standardized cognitive and language assessments that have previously been used with PWA (DeDe et al., 2014; Fillingham et al., 2005b; Laures-Gore & Rice, 2019; Martin et al., 2018; L. L. Murray, 2012; Simic et al., 2020; Vallila-Rohter & Kiran, 2013; Villard & Kidd, 2019). Language and cognitive behavioural measures are described in Table 2. Previous research has identified that language variables including phonological processing and verbal lexical-semantic short-term memory (STM) are correlated with novel word learning ability (Gupta et al., 2006; Peñaloza et al., 2016, 2017; Tuomiranta et al., 2011). Thus, these linguistic variables were used to account for the effect of language severity on novel word learning rather than an overall WAB-R aphasia quotient which (1) includes sub-tests of language domains less central to our novel word learning task (e.g., spontaneous speech content and fluency) and (2) collapses performance across several language domains which can be affected to varying degrees in a single individual. To increase the reliability and validity of the measures we created composite scores (Murray et al., 2018). Of note, for the Berg Card Sorting Test (BCST, Fox et al., 2013), administered to assess mental set switching, nine participants completed the 128-card version of the BCST which was later reduced to the 64-card version due to participant fatigue. Scores on the 128-card and 64-card versions of the BCST are comparable (Fox et al., 2013).
Table 2.
Assessments administered to evaluate cognitive and language domains.
| Phonological Processing | Phoneme Discrimination (Novel words, 1SUF)1: Participants judged if two novel words were the same or different. |
| Rhyme Judgment (Novel words, 1SUF)1: Participants judged if two novel words did or did not rhyme. | |
| Verbal STM | Digit Span (Pointing)1: Participants pointed to digits in serial order for lists of increasing length (1 through 7). Discontinued when list accuracy was <50%. |
| Word Span (Pointing)1: Participants pointed to words in serial order for lists of increasing length (1 through 7). Discontinued when list accuracy was <50%. | |
| Selective Attention | Map Search2: Participants identified as many target symbols as they could on a map in 2-minutes. |
| Elevator Counting with Distractions2: Participants reported how many times they heard a target tone while ignoring a distractor tone. | |
| Information Updating | 1-Back (Tones)3: Participants indicated if the tone they just heard was the same as the tone presented 1-back. |
| 2-Back (Fruit)4: Participants indicated if the fruit they saw was the same as the fruit presented 2-back. | |
| Mental Set Switching | BCST5: Participants sorted cards into piles using one of four rules which changed intermittently. |
Note.
Temple Assessment of Language and Short-Term Memory in Aphasia (TALSA, Martin et al., 2018). The 1 Second Unfilled (1SUF) interval condition of the tasks includes a 1-second interval between the two stimuli being compared and between the probe and response screen. Unfilled means there is no distractor task during the 1-second intervals.
Test of Everyday Attention (TEA, Robertson et al., 1996).
Berg Card Sorting Test via PEBL (Fox et al., 2013; Grant & Berg, 1948)
Novel word Learning Task
Participants underwent training to learn the novel word names of 30 unfamiliar objects (see Figure 1). The unfamiliar objects were randomly selected from Kroll and Potter (1984). To control for naturalness, any items rated on average by Kroll and Porter (1984) as looking “very much” (< 2 on a 7-point scale) or “nothing at all” (> 6 on a 7-point scale) like a real object were removed. Next, the first author identified objects that were visually similar to one another and replaced them with the next randomly selected image.
Figure 1.
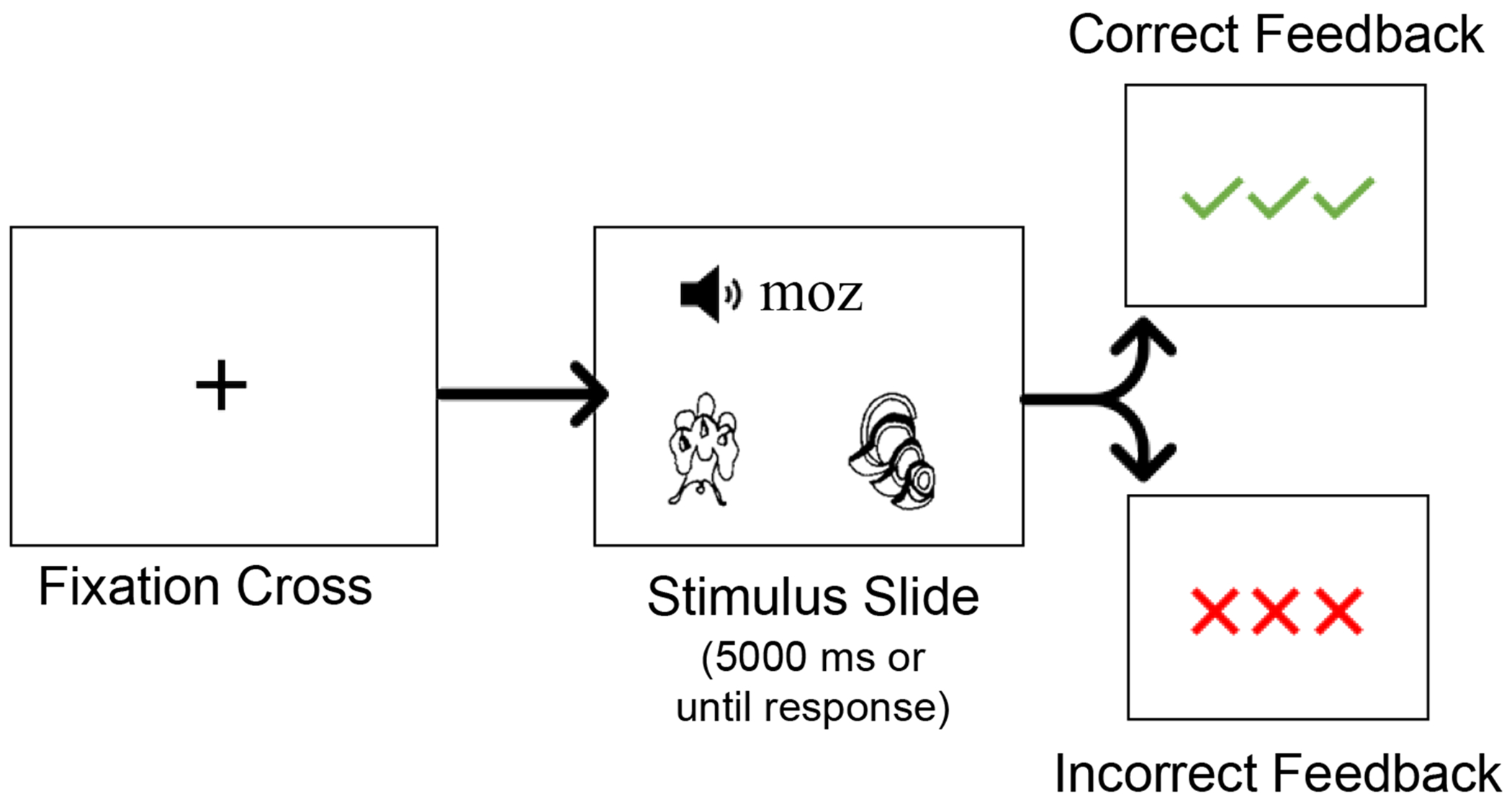
Illustration of a training trial with positive or negative feedback.
The novel words were from the ARC Novel Word Database (Rastle et al., 2002). All novel words were three-letter consonant-vowel-consonant (CVC) forms that followed English phonological and orthographic constraints (e.g., /tæm/, spelled “tam”). A list of generated novel words was evaluated by three independent reviewers who identified whether any of the novel words were pre-existing words, abbreviations, or slang. Once the thirty novel words and objects were selected, they were divided into three sets of ten. Each set contained two words with each vowel, ≤ 2 words with the same onset, and no minimal pairs. The naturalness rating of the unfamiliar objects across sets was not significantly different (p > .05).
Training
Each set (ten novel words per set, three sets total) was trained separately such that training for the next set did not begin until training for the previous set had been completed. Sets were trained over seven learning rounds and each round consisted of 10 trials (i.e., one trial per novel word). Altogether, each novel word was presented seven times resulting in a total of 210 trials during training over the three sets (See Figure 2). During training, participants were instructed to use feedback to learn the correct names for the unfamiliar objects. In each trial, participants heard and saw a novel word and saw two unfamiliar objects – a target and a foil – which remained on the screen for 5000 ms or until a response. Participants selected the object they believed was referred to by the novel word via a button-press and received immediate visual feedback on the accuracy of their response (See Figure 1). Of note, for each trial, the foil object was the correct referent of another novel word trained in the same set. During training, each target was only paired with each foil once. The order of the novel words and images was pseudorandomized to ensure that there were at least two trials between subsequent presentations of the same object.
Figure 2.
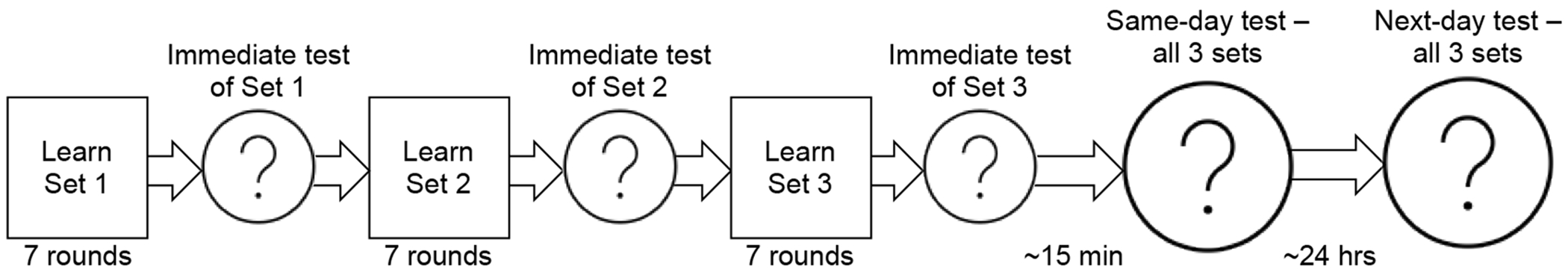
Training structure and testing intervals.
Testing
Testing trials mirrored training except that feedback was not provided. To reduce inflated accuracy due to chance, during each test, each novel word was probed twice. Novel words were only considered “learned” if they were identified correctly on both probes within a test. Thus, chance performance was 25%. Learning was probed at three time points (See Figure 2): immediately after the training of that set (immediate test), after a ~15-minute delay (same day test), and the next day, ~24 hours (next day test). For one participant, the next day test occurred ~48 hours after training due to scheduling constraints.
EEG Recording and Signal Processing
EEG was collected during training using a GES 400 system by Electrical Geodesics, Inc to assess feedback processing. Prior to training, participants were fitted with a 32-channel HydroCel Geodesic Sensor Net from EGI. During training, the electrode impedances were kept below 50 kΩ. EEG was continuously recorded at a 1000 Hz sampling rate and amplifiers were set to a band-pass of 0.1 Hz to 30 Hz. The continuous EEG signal was time-locked to the onset of feedback and segmented into 1000 ms long epochs (200 ms before and 800 ms after feedback) which yielded ERPs that reflected cortical activity associated with feedback processing.
Offline EEG data processing was performed using MATLAB scripts (The MathWorks Inc., 2022) and EEGLAB toolbox (Delorme & Makeig, 2004). The channel spectra were plotted and used to identify and remove noisy channels (e.g., channels that may have made contact with participants’ face masks). Next, the epochs were visually inspected to identify and remove movement artifacts. On average, 2.5 epochs were removed per participant. The signal was re-referenced to the average reference and baseline corrected using the signal 200 ms prior to feedback presentation. Independent component analysis was then used to detect and remove eye movements and blinks.
EEG Data Analysis
To identify the electrode that best captured the FRN, a topographic plot was created and represented the brain activation across all electrode channels using the activity between 200 and 330 ms after feedback (Figure 3). FCz is often used to capture the FRN in healthy adults (Sambrook & Goslin, 2015) as well as adults with neurologic injury (Larson et al., 2007; Osborne-Crowley et al., 2016). In the current sample, FCz was detected as the electrode which showed the greatest difference in activation for positive and negative feedback. Therefore, ERP data from FCz in response to positive and negative feedback were extracted for each participant. Latency correction was performed to compensate for latency variability which is common in older adults. A time-window selection method was used (Luck & Gaspelin, 2017; Picton et al., 2000). Via visual inspection, we identified that the FRN peak occurred between 200 and 330 ms. Thus, for each participant, the most negative value between 200 and 330 ms after feedback was identified and aligned across participants resulting in epochs that were 870ms long.2 Averaged data from each participant was then submitted to a temporal principal component analysis with Promax rotation (Dien, 2010; Spencer et al., 2001). Temporal principal component analysis facilitated the analytic reduction of the temporal dimensionality of the data (Spencer et al., 2001). Seven temporal factors accounted for 74.36% of the variance in the data. The first temporal factor was identified as aligning with the activity reflecting the FRN. Factor scores from temporal factor one were extracted and represented the relative magnitude of each participant’s FRN to negative and positive feedback.
Figure 3.
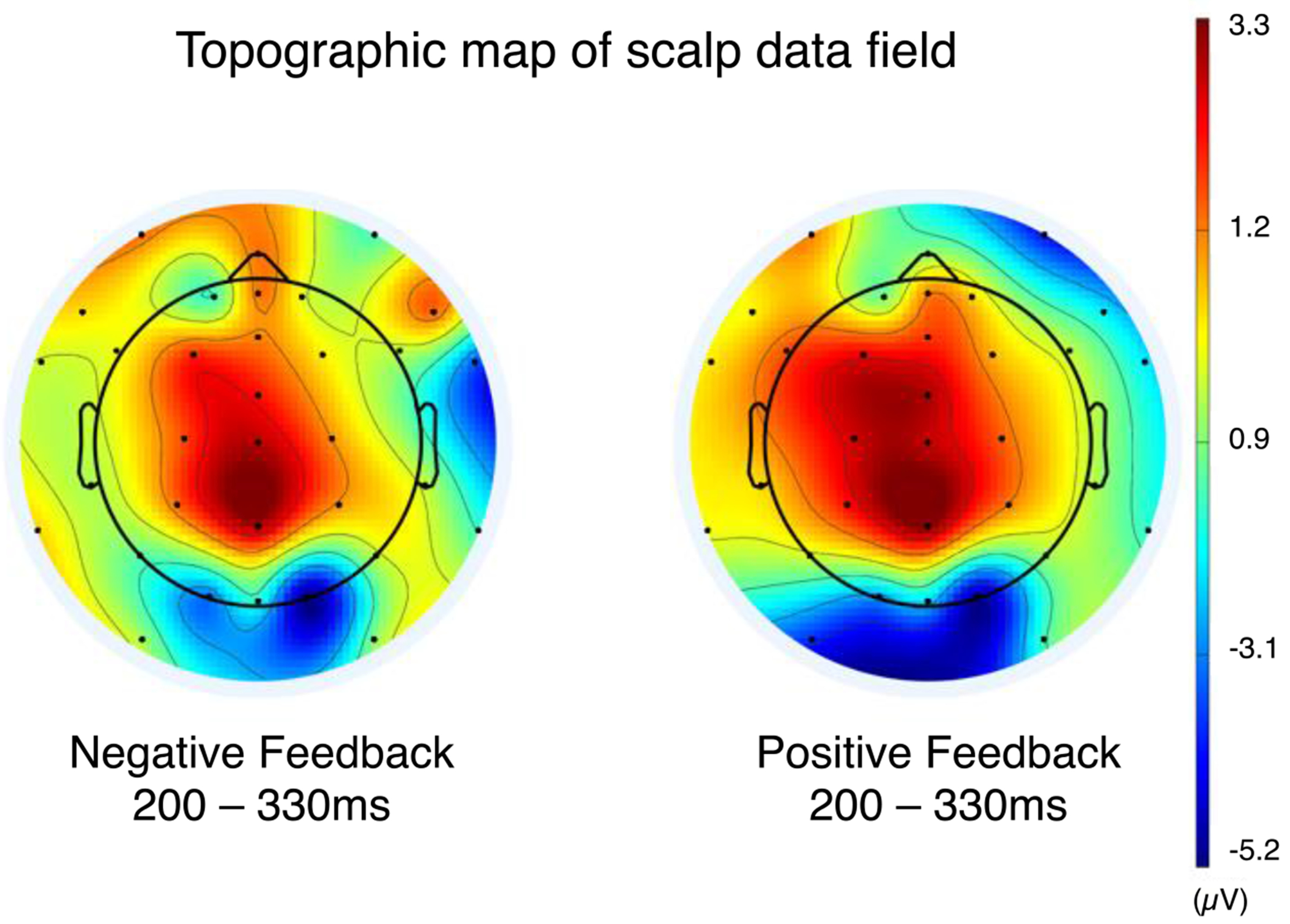
Topographic plots of activation across all channels between 200 and 330 ms after feedback. The difference in activation between positive and negative feedback is largest at FCz.
Statistical Analysis
Training accuracy was calculated as percent correct and averaged across all three sets to obtain a single score for training. For the testing data, the dependent variable was proportion learned at each testing point. The number of times a target was identified accurately was calculated. Targets that were identified accurately two out of two times in a test were scored as “learned”. The proportion of novel words learned out of 30 was then calculated for each participant. For the immediate test, the proportion learned was averaged across all three sets to obtain a single immediate test score. In calculating composite scores for cognitive measures, we examined correlations between the raw sub-assessment scores. All sub-assessments within proposed composites showed medium to large positive correlations among one another. Thus, it was deemed acceptable to create composite scores with the intended sub-assessments. Raw sub-assessment scores were then converted to z scores and z scores were averaged together for each participant to create composites.
All statistical analyses were performed in R Studio (R Core Team, 2021). To evaluate whether response accuracy improved over training a repeated measures ANOVA (DV: accuracy in training, IV: training rounds 1 through 7) was conducted. To determine if the proportion of novel words learned correlated with the FRN magnitude, partial correlations were calculated to control for previously identified predictors of novel word learning (phonological processing, verbal STM). Pearson’s r correlation coefficients were calculated to measure the relationship between (1) executive functioning and the FRN magnitude and (2) language and cognitive behavioural measures and learning.
Results
Non-Word Learning
The assumption of Mauchly’s test for sphericity was not significant (p = .11) allowing for the planned repeated measures ANOVA to evaluate learning during training. Examining accuracy across the seven rounds revealed a main effect of training round, F (1, 16) = 17.1, p = .001, η2 = 0.52. Pairwise comparisons with a Holm correction revealed significantly lower accuracy on round one (M = .44, SD = .08) relative to rounds five (M = 0.60, SD = 0.12, p = .01) and seven (M = 0.65, SD = 0.14, p < .001); round two (M = 0.53, SD = 0.10) relative to round seven (p = .049); and round four (M = 0.53, SD = 0.15) relative to round seven (p = .01). Therefore, participant performance at the group level improved over the training rounds (Figure 4).
Figure 4.
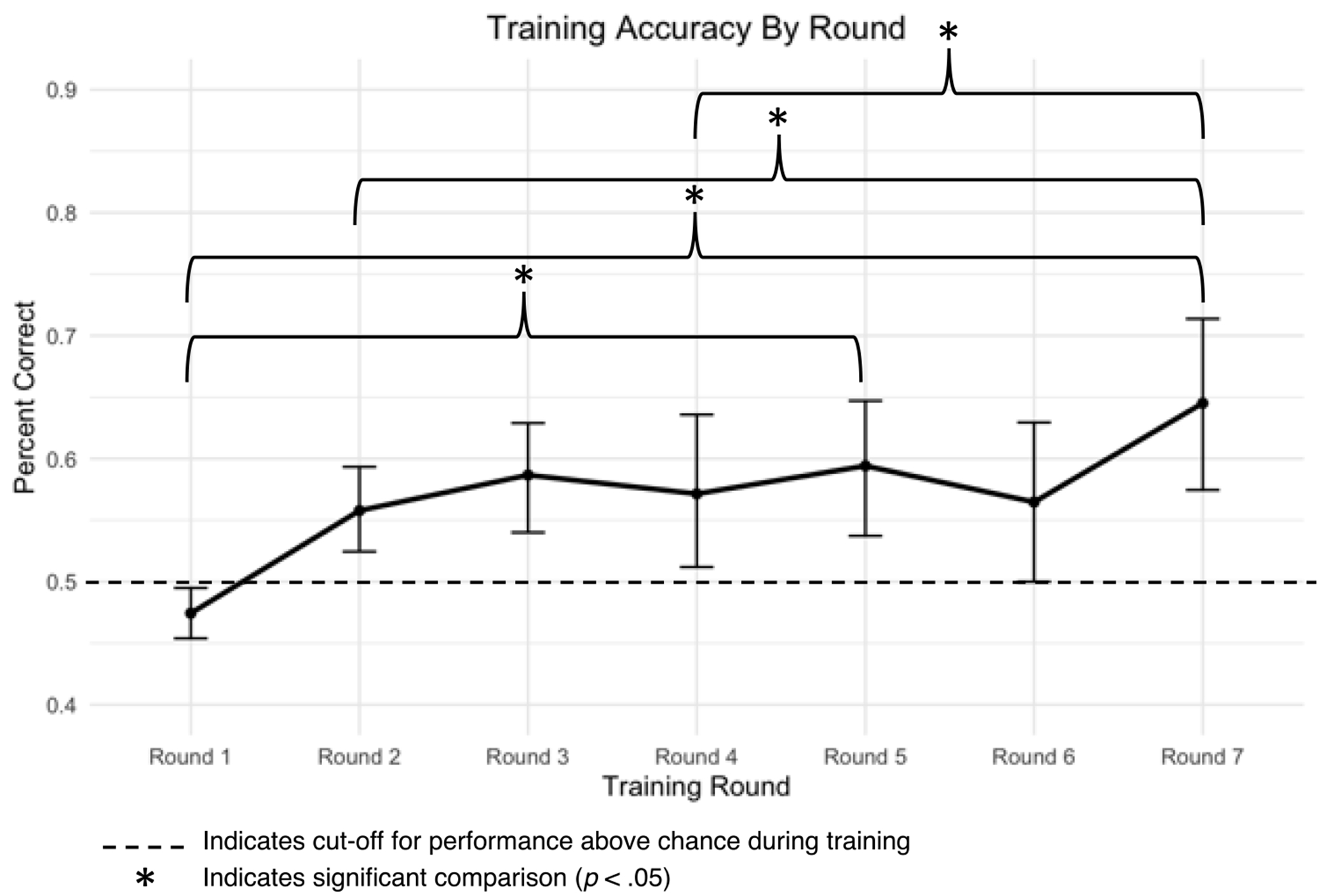
Mean response accuracy over training rounds one through seven.
To ensure that the proportion of novel words learned did not differ across training sets, we conducted a three (item set: set one, set two, set three) by three (test interval: immediate, same day, next day) repeated measures ANOVA. There were no significant main or interaction effects suggesting equivalent performance across all sets at all testing intervals.
The proportion of items learned was above chance (25%) on the immediate test (M = 0.37, SD = 0.19), same day test (M = 0.41, SD = 0.22), and the next day test (M = 0.40, SD = 0.16). See Figure 5 for a boxplot showing the distribution of the data across testing intervals. Pairwise comparisons revealed no significant differences in the proportion of novel words learned across testing intervals (p > .05). Performance was variable across participants, with a small subset of participants performing below chance: immediate test (n = 5), same day test (n = 4), and next day test (n = 2). Only one participant performed below chance across all test points. All participants who performed below chance at any test point demonstrated they understood the task by consistently and accurately responding (>75% accuracy) to a small portion of items (median: 6, min: 2, max: 18) during training. See Appendix 1 for individual learning and composite scores.
Figure 5.
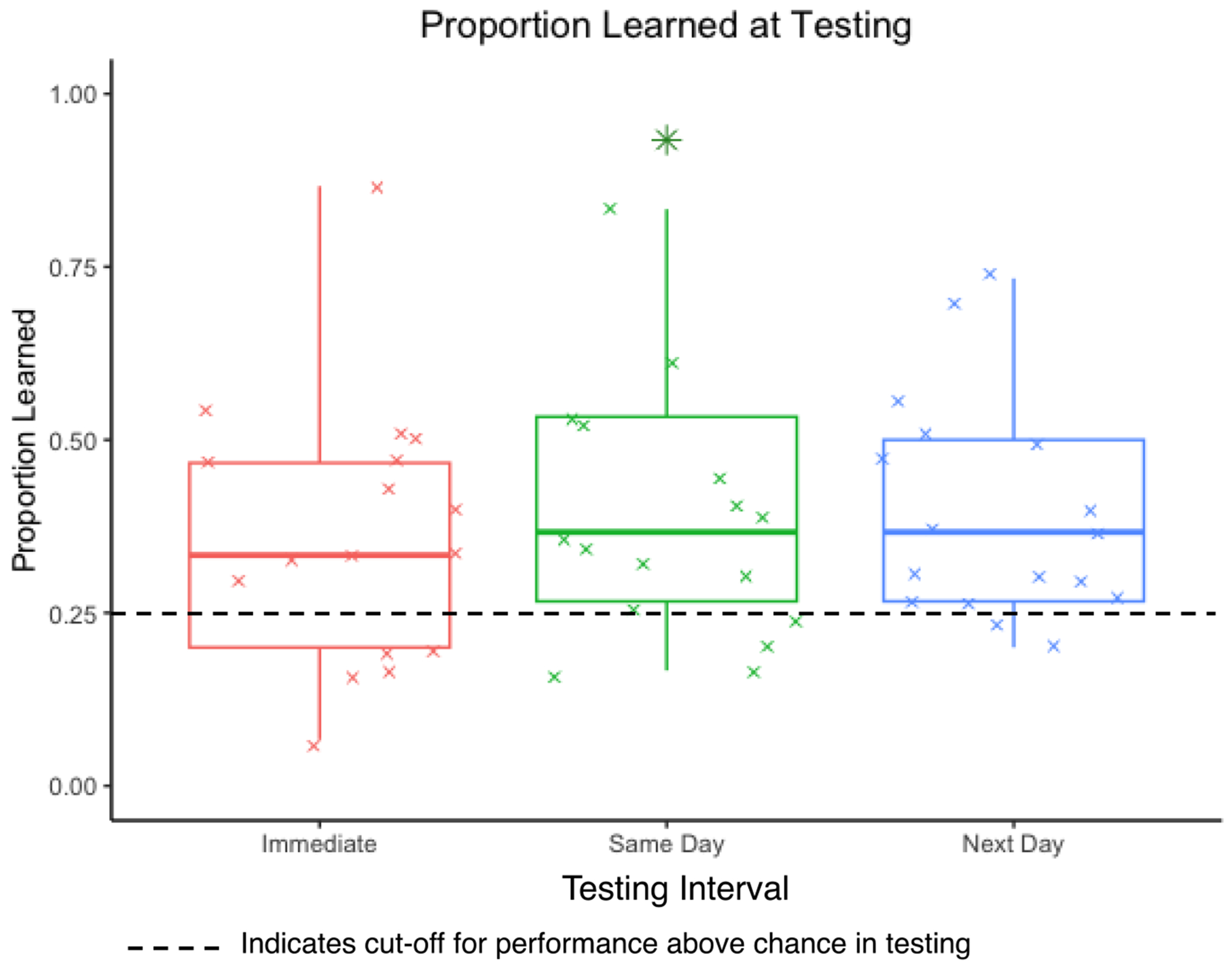
Boxplot illustrating the distribution of the proportion of novel words learned across testing intervals.
Feedback Processing
The grand average waveform (Figure 6) represents the average FRN for all 17 participants. A negative going deflection in the expected window of the FRN peaked at approximately 270 ms after feedback. A two-tailed dependent samples t-test revealed a larger (more negative-going) FRN magnitude in response to negative feedback (M = 0.35, SD = 1.00) relative to positive feedback (M = 0.54, SD = 1.01), t (16) = 2.84, p = .01 (Figure 7), as is typically observed with the FRN (Miltner et al., 1997; Sambrook & Goslin, 2015; Williams et al., 2021).
Figure 6.
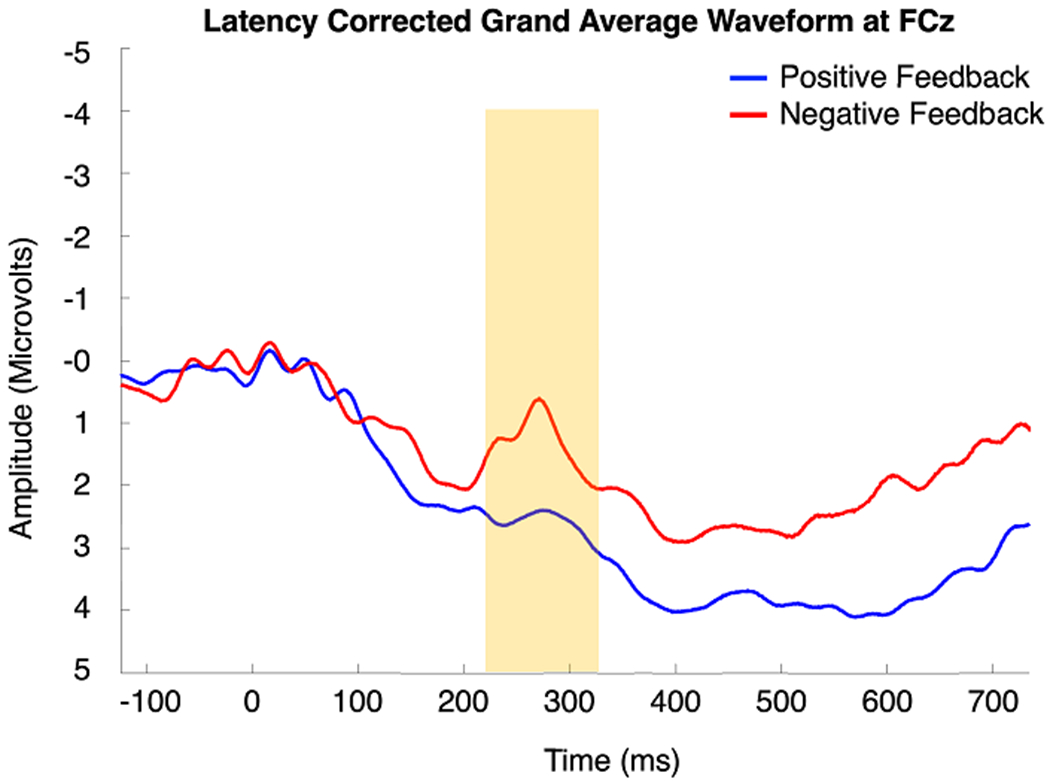
Latency corrected grand average waveform measured at FCZ. The feedback-related negativity (FRN) is highlighted in yellow.
Figure 7.
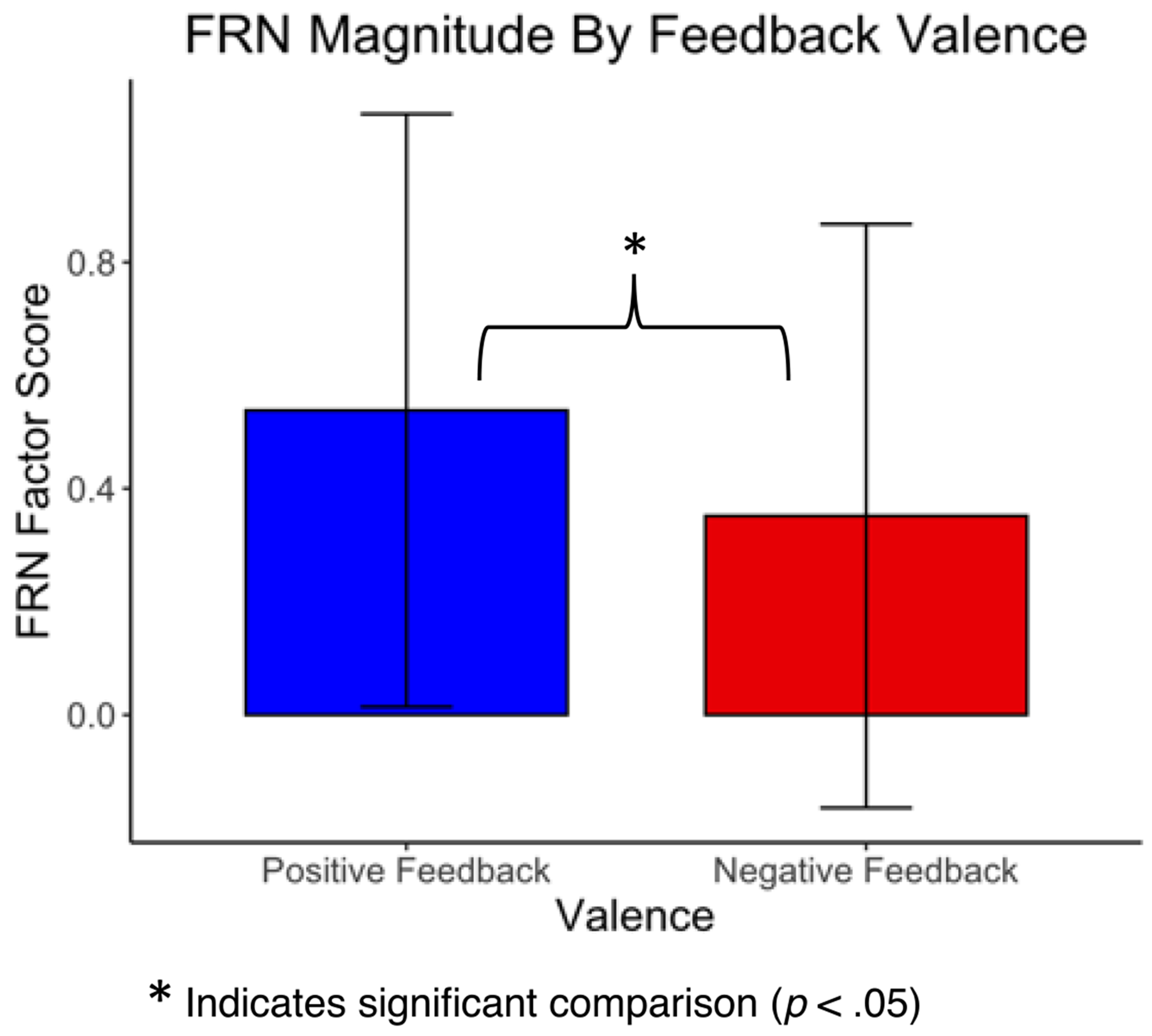
FRN factors scores to positive and negative feedback. Of note, because the FRN is a negative-going waveform, a smaller factor score represents a larger magnitude FRN.
Feedback Processing and Learning
We computed partial correlations to determine whether the FRN magnitude to positive or negative feedback was associated with learning when controlling for phonological processing and verbal STM. No partial correlations were statistically significant (Table 3). Zero-order correlations (Pearson’s r) were also non-significant.
Table 3.
Partial correlations between FRN factor scores and learning controlling for phonological processing and verbal STM
| Immediate Test (r) | Same Day Test (r) | Next Day Test (r) | |
|---|---|---|---|
| FRN to Positive Feedback | −.07 (p = .80) | −.13 (p = .65) | −.21(p = .45) |
| FRN to Negative Feedback | .01 (p = .97) | −.01 (p = .98) | −.11 (p = .71) |
Cognitive Correlates of Feedback Processing
To evaluate whether feedback processing was associated with cognitive scores, we computed Pearson’s r correlation coefficients between cognitive measures and the FRN magnitude to positive and negative feedback. Correlations between the FRN to positive and negative feedback and the updating composite, positive: r = −.21, p = .41; negative: r = −.18, p = .48 and attention composite, positive: r = −.05, p = .85; negative: r < .001, p = 1 were small and not significant. However, the correlation between the percent correct on the BCST and the FRN to positive feedback was large and significant, r = −.52, p = .03 (Figure 8). A negative correlation coefficient indicates that higher scores on the BCST were associated with a larger magnitude FRN to positive feedback. There was a trending but non-significant relationship between BCST and the FRN to negative feedback, r = −.40, p = .11. The relationship between the BCST and FRN positive feedback did not remain significant after a Holm correction for multiple comparisons was applied.
Figure 8.
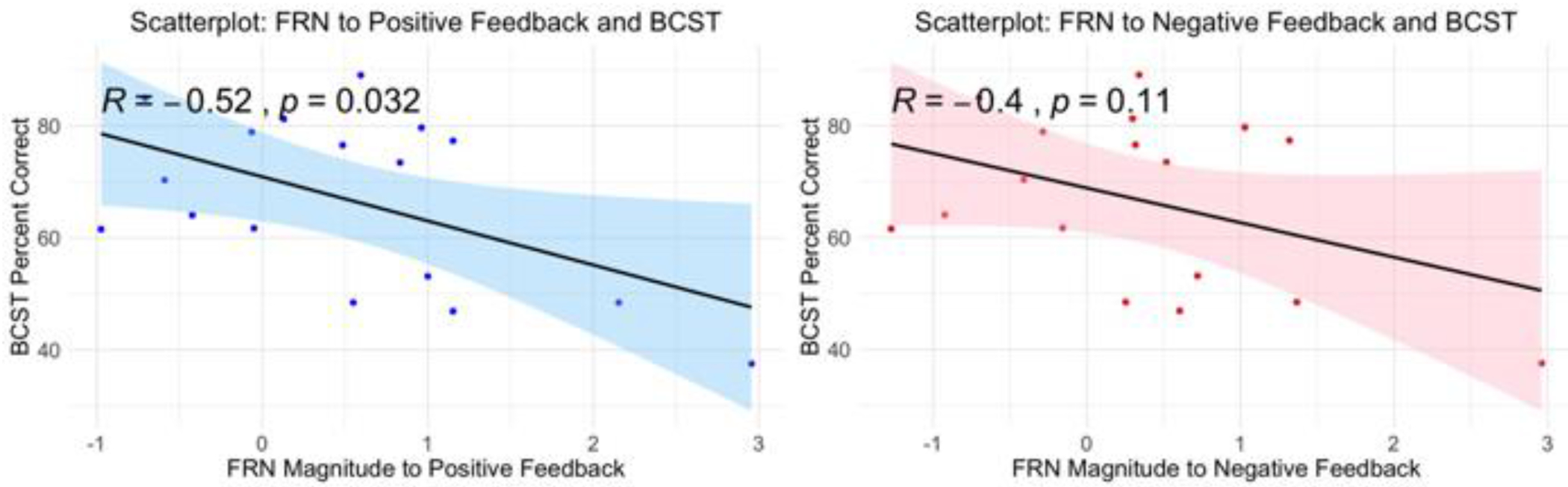
Scatterplot illustrating the relationship between the FRN factor scores to positive (left) and negative (right) feedback and the percent correct on the Berg Card Sorting Test.
Given that the relationship between the FRN and learning is well-established, it was reasonable to consider that a third variable may have influenced the relationship between these two variables. Verbal STM, for example, may support individuals in keeping novel words in memory making feedback that is detected more useful. To inform future quantitative work, we evaluated and qualitatively described interaction plots between behavioural composites and feedback processing. Data indicated a potential cross-over interaction between verbal STM and FRN magnitude and attention and the FRN magnitude at the same-day and next-day tests, respectively (Figure 9). Individuals who had verbal STM or attention composites at least 1 SD above the mean appeared to show the expected negative relationship between the FRN magnitude and learning, while individuals with scores 1 SD below the mean did not.
Figure 9.
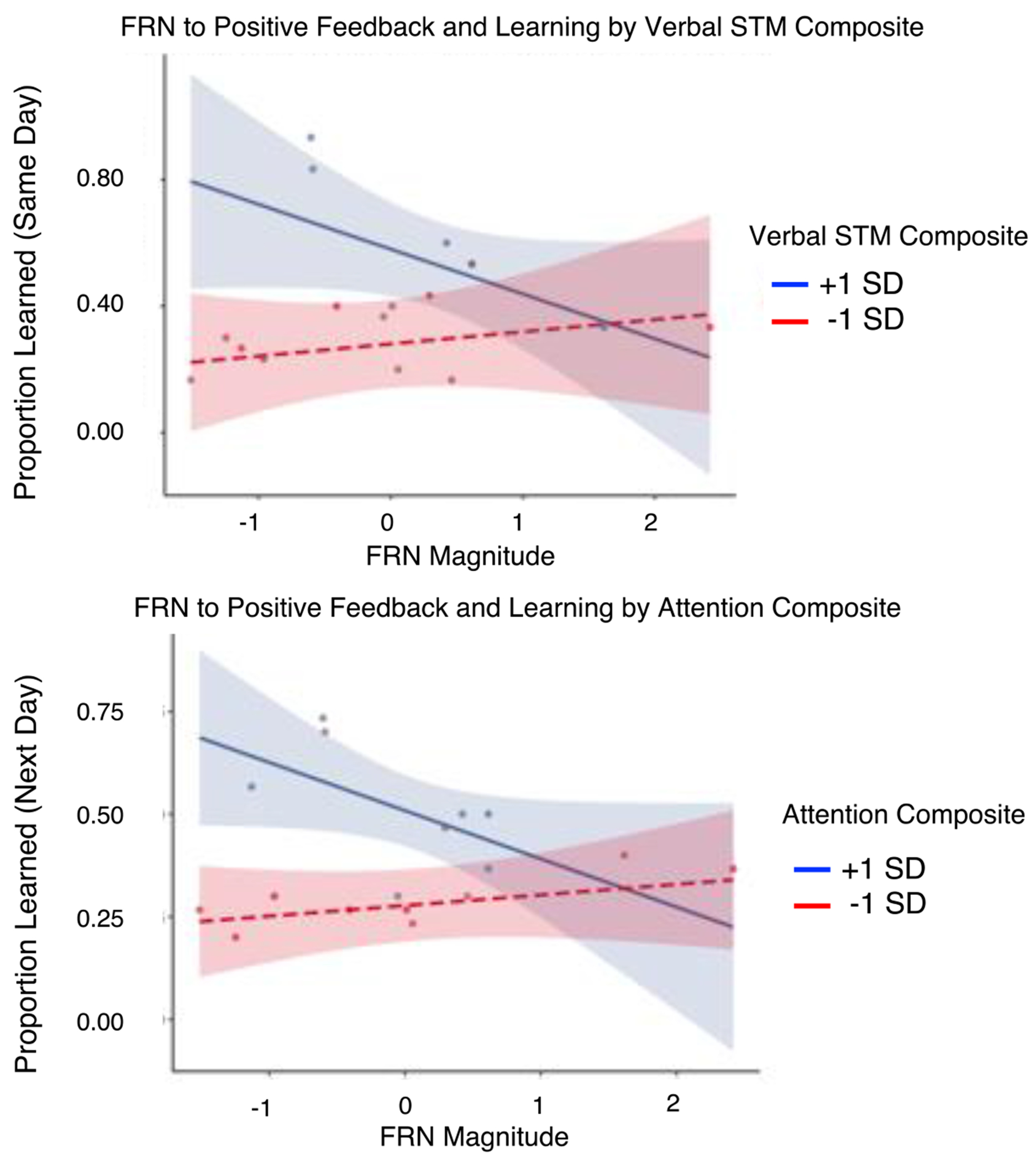
Visualization of the relationship between the FRN factor scores to positive feedback and learning across participants with higher (blue) and lower (red) composite scores for verbal short-term memory (top) and attention (bottom).
Behavioural Correlates of Learning
Figure 10 displays a correlation matrix between all dependent and independent variables and Table 4 contains the mean sub-assessment scores used to calculate composites. Pearson’s r correlation coefficients were computed to determine whether cognitive or linguistic variables were associated with the proportion of novel words learned. There were no significant correlates of performance at the immediate test. At the same-day test, the relationship between verbal STM and the proportion of items learned was no longer significant once we applied a Holm correction for multiple comparisons, r = .51, p = .038. At the next-day test, attention, r = .62, p = .004 and information updating, r = .65, p = .008 showed large positive correlations with the proportion of novel words learned which remained significant after correcting for multiple comparisons. The correlation between proportion learned at the next day test and phonological processing approached significance, r = .48, p = .05.
Figure 10.
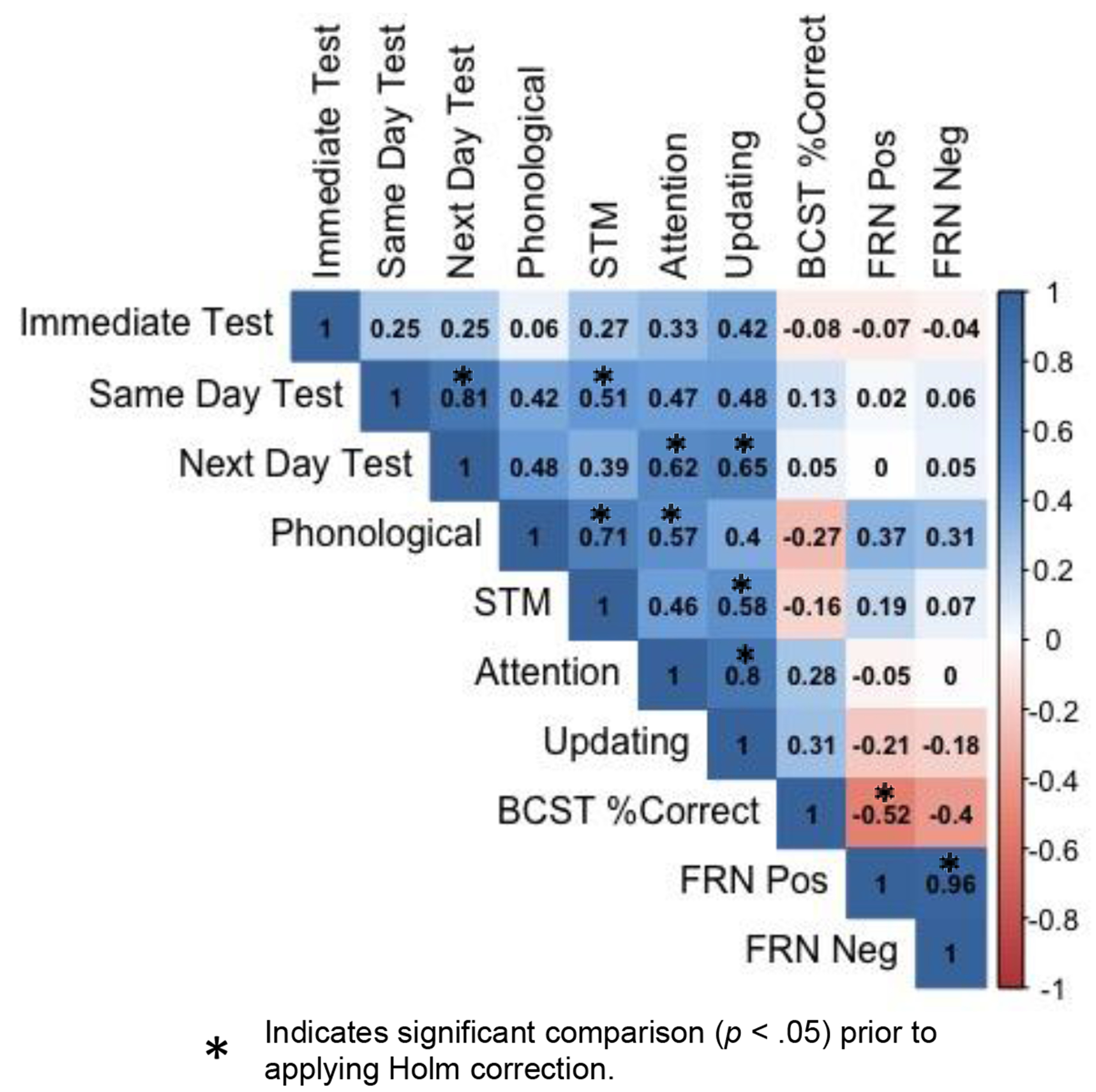
Correlation matrix showing Pearson’s r correlation coefficients all independent and dependent variables.
Table 4.
Means and standard deviations of cognitive and language assessments
| Measure | M | SD |
|---|---|---|
| Phonological Composite | -- | -- |
| Phonological Discrimination1 (--/1) | 0.77 | 0.12 |
| Rhyme Judgement1 (--/1) | 0.83 | 0.12 |
| Verbal STM Composite | -- | -- |
| Word Span (Pointing)1 (--/7) | 2.84 | 0.96 |
| Digit Span (Pointing)1 (--/7) | 3.73 | 1.55 |
| Attention Composite | -- | -- |
| Map2 (--/80) | 36.65 | 17.32 |
| Elevator Counting2 (--/10) | 3.71 | 2.31 |
| Updating Composite | -- | -- |
| Auditory 1-back3 (--/1) | 0.91 | 0.09 |
| Visual 2-back4 (--/1) | 0.80 | 0.09 |
| Switching (BCST %Correct5) (--/100) | 66.66 | 15.39 |
Notes.
Temple Assessment of Language and Short-Term Memory in Aphasia (TALSA) (Martin et al., 2018).
Test of Everyday Attention (TEA) (Robertson et al., 1996).
Berg Card Sorting Test (Fox et al., 2013; Grant & Berg, 1948)
Discussion
The current study evaluated the relationship between feedback processing and novel word learning in PWA. First, PWA demonstrated above-chance performance, thus demonstrating successful learning. The number of novel words learned varied across participants and only one participant performed below chance across all three test points. Overall, the novel word learning results provide additional evidence that individuals with post-stroke aphasia can learn in feedback-based contexts (Peñaloza et al., 2016). There was no significant difference in the proportion of novel words learned across the three testing intervals, indicating maintenance of novel words learned. The design of the feedback-based training may have supported retention by engaging individuals in repeated instances of testing (i.e., opportunities to retrieve the correct object for a novel word from memory). The “testing effect” is a well-studied phenomenon in education that demonstrates that learned information is retained better when it is “tested” rather than “restudied” (i.e., reviewed without opportunities for retrieval) (Rowland, 2014) and is applicable to naming treatment for aphasia (Middleton et al., 2015). The current study did not have a restudy condition and thus, we cannot conclude that the testing itself supported retention; however, future comparative studies may provide useful insights into components of feedback-based learning conditions that may be beneficial for learning for PWA.
The current paper provides the first electrophysiological investigation of feedback-based learning in aphasia. A frontocentral negative-going waveform was identified within the time window of the FRN. This waveform was larger for negative relative to positive feedback, as is typical with the FRN (Miltner et al., 1997; Sambrook & Goslin, 2015; Williams et al., 2021). These findings suggest that the FRN can be measured in people with chronic post-stroke aphasia and that it follows a similar time course and sensitivity to feedback valence as adults without stroke. Niessen et al. (2020) found similar findings when evaluating response monitoring via the error-related negativity (ERN) in individuals with left hemisphere stroke, some of whom had aphasia. The ERN is hypothesized to be generated by the same neural system as the FRN but is elicited when an individual realizes they have made an erroneous response (Holroyd & Coles, 2002; Miltner et al., 1997). In their study, the ERN was typical in those with chronic stroke but impaired in those in the subacute phase (< 4 weeks). The findings of the current study and Niessen et al. (2020) suggest that error monitoring as measured via ERPs may be intact in individuals with chronic aphasia due to left hemisphere stroke. However, how error monitoring may change across stages of stroke recovery warrants exploration, especially considering that much of language therapy occurs during the subacute phase.
Models of gated Hebbian learning have described learning in the presence of errors as requiring three major components: detection of errant behavior, memory and coding of responses, and attention-executive skills important for correcting errors (Lambon Ralph & Fillingham, 2007; Nunn et al., 2023). Our findings provide support for attention and information updating being important for the retention of novel words learned in feedback-based contexts. However, contrary to our expectations and previous research, we did not find that the FRN, a measure of error detection, was associated with learning.
The FRN magnitude was strongly correlated with performance on another feedback-based task within our study, the BCST. Interestingly, these findings suggest that the FRN is associated with PWA’s performance in some but not all feedback-dependent tasks. One potential explanation for the absence of an association between the FRN and performance in the novel word learning task is that the FRN did not entirely capture the kind of feedback processing that was necessary for PWA to learn in this task specifically. Broadly, feedback processing can be described as occurring in temporally distinct stages (West et al., 2012). First, learners must detect feedback and extract information regarding the outcome of their action (captured via the FRN); then, feedback must be used to update associations between a stimulus and responses (Butterfield & Mangels, 2003; Li et al., 2018). The cognitive requirements necessary to use feedback to update associations in memory may have varied across tasks and affected the relationship between feedback processing (measured using the FRN) and learning. Potentially relevant task differences are described below:
Mental operations required to associate feedback with responses: In the novel word learning task, training stimuli were not visible when feedback was presented. Thus, an individual needed to maintain novel words and unfamiliar objects in STM so that feedback could reinforce the correct response. Attention also had to be allocated to relevant aspects of stimuli that had to be recalled later. In the BCST, stimuli were visible when feedback is presented reducing the need to maintain previous responses in memory.
Time until presented feedback could be applied on future trials: In the BCST, feedback could be used immediately on the next trial. Feedback in the novel word learning task informed responses that occurred after several intervening trials. If the memory trace of the correct association had faded prior to the next presentation of the novel word, learners would be unable to reinforce and further strengthen the correct stimulus-response association (see Maddox et al., 2011 for discussion on forgetting rates and spacing of training trials in older adults).
Timing of assessment: Performance on the BCST was assessed during training while performance on the novel word learning task was assessed at testing intervals that ranged from immediately after training to the next day. Measuring outcomes during testing and not training required that novel words were consolidated in long-term memory.
Considering these key differences, we propose that in the novel word learning task, after feedback had been detected, updating associations in memory required additional cognitive computations relative to the BCST. For some individuals, these cognitive demands may have negatively affected performance, even if feedback was detected, as measured by the FRN. As a result, the relationship between the FRN and learning was not observed in the novel word learning task. Alternatively, in the BCST, there were fewer cognitive demands necessary to update action-outcome contingencies and thus those who detected feedback were more likely to learn, even if they had concomitant cognitive deficits.
To better understand the relationship between feedback processing and learning across individuals with different cognitive profiles, we plotted the relationship between feedback processing and learning for individuals with behavioural composites one SD above and below the mean (Figure 9). Visual inspection indicated that those with better attention and verbal STM (1 SD above the mean) may show the expected relationship between the FRN and learning while individuals with lower scores (1 SD below the mean) may not. While this relationship is anecdotal, it does provide a logical explanation of the current findings that considers the cognitive skills necessary to learn from feedback. Future evaluations of this hypothesis may aid in identifying which cognitive variables interact with the relationship between feedback processing and learning, with the ultimate goal of understanding how treatment tasks may be altered to reduce unnecessary cognitive demands that may hinder learning. Figure 11 illustrates the potential relationship between feedback processing, learning, and cognition and its implications for future research.
Figure 11.
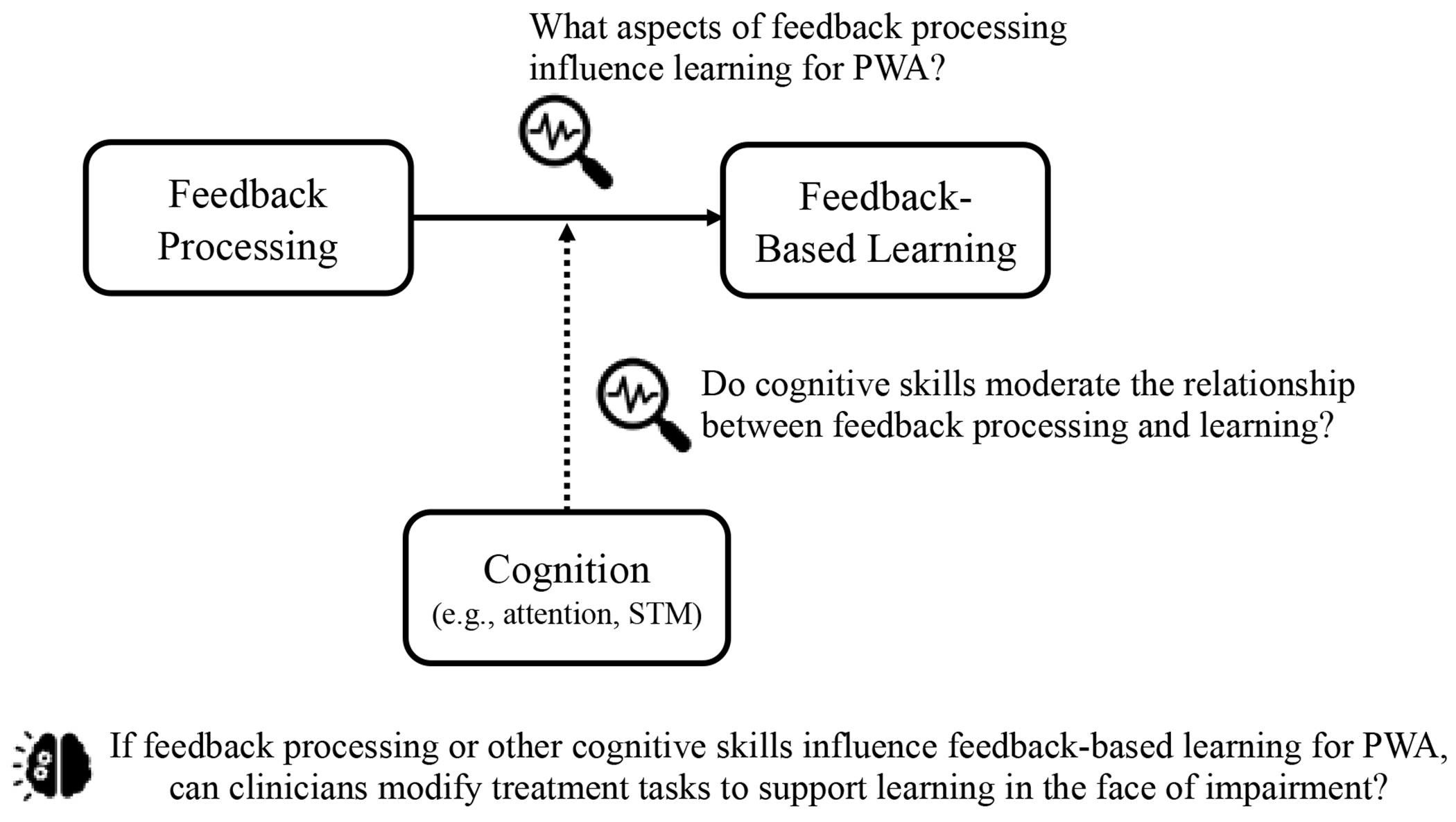
Schematic illustrating the potential relationship between feedback processing, feedback-based learning, and cognition. Areas for future research are identified.
As stated above, these findings differ from previous research evaluating learning and the FRN in adults without neurologic injury (Arbel et al., 2013; Arbel & Wu, 2016; Luft, 2014). The dissimilarity in findings may provide additional rationale for the evaluation of ERPs within populations with varying cognitive-linguistic abilities. Interestingly, in children between 8 and 11 years old, Arbel and Fox (2021) also found that the relationship between the FRN and learning was affected by a third variable. Children who were older (> 10.5 years) showed the expected relationship between feedback processing and learning while children who were younger (< 8.4 years) showed the opposite pattern. Conversely, the P3a, an ERP associated with the post-processing of feedback and the updating of action-outcome contingencies, predicted learning with no significant interaction with age. Age-related changes in executive functioning are common during the early school years (Welsh et al., 1991) and similar to PWA, differences in cognition may explain why the FRN may not be associated with learning. How cognitive profiles influence learning in feedback-based contexts warrants ongoing investigation.
Prior research in people with aphasia has found relationships between phonological processing and learning, which was not observed in the current study, potentially due to differences in novel word learning tasks. Phonological processing, for example, has been found to be a stronger predictor of learning when the assessment or trained material places significant demands on the phonological system. Gupta et al. (2006) identified that phonological processing was associated with performance when learning was assessed via confrontation naming but not when assessed via receptive recognition. In the current task, participants were not asked to produce novel words, only to indicate via button-press the association between a novel word and a picture. Researchers who have found phonological processing abilities to predict receptive performance have trained bisyllabic and trisyllabic words (Peñaloza et al., 2016, 2017) which likely require more phonological skills relative to the CVC words used in the current task.
Limitations
The current study aimed to specifically evaluate the contribution of feedback processing to learning and thus did not include a task without feedback. It is possible that behavioural variables that correlated with the current feedback-based task also correlate with performance on non-feedback-based tasks. However, this relationship cannot be evaluated within this study. Determining how to best identify the unique demands of learning with and without feedback and how they relate to cognitive-linguistic skills in people with aphasia is an important area of future research that may help guide treatment selection.
The sample size limited our ability to investigate interaction effects between variables. However, the current study may guide future research as to the sample size required to detect an interaction between feedback processing and cognition. Additionally, our sample does not reflect the diversity of the post-stroke aphasia population limiting our ability to understand how individuals with varied linguistic, cultural, racial, ethnic, and educational backgrounds may perform in feedback-based learning contexts. Importantly, we recognize that there are known systemic racial biases in EEG research. Contact with the electrode and scalp is affected by hair thickness, texture, and styling which predominantly influences the signal quality and recruitment of Black and African American participants (Choy et al., 2022). We were able to achieve acceptable scalp conductance with our participants identifying as Black and African American; however, it is unknown if previous negative experiences with EEG influenced the participant pool (see Choy et al. 2022 for potential developments for reducing this bias in EEG research).
Finally, while more than 100 studies have been published using ERPs with individuals with aphasia (Silkes & Anjum, 2021), additional work is needed to fully understand how variables such as stroke location and time-post stroke influence the EEG signal and interpretation.
Future Directions
Our findings indicate that for PWA, feedback-based learning may not only depend on feedback processing but also on cognitive skills that facilitate the extraction of meaningful information from feedback. Future research can further investigate this hypothesis by evaluating the relationship between the FRN and learning with a larger sample of PWA who vary on relevant cognitive variables. Alternatively, other ERPs may provide insight into the cognitive skills that support the post-processing of feedback and can also be evaluated in PWA. Broadly, our study has identified that PWA can learn from feedback but that outcomes are variable. Feedback is ubiquitous in aphasia interventions and an ingredient that in other fields, is a known driver of learning (Kearney et al., 2019; Maas et al., 2008; Wisniewski et al., 2020). Thus, understanding how feedback may affect learning during aphasia rehabilitation and how clinicians can modify tasks to support learning from feedback warrants ongoing exploration.
Conclusion
The current study evaluated the relationship between feedback processing and learning in individuals with post-stroke aphasia. PWA showed an FRN that was sensitive to feedback valence (larger for negative relative to positive feedback). The FRN magnitude was not associated with novel word learning but was strongly correlated with performance on another feedback-based task, the BCST. These findings suggest that in PWA, the relationship between feedback processing and learning may vary across tasks. Inspecting the relationship between feedback processing and novel word learning across individuals with varied cognitive composite scores suggests that even those who can effectively process feedback may not learn if using feedback has added cognitive demands (e.g., holding previous stimuli in memory, attending to relevant stimulus dimensions). Broadly, these findings indicate that PWA can process feedback and that this ability may be associated with performance in some feedback-based tasks. This work contributes to a growing area of research that aims to understand how language and learning systems work with language systems to influence language recovery. Future research can further characterize the relationship between individual cognitive profiles, feedback processing, and learning in aphasia rehabilitation.
Acknowledgements:
Thank you, Asiya Gul, for your support with EEG data processing; Kesi Cania, for assistance with behavioural data processing and double scoring; and Victoria Tilton-Bolowsky for your feedback on this manuscript. Thank you to the individuals with aphasia who participated in this research.
Funding Statement:
This work was supported by the National Institute on Deafness and Other Communication Disorders of the National Institute of Health awarded to Sofia Vallila-Rohter [R21 DC019203] and Yael Arbel [R15 DC016438]. A portion of Kristen Nunn’s time was also supported by the Department of Veterans Affairs Office of Academic Affiliations, Advanced Fellowship in Geriatrics of the Veterans Affairs Pittsburgh Health Care System and the Department of Veterans Affairs Pittsburgh Geriatric Research, Education, and Clinical Center (GRECC). The content is solely the responsibility of the authors and does not represent the views of the National Institute of Health, VA, or United States Government.
Appendix 1. Individual participant scores for language, cognitive, electrophysiological, and learning measures.
| ID | WAB-R AQ | Phon. Comp | V-STM Comp | Attention Comp | Updating Comp | BCST %acc | FRN (Pos) | FRN (Neg) | Immediate Test | Same Day Test | Next Day Test |
|---|---|---|---|---|---|---|---|---|---|---|---|
| A1 | 82.5 | 0.68 | 0.24 | −0.83 | −1.17 | 48.44 | 0.55 | 0.25 | 0.30 | 0.40 | 0.27 |
| A2 | 64.0 | −0.25 | −1.23 | −1.43 | −1.48 | 37.50 | 2.95 | 2.96 | 0.20 | 0.33 | 0.37 |
| A4 | 93.5 | 1.22 | 1.04 | 0.67 | 0.47 | 78.90 | −0.06 | −0.29 | 0.70 | 0.93 | 0.73 |
| A5 | 91.7 | 0.19 | 0.95 | 0.32 | 1.17 | 61.72 | −0.05 | −0.16 | 0.87 | 0.83 | 0.70 |
| A8 | 85.1 | −1.05 | −0.17 | −0.69 | −0.03 | 61.54 | −0.97 | −1.27 | 0.17 | 0.17 | 0.27 |
| A9 | 89.5 | 0.61 | 1.63 | −0.34 | 0.54 | 48.44 | 2.15 | 1.37 | 0.33 | 0.33 | 0.40 |
| A10 | 91.6 | 1.12 | 1.31 | 0.92 | 0.32 | 46.88 | 1.15 | 0.60 | 0.47 | 0.53 | 0.37 |
| A11 | 70.9 | −0.46 | −0.86 | 0.35 | 0.03 | 73.44 | 0.83 | 0.52 | 0.20 | 0.43 | 0.47 |
| A12 | 87.6 | 1.01 | 0.13 | 1.50 | 0.93 | 77.34 | 1.15 | 1.32 | 0.47 | 0.53 | 0.50 |
| A13 | 60.8 | −1.00 | −0.86 | −0.84 | −0.89 | 64.06 | −0.42 | −0.92 | 0.50 | 0.23 | 0.30 |
| A14 | 50.0 | −2.14 | −1.86 | −0.94 | −1.17 | 81.25 | 0.13 | 0.30 | 0.33 | 0.40 | 0.27 |
| A15 | 84.6 | −0.72 | 0.34 | 0.54 | 0.32 | 76.56 | 0.49 | 0.32 | 0.50 | 0.37 | 0.30 |
| A16 | 66.3 | 0.16 | −0.94 | 1.08 | 1.10 | 70.31 | −0.59 | −0.41 | 0.40 | 0.27 | 0.57 |
| A17 | 79.0 | −0.80 | −0.57 | −0.82 | 0.13 | 85.00 | −0.71 | −0.70 | 0.33 | 0.30 | 0.20 |
| A18 | 83.0 | 0.22 | −0.10 | −0.34 | −0.45 | 89.06 | 0.59 | 0.34 | 0.17 | 0.20 | 0.23 |
| A19 | 85.1 | 0.46 | 1.14 | 0.81 | 0.89 | 79.69 | 0.96 | 1.03 | 0.53 | 0.60 | 0.50 |
| A20 | 68.6 | 0.74 | −0.19 | 0.04 | −0.72 | 53.13 | 1.00 | 0.72 | 0.43 | 0.17 | 0.30 |
| Mean (SD) | 78.46 (12.69) | -- | -- | -- | -- | 66.66 (15.39) | 0.54 (1.02) | 0.35 (1.00) | .37 (.19) | .41 (.22) | .40 (.16) |
WAB-R AQ: Western Aphasia Battery – Revised Aphasia Quotient. Phon Comp: Phonological Composite. V-STM Comp: Verbal Short-Term Memory Composite. Attention Comp: Attention Composite. Updating Comp: Updating Composite. BCST %acc: Berg Card Sorting Task, percent accurate. FRN (Pos): FRN to Positive Feedback. FRN (Neg): FRN to Negative Feedback.
Footnotes
Declaration of interest statement
The authors have no relevant financial or nonfinancial conflicts of interest to disclose.
To verify that this participant did not have a response preference, we calculated the percent of responses that were for the target on the left and the right. The participant chose the item on the left 48% of the time and the item on the right 52% of the time indicating no response bias secondary to a history of visual inattention.
To ensure that latency correction did not impact findings, analyses were run with both the latency and non-latency corrected data. Significant findings were the same across data sets. Latency corrected data is presented here for visual clarity of figures.
References
- Abel S, Weiller C, Huber W, Willmes K, & Specht K (2015). Therapy-induced brain reorganization patterns in aphasia. Brain, 138(4), 1097–1112. 10.1093/brain/awv022 [DOI] [PubMed] [Google Scholar]
- Arbel Y, & Fox AB (2021). Electrophysiological examination of feedback-based learning in 8–11-year-old children. Frontiers in Psychology, 12, 640270. 10.3389/fpsyg.2021.640270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbel Y, Goforth K, & Donchin E (2013). The good, the bad, or the useful? The examination of the relationship between the feedback-related negativity (FRN) and long-term learning outcomes. Journal of Cognitive Neuroscience, 25(8), 1249–1260. 10.1162/jocn_a_00385 [DOI] [PubMed] [Google Scholar]
- Arbel Y, Hong L, Baker TE, & Holroyd CB (2017). It’s all about timing: An electrophysiological examination of feedback-based learning with immediate and delayed feedback. Neuropsychologia, 99, 179–186. 10.1016/j.neuropsychologia.2017.03.003 [DOI] [PubMed] [Google Scholar]
- Arbel Y, Murphy A, & Donchin E (2014). On the utility of positive and negative feedback in a paired-associate learning task. Journal of Cognitive Neuroscience, 26(7), 1445–1453. 10.1162/jocn_a_00617 [DOI] [PubMed] [Google Scholar]
- Arbel Y, & Wu H (2016). A Neurophysiological examination of quality of learning in a feedback-based learning task. Neuropsychologia, 93, 13–20. 10.1016/j.neuropsychologia.2016.10.001 [DOI] [PubMed] [Google Scholar]
- Baddeley A, & Wilson BA (1994). When implicit learning fails: Amnesia and the problem of error elimination. Neuropsychologia, 32(1), 53–68. 10.1016/0028-3932(94)90068-X [DOI] [PubMed] [Google Scholar]
- Becker MPI, Nitsch AM, Miltner WHR, & Straube T (2014). A single-trial estimation of the feedback-related negativity and its relation to BOLD responses in a time-estimation task. Journal of Neuroscience, 34(8), 3005–3012. 10.1523/JNEUROSCI.3684-13.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady MC, Kelly H, Godwin J, Enderby P, & Campbell P (2016). Speech and language therapy for aphasia following stroke. Cochrane Database of Systematic Reviews, 2016(6). 10.1002/14651858.CD000425.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitenstein C, Grewe T, Flöel A, Ziegler W, Springer L, Martus P, Huber W, Willmes K, Ringelstein EB, Haeusler KG, Abel S, Glindemann R, Domahs F, Regenbrecht F, Schlenck K-J, Thomas M, Obrig H, de Langen E, Rocker R, … Bamborschke S (2017). Intensive speech and language therapy in patients with chronic aphasia after stroke: A randomised, open-label, blinded-endpoint, controlled trial in a health-care setting. The Lancet, 389(10078), 1528–1538. 10.1016/S0140-6736(17)30067-3 [DOI] [PubMed] [Google Scholar]
- Breitenstein C, Kamping S, Jansen A, Schomacher M, & Knecht S (2004). Word learning can be achieved without feedback: Implications for aphasia therapy. Restorative Neurology & Neuroscience, 22(6), 445–458. [PubMed] [Google Scholar]
- Brownsett SLE, Warren JE, Geranmayeh F, Woodhead Z, Leech R, & Wise RJS (2014). Cognitive control and its impact on recovery from aphasic stroke. Brain, 137(1), 242–254. 10.1093/brain/awt289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler AC, & Roediger HL (2008). Feedback enhances the positive effects and reduces the negative effects of multiple-choice testing. Memory & Cognition, 36(3), 604–616. 10.3758/MC.36.3.604 [DOI] [PubMed] [Google Scholar]
- Butterfield B, & Mangels JA (2003). Neural correlates of error detection and correction in a semantic retrieval task. Cognitive Brain Research, 17(3), 793–817. 10.1016/S0926-6410(03)00203-9 [DOI] [PubMed] [Google Scholar]
- Charidimou A, Kasselimis D, Varkanitsa M, Selai C, Potagas C, & Evdokimidis I (2014). Why is it difficult to predict language impairment and outcome in patients with aphasia after stroke? Journal of Clinical Neurology, 10(2), 75–83. 10.3988/jcn.2014.10.2.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choy T, Baker E, & Stavropoulos K (2022). Systemic Racism in EEG Research: Considerations and Potential Solutions. Affective Science, 3(1), 14–20. 10.1007/s42761-021-00050-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen SC, & Wright HH (2010). Verbal and non-verbal working memory in aphasia: What three n-back tasks reveal. Aphasiology, 24, 752–762. [Google Scholar]
- Conroy P, Sage K, & Lambon Ralph MA (2009b). Errorless and errorful therapy for verb and noun naming in aphasia. Aphasiology, 23(11), 1311–1337. 10.1080/02687030902756439 [DOI] [Google Scholar]
- DeDe G, Ricca M, Knilans J, & Trubl B (2014). Construct validity and reliability of working memory tasks for people with aphasia. Aphasiology, 28(6), 692–712. 10.1080/02687038.2014.895973 [DOI] [Google Scholar]
- Delorme A, & Makeig S (2004). EEGLAB: An open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. Journal of Neuroscience Methods, 134(1), 9–21. 10.1016/j.jneumeth.2003.10.009 [DOI] [PubMed] [Google Scholar]
- Dien J (2010). Evaluating two-step PCA of ERP data with Geomin, Infomax, Oblimin, Promax, and Varimax rotations. Psychophysiology, 47(1), 170–183. 10.1111/j.1469-8986.2009.00885.x [DOI] [PubMed] [Google Scholar]
- Dignam J, Copland D, Rawlings A, O’Brien K, Burfein P, & Rodriguez AD (2016). The relationship between novel word learning and anomia treatment success in adults with chronic aphasia. Neuropsychologia, 81, 186–197. 10.1016/j.neuropsychologia.2015.12.026 [DOI] [PubMed] [Google Scholar]
- El Hachioui H, Visch-Brink EG, Lingsma HF, van de Sandt-Koenderman MWME, Dippel DWJ, Koudstaal PJ, & Middelkoop HAM (2014). Nonlinguistic cognitive impairment in poststroke aphasia: A prospective study. Neurorehabilitation and Neural Repair, 28(3), 273–281. 10.1177/1545968313508467 [DOI] [PubMed] [Google Scholar]
- Exton-McGuinness MT, Lee JL, & Reichelt AC (2015). Updating memories—The role of prediction errors in memory reconsolidation. Behavioural Brain Research, 278, 375–384. 10.1016/j.bbr.2014.10.011 [DOI] [PubMed] [Google Scholar]
- Fillingham J, Hodgson C, Sage K, & Lambon Ralph MA (2003). The application of errorless learning to aphasic disorders: A review of theory and practice. Neuropsychological Rehabilitation, 13(3), 337–363. 10.1080/09602010343000020 [DOI] [PubMed] [Google Scholar]
- Fillingham J, Sage K, & Lambon Ralph M (2005b). Treatment of anomia using errorless versus errorful learning: Are frontal executive skills and feedback important? International Journal of Language & Communication Disorders, 40(4), 505–523. 10.1080/13682820500138572 [DOI] [PubMed] [Google Scholar]
- Fillingham J, Sage K, & Lambon Ralph MA (2006). The treatment of anomia using errorless learning. Neuropsychological Rehabilitation, 16(2), 129–154. 10.1080/09602010443000254 [DOI] [PubMed] [Google Scholar]
- Fonseca J, Raposo A, & Martins IP (2019). Cognitive functioning in chronic post-stroke aphasia. Applied Neuropsychology: Adult, 26(4), 355–364. 10.1080/23279095.2018.1429442 [DOI] [PubMed] [Google Scholar]
- Fox CJ, Mueller ST, Gray HM, Raber J, & Piper BJ (2013). Evaluation of a short-form of the berg card sorting test. PLoS ONE, 8(5), e63885. 10.1371/journal.pone.0063885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman ML, & Martin RC (2001). Dissociable components of short-term memory and their relation to long-term learning. Cognitive Neuropsychology, 18(3), 193–226. 10.1080/02643290126002 [DOI] [PubMed] [Google Scholar]
- Fridriksson J, Bonilha L, Baker JM, Moser D, & Rorden C (2009). Activity in preserved left hemisphere regions predicts anomia severity in aphasia. Cerebral Cortex, 20, 1013–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson J, Nettles C, Davis M, Morrow L, & Montgomery A (2006). Functional communication and executive function in aphasia. Clinical Linguistics & Phonetics, 20(6), 401–410. 10.1080/02699200500075781 [DOI] [PubMed] [Google Scholar]
- Gilmore N, Meier EL, Johnson JP, & Kiran S (2019). Nonlinguistic cognitive factors predict treatment-induced recovery in chronic poststroke aphasia. Archives of Physical Medicine and Rehabilitation, 100(7), 1251–1258. 10.1016/j.apmr.2018.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Yuan J, Wang S, Shi L, Cui X, & Luo X (2014). Feedback-related negativity in children with two subtypes of attention deficit hyperactivity disorder. PLoS ONE, 9(6), e99570. 10.1371/journal.pone.0099570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant DA and Berg E. (1948). A behavioral analysis of degree of reinforcement and ease of shifting to new responses in a Weigl-type card-sorting problem. Journal of Experimental Psychology, 38(4), 404–411. 10.1037/h0059831 [DOI] [PubMed] [Google Scholar]
- Gupta P, Martin N, Abbs B, Schwartz M, & Lipinski J (2006). New word learning in aphasic patients: Dissociating phonological and semantic components. Brain and Language, 99(1–2), 8–9. 10.1016/j.bandl.2006.06.015 [DOI] [Google Scholar]
- Hauser TU, Iannaccone R, Stämp P, Walitza S, & Brem S (2014). The feedback-related negativity (FRN) revisited: New insights into the localization, meaning and network organization. NeuroImage, 84, 159–168. [DOI] [PubMed] [Google Scholar]
- Holroyd CB, & Coles MGH (2002). The neural basis of human error processing: Reinforcement learning, dopamine, and the error-related negativity. Psychological Review, 109(4), 679–709. 10.1037/0033-295X.109.4.679 [DOI] [PubMed] [Google Scholar]
- Kearney E, Shellikeri S, Martino R, & Yunusova Y (2019). Augmented visual feedback-aided interventions for motor rehabilitation in Parkinson’s disease: A systematic review. Disability and Rehabilitation, 41(9), 995–1011. 10.1080/09638288.2017.1419292 [DOI] [PubMed] [Google Scholar]
- Kelly H, & Armstrong L (2009). New word learning in people with aphasia. Aphasiology, 23(12), 1398–1417. 10.1080/02687030802289200 [DOI] [Google Scholar]
- Kertesz A (2007). The Western Aphasia Battery—Revised. Grune & Stratton. [Google Scholar]
- Kóbor A, Takács Á, Janacsek K, Németh D, Honbolygó F, & Csépe V (2015). Different strategies underlying uncertain decision making: Higher executive performance is associated with enhanced feedback-related negativity: The role of cognitive control in risk taking. Psychophysiology, 52(3), 367–377. 10.1111/psyp.12331 [DOI] [PubMed] [Google Scholar]
- Kristinsson S, den Ouden DB, Rorden C, Newman-Norlund R, Neils-Strunjas J, & Fridriksson J (2022). Predictors of therapy response in chronic aphasia: Building a foundation for personalized aphasia therapy. Journal of Stroke, 24(2), 189–206. 10.5853/jos.2022.01102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroll J, & Potter M (1984). Recognizing words, pictures, and concepts: A comparison of lexical, object, and reality decisions. Journal of Verbal Learning and Verbal Behavior, 23, 39–66. [Google Scholar]
- Lacey E, Glezer L, Lott S, & Friedman R (2004). The role of effort in errorless and errorful learning. Brain and Language, 91(1), 189–190. 10.1016/j.bandl.2004.06.097. [DOI] [Google Scholar]
- Laiacona M, Inzaghi M, De Tanti A, & Capitani E (2000). Wisconsin card sorting test: A new global score, with Italian norms, and its relationship with the Weigl sorting test. Neurological Sciences, 21, 279–291. [DOI] [PubMed] [Google Scholar]
- Lam JMC, & Wodchis WP (2010). The relationship of 60 disease diagnoses and 15 conditions to preference-based health-related quality of life in ontario hospital-based long-term care residents. Medical Care, 48(4), 380–387. 10.1097/MLR.0b013e3181ca2647 [DOI] [PubMed] [Google Scholar]
- Lambon Ralph MA, & Fillingham JK (2007). The importance of memory and executive function in aphasia: Evidence from the treatment of anomia using errorless and errorful learning. In Meyer AS, Wheeldon LR, & Krott A (Eds.), Advances in Behavioural Brain Science. Automaticity and Control in Language Processing. (pp. 193–216). Psychology Press. [Google Scholar]
- Lambon Ralph MA, Snell C, Fillingham JK, Conroy P, & Sage K (2010). Predicting the outcome of anomia therapy for people with aphasia post CVA: Both language and cognitive status are key predictors. Neuropsychological Rehabilitation, 20(2), 289–305. 10.1080/09602010903237875 [DOI] [PubMed] [Google Scholar]
- Larson MJ, Kelly KG, Stigge-Kaufman DA, Schmalfuss IM, & Perlstein WM (2007). Reward context sensitivity impairment following severe TBI: An event-related potential investigation. Journal of the International Neuropsychological Society, 13(4), 615–625. 10.1017/S1355617707070762 [DOI] [PubMed] [Google Scholar]
- Laures-Gore J, & Rice KG (2019). The Simple Aphasia Stress Scale. Journal of Speech, Language, and Hearing Research, 62(8), 2855–2859. 10.1044/2019_JSLHR-L-19-0053 [DOI] [PubMed] [Google Scholar]
- Lazar RM, Speizer AE, Festa JR, Krakauer JW, & Marshall RS (2008). Variability in language recovery after first-time stroke. Journal of Neurology, Neurosurgery & Psychiatry, 79(5), 530–534. 10.1136/jnnp.2007.122457 [DOI] [PubMed] [Google Scholar]
- Levin MF, & Demers M (2021). Motor learning in neurological rehabilitation. Disability and Rehabilitation, 43(24), 3445–3453. 10.1080/09638288.2020.1752317 [DOI] [PubMed] [Google Scholar]
- Li F, Wang J, Du B, & Cao B (2018). Electrophysiological response to the informative value of feedback revealed in a segmented Wisconsin card sorting test. Frontiers in Psychology, 9, 57. 10.3389/fpsyg.2018.00057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lineweaver TT, Bondi MW, Thomas RG, & Salmon DP (1999). A normative study of Nelson’s (1976) modified version of the Wisconsin Card Sorting Test in healthy older adults. The Clinical Neuropsychologist, 13(3), 328–347. [DOI] [PubMed] [Google Scholar]
- Luck SJ, & Gaspelin N (2017). How to get statistically significant effects in any ERP experiment (and why you shouldn’t): How to get significant effects. Psychophysiology, 54(1), 146–157. 10.1111/psyp.12639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft CDB (2014). Learning from feedback: The neural mechanisms of feedback processing facilitating better performance. Behavioural Brain Research, 261(15), 356–368. 10.1016/j.bbr.2013.12.043 [DOI] [PubMed] [Google Scholar]
- Maas E, Robin DA, Austermann Hula SN, Freedman SE, Wulf G, Ballard KJ, & Schmidt RA (2008). Principles of motor learning in treatment of motor speech disorders. American Journal of Speech-Language Pathology, 17(3), 277–298. 10.1044/1058-0360(2008/025) [DOI] [PubMed] [Google Scholar]
- Maddox GB, Balota DA, Coane JH, & Duchek JM (2011). The role of forgetting rate in producing a benefit of expanded over equal spaced retrieval in young and older adults. Psychology and Aging, 26(3), 661–670. 10.1037/a0022942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli CV, Spaccavento S, Craca A, Marangolo P, & Angelelli P (2017). Different cognitive profiles of patients with severe aphasia. Behavioural Neurology, 2017, 1–15. 10.1155/2017/3875954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin N, Minkina I, Kohen FP, & Kalinyak-Fliszar M (2018). Assessment of linguistic and verbal short-term memory components of language abilities in aphasia. Journal of Neurolinguistics, 48, 199–225. 10.1016/j.jneuroling.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe J, & Eich TS (2019). Memory and truth: Correcting errors with true feedback versus overwriting correct answers with errors. Cognitive Research: Principles and Implications, 4, 221–233. 10.1186/s41235-019-0153-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton EL, Schwartz MF, Rawson KA, & Garvey K (2015). Test-enhanced learning versus errorless learning in aphasia rehabilitation: Testing competing psychological principles. Journal of Experimental Psychology: Learning, Memory, and Cognition, 41(4), 1253–1261. 10.1037/xlm0000091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miltner WHR, Braun CH, & Coles MGH (1997). Event-Related brain potentials following incorrect feedback in a time-estimation task: Evidence for a “generic” neural system for error detection. Journal of Cognitive Neuroscience, 9(6), 788–798. 10.1162/jocn.1997.9.6.788 [DOI] [PubMed] [Google Scholar]
- Mohapatra B, & Marshall RS (2020). Performance differences between aphasia and healthy aging on an executive function test battery. International Journal of Speech-Language Pathology, 22(4), 487–497. 10.1080/17549507.2019.1691262 [DOI] [PubMed] [Google Scholar]
- Murray LL (2012). Attention and other cognitive deficits in aphasia: Presence and relation to language and communication measures. American Journal of Speech-Language Pathology, 21(2), 51–64. 10.1044/1058-0360(2012/11-0067) [DOI] [PubMed] [Google Scholar]
- Murray L, Salis C, Martin N, & Dralle J (2018). The use of standardised short-term and working memory tests in aphasia research: A systematic review. Neuropsychological Rehabilitation, 28(3), 309–351. 10.1080/09602011.2016.1174718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Institute on Deafness and Other Communication Disorders. (2015). NIDCD fact sheet: Aphasia. [Google Scholar]
- Niessen E, Ant JM, Bode S, Saliger J, Karbe H, Fink GR, Stahl J, & Weiss PH (2020). Preserved performance monitoring and error detection in left hemisphere stroke. NeuroImage: Clinical, 27, 1–13. 10.1016/j.nicl.2020.102307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S, Ridderinkhof KR, Talsma D, Coles MGH, Holroyd CB, Kok A, & van der Molen MW (2002). A computational account of altered error processing in older age: Dopamine and the error-related negativity. Cognitive, Affective, & Behavioral Neuroscience, 2(1), 19–36. 10.3758/CABN.2.1.19 [DOI] [PubMed] [Google Scholar]
- Nunn K, Vallila-Rohter S, & Middleton EL (2023). Errorless, errorful, and retrieval practice for naming treatment in aphasia: A scoping review of learning mechanisms and treatment ingredients. Journal of Speech and Hearing Research, 66(2), 668–687. 10.1044/2022_JSLHR-22-00251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson S (1996). Learning from performance errors. Psychological Review, 103(2), 241–262. 10.1037/0033-295X.103.2.241 [DOI] [Google Scholar]
- O’Reilly RC, Hazy TE, & Herd SA (2017). The LEABRA cognitive architecture: How to play 20 principles with nature and win! In The Oxford handbook of cognitive science. (pp. 91–115). Oxford University Press. [Google Scholar]
- Osborne-Crowley K, McDonald S, & Rushby JA (2016). Role of reversal learning impairment in social disinhibition following severe traumatic brain injury. Journal of the International Neuropsychological Society, 22(3), 303–313. 10.1017/S1355617715001277 [DOI] [PubMed] [Google Scholar]
- Pashler H, Cepeda NJ, Wixted JT, & Rohrer D (2005). When does feedback facilitate learning of words? Journal of Experimental Psychology: Learning, Memory, and Cognition, 31(1), 3–8. 10.1037/0278-7393.31.1.3 [DOI] [PubMed] [Google Scholar]
- Peñaloza C, Mirman D, Cardona P, Juncadella M, Martin N, Laine M, & Rodríguez-Fornells A (2017). Cross-situational word learning in aphasia. Cortex, 93, 12–27. 10.1016/j.cortex.2017.04.020 [DOI] [PubMed] [Google Scholar]
- Peñaloza C, Mirman D, Tuomiranta L, Benetello A, Heikius I-M, Järvinen S, Majos MC, Cardona P, Juncadella M, Laine M, Martin N, & Rodríguez-Fornells A (2016). Novel word acquisition in aphasia: Facing the word-referent ambiguity of natural language learning contexts. Cortex, 79, 14–31. 10.1016/j.cortex.2016.03.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Miller GA, Ritter W, Ruchkin DS, Rugg MD, & Taylor MJ (2000). Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology, 37, 26. [PubMed] [Google Scholar]
- Postma A (2000). Detection of errors during speech production: A review of speech monitoring models. Cognition, 77, 97–131. 10.1016/S0010-0277(00)00090-1 [DOI] [PubMed] [Google Scholar]
- Purdy M (2002). Executive function ability in persons with aphasia. Aphasiology, 16(4–6), 549–557. 10.1080/02687030244000176 [DOI] [Google Scholar]
- R Core Team. (2021). R: A language and environment for statistical computing. R Foundation for Statistical Computing. https://www.R-project.org/ [Google Scholar]
- Rastle K, Harrington J, & Coltheart M (2002). 358,534 novel words: The ARC novel word database. The Quarterly Journal of Experimental Psychology Section A, 55(4), 1339–1362. 10.1080/02724980244000099 [DOI] [PubMed] [Google Scholar]
- Reitan RM (1958). Validity of the Trail Making Test as an indicator of organic brain damage. Perceptual and Motor Skills, 8(3), 271–276. [Google Scholar]
- Rmus M, McDougle SD, & Collins AG (2021). The role of executive function in shaping reinforcement learning. Current Opinion in Behavioral Sciences, 38, 66–73. 10.1016/j.cobeha.2020.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson IH, Ward T, Ridgeway V and Nimmo-Smith I. (1996). The structure of normal human attention: The Test of Everyday Attention. J Int Neuropsychol Soc, 2(6), 525–534. 10.1017/S1355617700001697 [DOI] [PubMed] [Google Scholar]
- Robey RR (1998). A meta-analysis of clinical outcomes in the treatment of aphasia. Journal of Speech, Language, and Hearing Research, 41(1), 172–187. 10.1044/jslhr.4101.172 [DOI] [PubMed] [Google Scholar]
- Rowland CA (2014). The effect of testing versus restudy on retention: A meta-analytic review of the testing effect. Psychological Bulletin, 140(6), 1432–1463. 10.1037/a0037559 [DOI] [PubMed] [Google Scholar]
- Sambrook TD, & Goslin J (2015). A neural reward prediction error revealed by a meta-analysis of ERPs using great grand averages. Psychological Bulletin, 141(1), 213–235. 10.1037/bul0000006 [DOI] [PubMed] [Google Scholar]
- Seniów J, Litwin M, & Leśniak M (2009). The relationship between non-linguistic cognitive deficits and language recovery in patients with aphasia. Journal of the Neurological Sciences, 283(1–2), 91–94. 10.1016/j.jns.2009.02.315 [DOI] [PubMed] [Google Scholar]
- Silkes JP, & Anjum J (2021). The role and use of event-related potentials in aphasia: A scoping review. Brain and Language, 219, 104966. 10.1016/j.bandl.2021.104966 [DOI] [PubMed] [Google Scholar]
- Simic T, Bitan T, Turner G, Chambers C, Goldberg D, Leonard C, & Rochon E (2020). The role of executive control in post-stroke aphasia treatment. Neuropsychological Rehabilitation, 30(10), 1853–1892. 10.1080/09602011.2019.1611607 [DOI] [PubMed] [Google Scholar]
- Simmons-Mackie N, Damico JS, & Damico HL (1999). A qualitative study of feedback in aphasia treatment. American Journal of Speech-Language Pathology, 8(3), 218–230. 10.1044/1058-0360.0803.218 [DOI] [Google Scholar]
- Spencer KM, Dien J, & Donchin E (2001). Spatiotemporal analysis of the late ERP responses to deviant stimuli. Psychophysiology, 38(2), 343–358. [PubMed] [Google Scholar]
- Sur S, & Sinha VK (2009). Event-related potential: An overview. Industrial Psychiatry Journal, 18(1), 70–73. 10.4103/0972-6748.57865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swinburn K, Porter G, & Howard D (2004). CAT: Comprehensive aphasia test. Psychology Press. [Google Scholar]
- Sze WP, Hameau S, Warren J, & Best W (2021). Identifying the components of a successful spoken naming therapy: A meta-analysis of word-finding interventions for adults with aphasia. Aphasiology, 35(1), 33–72. 10.1080/02687038.2020.1781419 [DOI] [Google Scholar]
- Talmi D, Atkinson R, & El-Deredy W (2013). The feedback-related negativity signals salience prediction errors, not reward prediction errors. The Journal of Neuroscience, 33(19), 8264–8269. 10.1523/JNEUROSCI.5695-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The MathWorks Inc. (2022). MATLAB (9.12.0). The MathWorks Inc. https://mathworks.com [Google Scholar]
- Tuomiranta L, Grönholm-Nyman P, Kohen F, Rautakoski P, Laine M, & Martin N (2011). Learning and maintaining new vocabulary in persons with aphasia: Two controlled case studies. Aphasiology, 25(9), 1030–1052. 10.1080/02687038.2011.571384 [DOI] [Google Scholar]
- Vallila-Rohter S, & Kiran S (2013). Non-linguistic learning and aphasia: Evidence from a paired associate and feedback-based task. Neuropsychologia, 51(1), 79–90. 10.1016/j.neuropsychologia.2012.10.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Helden J, Boksem MAS, & Blom JHG (2010). The importance of failure: Feedback-related negativity predicts motor learning efficiency. Cerebral Cortex, 20(7), 1596–1603. 10.1093/cercor/bhp224 [DOI] [PubMed] [Google Scholar]
- Villard S, & Kidd G (2019). Effects of acquired aphasia on the recognition of speech under energetic and informational masking conditions. Trends in Hearing, 23, 233121651988448. 10.1177/2331216519884480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh MC, Pennington BF, & Groisser DB (1991). A normative-developmental study of executive function: A window on prefrontal function in children. Developmental Neuropsychology, 7(2), 131–149. [Google Scholar]
- West R, Bailey K, Tiernan BN, Boonsuk W, & Gilbert S (2012). The temporal dynamics of medial and lateral frontal neural activity related to proactive cognitive control. Neuropsychologia, 50(14), 3450–3460. 10.1016/j.neuropsychologia.2012.10.011 [DOI] [PubMed] [Google Scholar]
- Williams CC, Ferguson TD, Hassall CD, Abimbola W, & Krigolson OE (2021). The ERP, frequency, and time–frequency correlates of feedback processing: Insights from a large sample study. Psychophysiology, 58(2). 10.1111/psyp.13722 [DOI] [PubMed] [Google Scholar]
- Wisniewski B, Zierer K, & Hattie J (2020). The power of feedback revisited: A meta-analysis of educational feedback research. Frontiers in Psychology, 10, 1–14. 10.3389/fpsyg.2019.03087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y, Ma F, Lv Y, Cai G, Teng P, Xu F, & Chen S (2015). Sustained attention is associated with error processing impairment: Evidence from mental fatigue Study in four-choice reaction time task. PLOS ONE, 10(3), e0117837. 10.1371/journal.pone.0117837 [DOI] [PMC free article] [PubMed] [Google Scholar]


