Abstract
INTRODUCTION:
As several behaviors captured by the Lifestyle Risk Factor Index (LSRI) are protective against Type 2 diabetes (T2D) and may affect body fat distribution, we examined its relation with both outcomes.
METHODS:
In a subset of the Multiethnic Cohort, participants from five ethnic groups (60–77 years) were assigned LSRI scores (one point each for consuming <1 (women)/<2 (men) alcoholic drinks/day, ≥1.5 physical activity hours/week, not smoking, and adhering to ≥3/7 dietary recommendations). All participants completed an extensive Quantitative Food Frequency Questionnaire to allow estimation of adherence to intake recommendations for fruits, vegetables, refined and whole grains, fish, processed and non-processed meat. Glycemic/T2D status was classified according to self-reports and fasting glucose. We estimated prevalence odds ratios (POR) of LSRI with glycemic/T2D status and DXA- and MRI-based body fat distribution using logistic regression.
RESULTS:
Of 1713 participants, 43% had normoglycemia, 30% Pre-T2D, 9% Undiagnosed T2D, and 18% T2D. Overall, 39% scored 0–2, 49% 3, and 12% 4 LSRI points. T2D prevalence was 55% (POR 0.45; 95% confidence intervals 0.27, 0.76) lower for 4 vs. 0–2 LSRI points with weaker associations for abnormal glycemic status. Despite the low adherence to dietary recommendations (22%), this was the only component related to lower T2D prevalence. The inverse LSRI-T2D association was only observed among Latinos and Japanese Americans in ethnic-specific models. Visceral fat measures were higher in T2D patients and attenuated the LSRI-T2D association.
CONCLUSION:
These findings support the role of a healthy lifestyle, especially diet, in T2D prevention with differences across ethnicity.
INTRODUCTION
A global diabetes prevalence of 10.5% in 2021 (536.6 million people) and a projected trend towards 12.2% in 2045 (783.2 million people) has been estimated by the International Diabetes Federation (IDF) [1]. Type 2 diabetes (T2D) is one of the most common non-communicable diseases around the world [1] and confers a high cardiovascular disease risk [2] and a reduced life expectancy [3]. There is evidence that adherence to specific dietary recommendations [4, 5], adequate physical activity [6], avoidance of smoking and moderate alcohol consumption [7] protect against T2D. Thus, analyzing those individual behaviors in combination as part of a composite Lifestyle Risk Factor Index (LSRI), which predicted a lower dementia risk [8], may give additional insight into the development of T2D and other chronic diseases. In general, these indices include smoking, physical activity, diet, and alcohol intake although the choice of risk factors and the scoring patterns differ across indices [8–11]. For example, among T2D patients in the Nurses’ Health Study and the Health Professionals Follow-Up Study, higher scores for a healthy lifestyle were associated with lower incidence and mortality of cardiovascular diseases [9]. Data from the UK Biobank population showed an 8 to 11-fold higher risk of developing T2D for individuals with a low score for a healthy lifestyle index [10]. A recent report showed that favorable scoring among T2D patients was associated with cardiovascular biomarkers and body fat distribution [11].
Across different cohorts [12–14], the importance of Body Mass Index (BMI) in T2D etiology has been established and a mediating role of BMI in the association between diet and T2D incidence has been described [7]. Moreover, as previous reports have shown, body fat distribution pattern, especially a high ratio of visceral (VAT) vs. subcutaneous adipose tissue (SAT) appears to play a key role in T2D etiology beyond BMI, in particular among populations with Asian ancestry [15–17]. Evidence for the hypothesis that VAT is an important predictor of morbidity and mortality beyond BMI [18–20] comes from the stronger association of body weight with T2D among individuals with Asian ancestry [15] who have the highest levels of VAT across ethnic groups after adjusting for total adiposity [21]. A positive association between both regional and total fat-to-muscle mass ratio with T2D after adjusting for BMI was also shown in the UK Biobank [22]. As trunk fat has been linked to higher and leg fat to lower glucose levels [23, 24], the Trunk-to-Leg Fat Ratio (TLFR) has frequently been applied as a DXA-based surrogate for visceral fat proportion [25, 26]. This cross-sectional analysis aimed to understand the relation of the LSRI, a combination of known T2D lifestyle risk factors, with body fat distribution as assessed by the VAT/SAT ratio and TLFR as well as with glycemic status/T2D in a subset of the elderly and ethnically diverse Multiethnic Cohort (MEC) participants.
METHODS
Participants and Study Design
The MEC is a prospective population-based cohort, which follows more than 215,000 men and women of five ethnic groups (African American, White, Japanese American, Native Hawaiian, and Latino) in Hawai’i and Los Angeles, California, to investigate diet, lifestyle and genetic risk factors for cancer and other chronic diseases since 1993 [27]. After 20 years of follow-up, the cross-sectional Adiposity Phenotype Study (APS) with detailed imaging for body composition was established with ∼2,000 MEC members. As age at recruitment ranged from 45–75 years in 1993–1996, potential participants were 60–77 years old in 2013–2016 when data collection occurred [21]. The APS study design and exclusion criteria have been described in detail in previous publications [21]. Individuals with a self-reported BMI outside a range of 18.5–40 kg/m2, current or former smoking in the past two years, or general serious medical conditions were excluded from the study as these conditions might have interfered with optimal imaging or ‘omics biomarker prediction of body fat distribution. The overall response rate was 15% among the 12,602 individuals invited and then 23% after excluding 4624 persons who were willing but ineligible. A balanced distribution of participants was ensured by stratification on sex, ethnicity, and six BMI categories. The study protocol was approved by the Institutional Review Boards at University of Hawai’i (CHS#17200) and University of Southern California (#HS-12–00623). Informed consent forms were signed by all participants.
Classification of T2D status
Based on self-reports of T2D diagnosis and medication plus fasting blood glucose levels obtained at clinic visit for all participants and measured with a Cobas Mira Plus Chemistry autoanalyzer (Randox Laboratories, Crumlin, United Kingdom) the following four glycemic/ T2D status categories were assigned: “Normoglycemic” (fasting glucose <100 mg/dl), “Pre-T2D” (fasting glucose 100–125 mg/dl), “Undiagnosed T2D” (fasting glucose >125 mg/dl) and “T2D” (self-reported T2D and/or taking diabetes medication).
Assessment of adiposity
The TLFR as an alternate measure of body fat distribution [25, 26], was calculated from fat mass (kg) in the trunk and legs obtained from dual-energy X-ray absorptiometry (DXA). As previously described in detail [21], a whole-body DXA scan (Hologic Discovery A) was used to measure total and regional body composition at clinic visit. The VAT/SAT ratio was computed from abdominal measurements of average VAT and SAT areas. To assess localized VAT and SAT areas at the four cross-sectional lumbar sites (L1-L2, L2-L3, L3-L4, and L4-L5), abdominal magnetic resonance imaging (MRI) scans were obtained on 3-T scanners (Siemens TIM Trio, Erlangen, Germany, software version VB13 at UH; General Electric HDx, Milwaukee, WI, software release 15M4 at USC) using an axial gradient-echo sequence with water-suppression and breath-holds (25 slices, 10 mm thickness, 2.5 mm gap, TR/TE = 140/2.6 ms, 70° flip angle).
Lifestyle Risk Factor Index
All components of the LSRI, i.e., typical diet, smoking, physical activity, and alcohol consumption were assessed by questionnaires including a detailed Quantitative Food Frequency Questionnaire (QFFQ) for the past year [28]. The LSRI ranging from 0 to 4 was originally created in the UK Biobank study to analyze the association of lifestyle and dementia as the sum of scores for smoking, physical activity, alcohol consumption and adherence to healthy diet [8], which individually have been associated with chronic disease incidence and mortality [10]. One point each was assigned for never smoking, moderate or vigorous physical activity of ≥1.5 hours per week, consuming <1 (women) or <2 (men) alcoholic drinks per day, and adhering to ≥3 of 7 dietary recommendations. The food recommendations were based on beneficial eating pattern comparable with dietary recommendations for cardiometabolic health [8] and included adherence to ≥3 servings per day of fruits, ≥3 servings per day of vegetables, ≥3 servings per day of whole grains, ≥2 servings per week of fish, ≤1.5 servings per day of refined grains, ≤1 serving per week of processed meat, ≤1.5 serving per week of non-processed red meat. One serving corresponded to one cup of fruits or vegetables or one ounce (28.35 g) of grains, fish or meat [29].
Statistical analysis
All statistical analyses were performed in SAS 9.4 (Cary, NC). Of the 1861 APS participants, those with missing risk factor information were excluded resulting in a final dataset with 1713 participants. The LSRI was classified into four levels: very low LSRI (score 0–1), low LSRI (score 2), intermediate LSRI (score 3) and high LSRI (score 4) based on distribution. Due to an overall low score for 0 or 1 point, very low and low LSRI scores were combined (score 0–2) for the categorical LSRI analysis and are subsequently referred to as lowest category. Descriptive statistics were performed by glycemic/T2D status. Differences across these groups were evaluated by general linear regression without adjustment. We estimated prevalence odds ratios (POR) with 95% confidence intervals (95%CI) and respective p-values for the association of continuous or categorical LSRI with T2D status (normoglycemia as reference) using polytomous logistic regression with adjustment for age, sex, ethnicity and BMI. To explore the mediating effect of body fat distribution, the VAT/SAT ratio and TLFR were added as covariates in separate models and the respective attenuation in POR was calculated. Individual LSRI components were tested using the same models. Finally, ethnic-specific PORs for T2D status vs. normoglycemia in relation to LSRI were estimated.
RESULTS
Among the total study population (Table 1) of 840 men and 873 women, 43% were normoglycemic, 30% had Pre-T2D, 9% Undiagnosed T2D, and 18% T2D. The mean (±SD) LSRI scores were 2.8 for normoglycemia and 2.6 for the three levels of T2D. Across ethnic groups, the mean LSRI score did not differ substantially. The best adherence to the recommendations for this LSRI, by scoring one point for the respective category, was observed for moderate/ vigorous physical activity (96%), followed by moderate alcohol consumption (89%), and never smoking (61%). Only 22% of the participants scored for the dietary component by adhering to 3 or more of 7 food recommendations, given the fact that the adherence to almost all recommendations was low e.g., red meat (6%), refined grains (10%), whole grains (14%), fruits (19%), processed meat (22%), vegetables (24%). Only the adherence to the fish recommendations was high (77%). The LSRI distribution showed 7% of the participants with 0–1 point (Table 1), 32% with 2 points, 49% with 3 points, and 12% with 4 points with more favorable proportions for the normoglycemic group. Similarly, all adiposity measures differed by T2D status. Overall, the mean (±SD) for the VAT/SAT ratio was 0.8 (±0.5) with a range of 0.1–4.0 and the mean (±SD) for TLRF was 1.6 (±0.4) with a range of 0.5–5.2.
Table 1.
Characteristics of the Study Population by Type 2 Diabetes Status, Adiposity Phenotype Study in Hawai’i and Los Angeles, 2013–2016.
| Number, N (%) | All | Normoglycemiaa | Pre-T2Da | Undiagnosed T2Da | T2Da | p-valueb | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1713 | 741 (43) | 511 (30) | 154 (9) | 307 (18) | |||||||
| N | % | % | % | % | |||||||
| Male | 840 | 37 | 33 | 10 | 20 | <0001 | |||||
| Female | 873 | 50 | 26 | 8 | 16 | ||||||
| White | 389 | 48 | 37 | 9 | 6 | <0001 | |||||
| African American | 276 | 55 | 20 | 5 | 20 | ||||||
| Hawaiian | 271 | 38 | 33 | 10 | 19 | ||||||
| Japanese | 419 | 31 | 36 | 14 | 19 | ||||||
| Latino | 358 | 47 | 20 | 5 | 28 | ||||||
| Very low LSRI (0–1) | 115 | 44 | 37 | 7 | 12 | <0001 | |||||
| Low LSRI (2) | 553 | 36 | 30 | 11 | 23 | ||||||
| Intermediate LSRI (3) | 834 | 46 | 29 | 8 | 17 | ||||||
| High LSRI (4) | 211 | 54 | 26 | 9 | 11 | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||
| LSRI | 2.7 | 0.8 | 2.8 | 0.8 | 2.6 | 0.8 | 2.6 | 0.8 | 2.6 | 0.7 | 0.0004 |
| Age, years | 69.2 | 2.7 | 69.3 | 2.7 | 68.9 | 2.7 | 68.9 | 2.7 | 69.7 | 2.8 | 0.0002 |
| BMI, kg/m2 | 27.8 | 4.7 | 27.2 | 4.7 | 27.9 | 4.8 | 27.8 | 4.5 | 29.5 | 4.5 | <0.0001 |
| Total fat mass, kg | 25.3 | 8.5 | 25.2 | 8.7 | 24.4 | 8.4 | 24.1 | 7.9 | 27.6 | 8.2 | <0.0001 |
| SAT, cm2 | 229 | 102 | 229 | 101 | 221 | 99 | 220 | 101 | 246 | 108 | 0.006 |
| VAT, cm2 | 168 | 84 | 146 | 74 | 173 | 86 | 172 | 76 | 210 | 88 | <0.0001 |
| VAT/SAT ratio | 0.8 | 0.5 | 0.7 | 0.4 | 0.9 | 0.5 | 0.9 | 0.5 | 1.0 | 0.5 | <0.0001 |
| Leg Fat Mass, kgc | 8.8 | 3.3 | 9.2 | 3.4 | 8.5 | 3.2 | 8.3 | 3.0 | 8.7 | 3.2 | 0.002 |
| Trunk Fat Mass, kgc | 13.4 | 4.7 | 12.9 | 4.6 | 13.2 | 4.6 | 13.1 | 4.6 | 15.2 | 4.5 | <0.0001 |
| Trunk-to-Leg Fat Ratioc | 1.6 | 0.4 | 1.5 | 0.4 | 1.6 | 0.4 | 1.6 | 0.4 | 1.8 | 0.5 | <0.0001 |
Normoglycemia=fasting glucose <100 mg/dl; Pre-T2D=fasting glucose 100–125 mg/dl; Undiagnosed T2D=fasting glucose>125 mg/dl; T2D=Self-reported Type 2 Diabetes and/or taking diabetes medication
p-values obtained by chi-square tests for categorical variables and by general linear models for continuous variables.
Missing values for Leg Fat Mass: 116; Trunk Fat Mass: 50; Trunk-to-Leg Fat Ratio: 137.
A unit increase in the VAT/SAT ratio and the TLFR were associated with glycemic/T2D status (Table 2). In relation to the VAT/SAT ratio, the odds were 1.77 (95%CI: 1.23, 2.56) for Pre-T2D, 2.16 (95%CI: 1.29, 3.63) for Undiagnosed T2D, and 4.31 (95%CI: 2.87, 6.49) for T2D. The results for the TLFR were of similar magnitude: Pre-T2D 1.97 (95%CI: 1.37, 2.85), Undiagnosed T2D 2.30 (95%CI: 1.34, 3.95), and T2D 6.10 (95%CI: 4.00, 9.29). The LSRI was also associated with the two measures of body fat distribution: the estimated odds for the highest vs. lowest LSRI value were 0.59 (95%CI 0.35, 0.97) for the VAT/SAT ratio and 0.50 (95%CI: 0.30, 0.81) for the TLFR.
Table 2.
Association of MRI- and DXA-based Body Fat Distribution with T2D Status and the Lifestyle Risk Factor Index (LSRI), Adiposity Phenotype Study in Hawai’i and Los Angeles, 2013–2016.
| Measure | PORa | 95% CI | p-valuea | ||
|---|---|---|---|---|---|
| VAT/SAT ratio | Glycemic/T2D Status b | ||||
| Normoglycemia | 1.00 | – | – | <0.0001 | |
| Pre-T2D | 1.77 | 1.23 | 2.56 | ||
| Undiagnosed T2D | 2.16 | 1.29 | 3.63 | ||
| T2D | 4.31 | 2.87 | 6.49 | ||
| Lifestyle Risk Index | |||||
| Very Low–Low (0–2) | 1.00 | – | – | 0.004 | |
| Intermediate (3) | 0.63 | 0.46 | 0.84 | ||
| High (4) | 0.59 | 0.35 | 0.97 | ||
| Trunk-to-Leg Fat Ratio | Glycemic/T2D Status b | ||||
| Normoglycemia | 1.00 | – | – | <0.0001 | |
| Pre-T2D | 1.97 | 1.37 | 2.85 | ||
| Undiagnosed T2D | 2.30 | 1.34 | 3.95 | ||
| T2D | 6.10 | 4.00 | 9.29 | ||
| Lifestyle Risk Index | |||||
| Very Low-Low (0–2) | 1.00 | – | – | 0.01 | |
| Intermediate (3) | 0.76 | 0.56 | 1.02 | ||
| High (4) | 0.50 | 0.30 | 0.81 | ||
Prevalence Odds Ratios and 95% confidence intervals and p-values obtained by polytomous logistic regression adjusted for age, sex, ethnicity and BMI for one unit of VAT/SAT ratio and Trunk-to-Leg Fat Ratio respectively.
Normoglycemia=fasting glucose <100 mg/dl; Pre-T2D = fasting glucose 100–125 mg/dl; Undiagnosed T2D = fasting glucose>125 mg/dl; T2D = Self-reported Type 2 Diabetes and/or taking diabetes medication.
In logistic regression models adjusted for age, sex, ethnicity, and BMI (Table 3), one unit increase in LSRI was associated with a 21% lower prevalence for T2D (POR: 0.79; 95%CI: 0.65, 0.95) and 16% lower prevalence for Pre-T2D (POR: 0.84; 95%CI: 0.72, 0.98), but only 13% lower prevalence for Undiagnosed T2D (POR: 0.87; 95%CI: 0.69, 1.10). Adding VAT/SAT ratio or TLFR to the logistic model attenuated the associations by 5–8%. However, when participants with a LSRI score of 4 were compared to the lowest category, the odds were 0.45 (95%CI: 0.27, 0.76), which remained statistically significant after adjusting for VAT/SAT ratio or TLFR despite an attenuation of 9–16%. Looking at individual LSRI components showed that only diet was associated with T2D status. The T2D prevalence (POR: 0.55; 95%CI: 0.38, 0.81) was lower by 45% for adherence to the LSRI dietary recommendations, which was only attenuated minimally after adjustment for VAT/SAT ratio or TLFR. For the other LSRI components alone, no association was observed.
Table 3.
Association of the Lifestyle Risk Index (LSRI) with Diabetes Status, Adiposity Phenotype Study in Hawai’i and Los Angeles, 2013–2016a.
| Glycemic/T2D Status | N | PORb | 95% CI | p-value | PORc | 95% CI | p-value | PORd | 95% CI | p-value | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LSRI (cont.) | |||||||||||||
| Normoglycemia | 741 | 1.00 | – | – | 0.04 | 1.00 | – | – | 0.10 | 1.00 | – | – | 0.11 |
| Pre-T2D | 511 | 0.84 | 0.72 | 0.98 | 0.85 | 0.73 | 0.99 | 0.84 | 0.71 | 0.98 | |||
| Undiagnosed T2D | 154 | 0.87 | 0.69 | 1.10 | 0.89 | 0.70 | 1.12 | 0.84 | 0.65 | 1.07 | |||
| T2D | 307 | 0.79 | 0.65 | 0.95 | 0.83 | 0.68 | 1.00 | 0.85 | 0.70 | 1.04 | |||
| LSRI 3 vs. 0–2 | |||||||||||||
| Normoglycemia | 380 | 1.00 | – | – | 0.04 | 1.00 | – | – | 0.10 | 1.00 | – | – | 0.10 |
| Pre-T2D | 245 | 0.82 | 0.64 | 1.06 | 0.84 | 0.65 | 1.08 | 0.84 | 0.64 | 1.09 | |||
| Undiagnosed T2D | 68 | 0.72 | 0.49 | 1.06 | 0.74 | 0.50 | 1.09 | 0.68 | 0.46 | 1.01 | |||
| T2D | 141 | 0.70 | 0.52 | 0.94 | 0.75 | 0.55 | 1.01 | 0.72 | 0.52 | 1.00 | |||
| LSRI 4 vs. 0–2 | |||||||||||||
| Normoglycemia | 115 | 1.00 | – | – | 0.04 | 1.00 | – | – | 0.10 | 1.00 | – | – | 0.10 |
| Pre-T2D | 55 | 0.69 | 0.47 | 1.01 | 0.70 | 0.47 | 1.02 | 0.67 | 0.45 | 1.00 | |||
| Undiagnosed T2D | 18 | 0.73 | 0.41 | 1.32 | 0.75 | 0.42 | 1.35 | 0.64 | 0.34 | 1.19 | |||
| T2D | 23 | 0.45 | 0.27 | 0.76 | 0.49 | 0.29 | 0.82 | 0.52 | 0.30 | 0.89 | |||
| Individual Components of Lifestyle Risk Index (1 vs. 0 point) | |||||||||||||
| Diet | |||||||||||||
| Normoglycemia | 741 | 1.00 | – | – | 0.02 | 1.00 | – | – | 0.04 | 1.00 | – | – | 0.03 |
| Pre-T2D | 511 | 0.81 | 0.61 | 1.08 | 0.82 | 0.62 | 1.09 | 0.76 | 0.57 | 1.03 | |||
| Undiagnosed T2D | 154 | 0.77 | 0.49 | 1.21 | 0.79 | 0.50 | 1.24 | 0.71 | 0.44 | 1.15 | |||
| T2D | 307 | 0.55 | 0.38 | 0.81 | 0.58 | 0.40 | 0.85 | 0.58 | 0.39 | 0.87 | |||
| Smoking | |||||||||||||
| Normoglycemia | 741 | 1.00 | – | – | 0.63 | 1.00 | – | – | 0.79 | 1.00 | – | – | 0.79 |
| Pre-T2D | 511 | 0.97 | 0.76 | 1.24 | 0.97 | 0.76 | 1.25 | 0.94 | 0.73 | 1.22 | |||
| Undiagnosed T2D | 154 | 0.89 | 0.61 | 1.29 | 0.89 | 0.62 | 1.30 | 0.82 | 0.56 | 1.21 | |||
| T2D | 307 | 0.83 | 0.62 | 1.11 | 0.87 | 0.65 | 1.17 | 0.93 | 0.67 | 1.27 | |||
| Alcohol consumption | |||||||||||||
| Normoglycemia | 741 | 1.00 | – | . | 0.28 | 1.00 | – | – | 0.21 | 1.00 | – | – | 0.38 |
| Pre-T2D | 511 | 0.77 | 0.54 | 1.11 | 0.79 | 0.55 | 1.14 | 0.87 | 0.59 | 1.28 | |||
| Undiagnosed T2D | 154 | 0.91 | 0.52 | 1.62 | 0.95 | 0.54 | 1.69 | 1.05 | 0.57 | 1.91 | |||
| T2D | 307 | 1.24 | 0.74 | 2.09 | 1.39 | 0.82 | 2.38 | 1.45 | 0.82 | 2.57 | |||
| Physical activity | |||||||||||||
| Normoglycemia | 741 | 1.00 | – | – | 0.23 | 1.00 | – | – | 0.24 | 1.00 | – | – | 0.32 |
| Pre-T2D | 511 | 0.64 | 0.36 | 1.12 | 0.64 | 0.37 | 1.13 | 0.63 | 0.35 | 1.16 | |||
| Undiagnosed T2D | 154 | 1.78 | 0.52 | 6.02 | 1.79 | 0.53 | 6.07 | 1.48 | 0.43 | 5.06 | |||
| T2D | 307 | 0.80 | 0.43 | 1.50 | 0.78 | 0.42 | 1.46 | 0.72 | 0.36 | 1.42 | |||
Normoglycemia = fasting glucose <100 mg/dl; Pre-T2D = fasting glucose 100–125 mg/dl; Undiagnosed T2D = fasting glucose>125 mg/dl; T2D = Self-reported Type 2 Diabetes and/or taking diabetes medication.
Prevalence Odds Ratios and 95% confidence intervals obtained by polytomous logistic regression adjusted for age, sex, ethnicity, BMI.
b plus adjustment for VAT/SAT ratio.
b plus adjustment for Trunk-to-Leg Fat ratio.
After stratification by ethnicity (Fig. 1), the PORs for the highest vs. lowest LSRI category showed values below one for all groups except Native Hawaiians, although the distribution among LSRI categories was similar in Native Hawaiians compared to the other groups. In general, PORs for the highest vs. lowest LSRI category were only statistically significant among Latinos and Japanese Americans. The PORs among Native Hawaiians ranged between 1.30 and 1.60 but had very wide 95% CI due to the smaller sample size. Adding the VAT/SAT ratio or TLFR to the model did not substantially modify the estimated odds for T2D status in relation to the composite LSRI.
Fig. 1.
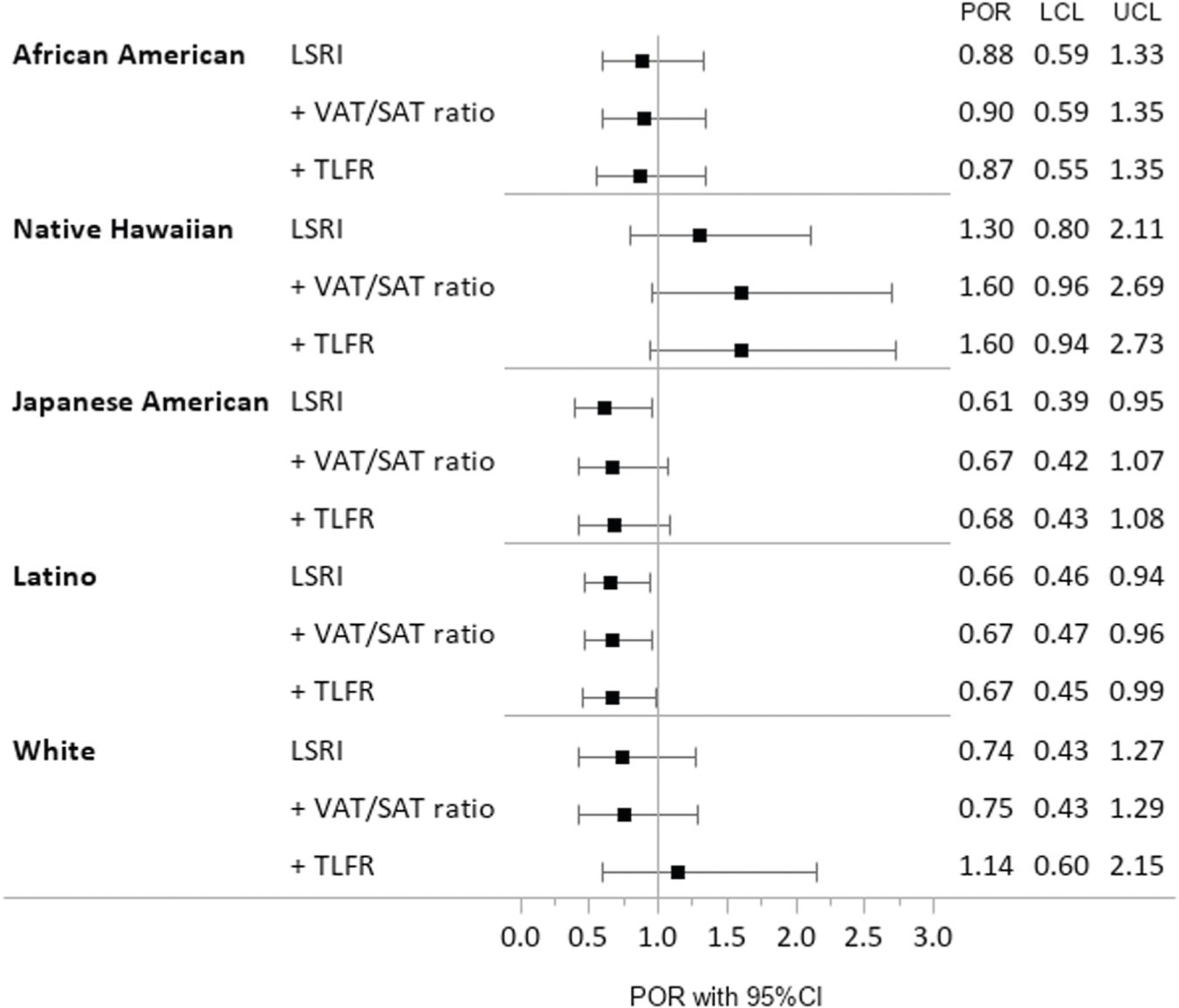
Association of the Lifestyle Risk Factor Index (LSRI) with Prevalence of Type 2 Diabetes (T2D) for the five ethnic groups, when adjusted for basic covariates or additionally adjusted for abdominal MRI- and DXA-based Body Fat Distribution, Adiposity Phenotype Study in Hawai’i and Los Angeles, 2013–2016. Prevalence Odds Ratios (POR) and 95% confidence intervals (95%CI) (lower (LCL) and upper (UCL) confidence limit) obtained by polytomous logistic regression after stratification by ethnicity adjusted for age, sex, ethnicity, BMI plus adjustment for VAT/SAT ratio and Trunk-to-Leg Fat Ratio (TLFR).
DISCUSSION
The current cross-sectional analysis indicated an inverse relation between a composite LSRI and T2D prevalence by showing that T2D prevalence was approximately half in participants with the highest as compared to the lowest LSRI score. When looking at individual score components, only adherence to the dietary recommendation, and not to the other three behaviors, was associated with a statistically significantly lower T2D prevalence. In general, the adherence to dietary recommendations was low (22%) in this population compared to the other lifestyle factors. The highest adherence among food components was achieved for fish intake (77%), reflecting the geographic location of the MEC, while adherence to all other food categories scored less than 24%. The highest BMI, VAT/ SAT ratio and TLFR values were observed among participants with T2D and the lowest in normoglycemic individuals. The relation of LSRI with T2D was not substantially attenuated when adding VAT/ SAT ratio or TLFR to the model despite the strong association of body fat distribution with T2D prevalence. This finding suggests a weak or no role of body fat distribution as a confounder of the lifestyle factors-T2D relation although this question cannot be conclusively answered in a cross-sectional study. The current findings suggest noteworthy differences across ethnic groups. The LSRI showed statistically significant negative associations with T2D status among Japanese Americans and Latinos, whereas the imprecise positive association among Native Hawaiians is difficult to interpret.
In regards to body composition, its relation to T2D prevalence confirms the results from previous studies that TLFR and VAT/SAT ratio are associated with T2D [24, 30], in particular a recent analysis of the German Diabetes Study that reported more favorable cardiometabolic markers and lower VAT in relation to an LSRI which included BMI as an additional component [11]. However, this study included only patients with T2D and no healthy individuals. The fact, that only adherence to diet recommendations was associated with T2D prevalence in the APS disagrees with other reports that described the impact of smoking, alcohol, and physical activity on T2D [7, 31, 32]. In our analysis, not smoking was compared to former smoking as current smokers were excluded from the study, but no association with T2D prevalence was observed. Also, a strong association between moderate alcohol consumption as compared to no consumption with a lower incidence of T2D was reported in a study on more than 40,000 male health professionals [7] and the Shanghai Men’s Health Study [31], which was also confirmed in women [33]. In the Honolulu Heart Program study, a strong inverse relation between physical activity and T2D was observed among 6815 Japanese-American men [34] and a meta-analysis of 81 cohort studies and randomized trials confirmed this inverse association for multiple types of physical activity with different levels of intensity [35].
In the current study, a diet adherence was related to a lower T2D prevalence, a finding that agrees with the IDF’s evaluation of poor diet as a risk factor for T2D development [36], and support the protective role of diet quality as assessed by the Mediterranean and the Dietary Approaches to Stop Hypertension diet pattern [5, 37]. Given that the general adherence to diet recommendations was influenced by a high adherence to fish intake, our results support previous findings in the UK Biobank, which stated that fish and fish-poultry but not vegetarian diets compared to meat diets were associated with a lower T2D risk, independent of sociodemographic and other lifestyle factors, including alcohol consumption, smoking, sleep duration and physical activity [38].
The multiethnic population with high incidence rates of T2D (Native Hawaiian and Japanese American) is a particular strength of this study [37]. This study used DXA to determine body fat, which is an easier, cheaper, and most importantly more comfortable method for patients compared to the gold standard, whole body potassium counting, by generating comparable results [39]. MRI imaging as the gold standard for VAT assessment, also provided valid data. The study limitations include selection bias and a low response rate of APS participants who were selected according to BMI, absence of serious medical conditions, and, in particular, non-smoking status as the original study aims focused on collecting MRI-based information on body fat distribution. As a result, the APS participants are not representative of the full MEC and the general population. In addition, the results are not generalizable to populations with different ancestries or a younger age given the ethnic and age distribution of the APS. Thus, verification and expansion of our findings in a prospective cohort are needed. Another concern is the reported disease status, which was determined by self-report or one single fasting blood glucose result at the clinic visit. The lack of repeated fasting glucose measures and/or HbA1c measurements may lead to an incorrect diagnosis and misclassification [40]. This may have been a more serious issue among African-American participants because fasting hyperglycemia is less common in early-stage T2D in this population [41]. The small sample sizes in the four glycemic/T2D categories and by ethnicity are a concern, but were a result of the challenges to obtain MRI images. In addition, people with more severe forms of T2D might be excluded from study participation due to strict exclusion criteria in regards to insulin treatment. Moreover, causality cannot be entertained due to the cross-sectional study design.
In conclusion, this study adds evidence to the protective role of a healthy lifestyle, in particular, diet with high adherence to fish intake of 2 or more servings per week, in T2D prevention. According to our findings, a high lifestyle score with a healthy diet, moderate physical activity, moderate alcohol consumption, and non-smoking was linked to lower T2D prevalence independent of body fat distribution pattern, with the greatest impact through adherence to a healthy diet. Notable ethnic differences in the association of the LSRI with T2D were observed. The results offer some evidence that there is little impact of body fat distribution as a confounder in the lifestyle-T2D relation. Nevertheless, body fat distribution as assessed by VAT/SAT ratio and TLFR should be considered in studies of the LSRI and T2D given its strong association with the LSRI. As our study includes five ethnic groups with substantial differences in the VAT/ SAT ratio, our results contribute to important findings for T2D prevention on a global scale.
FUNDING
This research was funded by grants from the US National Institutes of Health (P01 CA168530, U01CA164973, P30CA071789). GM was supported by Berlin Institute of Health Visiting Professorship, funded by Stiftung Charité. CG was supported by a postdoctoral fellowship, funded by the University of Hawai’i Cancer Center (T32CA229110).
Footnotes
COMPETING INTERESTS
The authors declare no competing interests.
ETHICAL APPROVAL
The study protocol was approved by the Institutional Review Boards at the University of Hawai’i (CHS# 17200) and the University of Southern California (#HS-12-00623). Informed consent forms were signed by all participants.
DATA AVAILABILITY
The data underlying this study cannot be made publicly available because they contain patient identifying information. Data are available from the Multiethnic Cohort study (http://www.uhcancercenter.org/research/the-multiethnic-cohort-study-mec/data-sharing-mec) for researchers who meet the criteria for access to data.
REFERENCES
- 1.Sun H, Saeedi P, Karuranga S, Pinkepank M, Ogurtsova K, Duncan BB, et al. IDF Diabetes Atlas: Global, regional and country-level diabetes prevalence estimates for 2021 and projections for 2045. Diabetes Res Clin Pract 2022;183:109119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pan Y, Chen W, Wang Y. Prediabetes and outcome of ischemic stroke or transient ischemic attack: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis 2019;28:683–92. [DOI] [PubMed] [Google Scholar]
- 3.Heald AH, Stedman M, Davies M, Livingston M, Alshames R, Lunt M, et al. Estimating life years lost to diabetes: outcomes from analysis of National Diabetes Audit and Office of National Statistics data. Cardiovasc Endocrinol Metab 2020;9:183–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aune D, Ursin G, Veierod MB. Meat consumption and the risk of type 2 diabetes: a systematic review and meta-analysis of cohort studies. Diabetologia 2009;52:2277–87. [DOI] [PubMed] [Google Scholar]
- 5.Jannasch F, Kroger J, Schulze MB. Dietary patterns and Type 2 diabetes: a systematic literature review and meta-analysis of prospective studies. J Nutr 2017;147:1174–82. [DOI] [PubMed] [Google Scholar]
- 6.Steinbrecher A, Erber E, Grandinetti A, Nigg C, Kolonel LN, Maskarinec G. Physical activity and risk of type 2 diabetes among Native Hawaiians, Japanese Americans, and Caucasians: the Multiethnic Cohort. J Phys Act Health. 2012;9:634–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rimm EB, Chan J, Stampfer MJ, Colditz GA, Willett WC. Prospective study of cigarette smoking, alcohol use, and the risk of diabetes in men. BMJ 1995;310:555–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lourida I, Hannon E, Littlejohns TJ, Langa KM, Hypponen E, Kuzma E, et al. Association of lifestyle and genetic risk with incidence of dementia. JAMA 2019;322:430–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu G, Li Y, Hu Y, Zong G, Li S, Rimm EB, et al. Influence of lifestyle on incident cardiovascular disease and mortality in patients with diabetes Mellitus. J Am Coll Cardiol 2018;71:2867–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Said MA, Verweij N, van der Harst P. Associations of combined genetic and lifestyle risks with incident cardiovascular disease and diabetes in the UK Biobank Study. JAMA Cardiol 2018;3:693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baechle C, Lang A, Strassburger K, Kuss O, Burkart V, Szendroedi J, et al. Association of a lifestyle score with cardiometabolic markers among individuals with diabetes: a cross-sectional study. BMJ Open Diabetes Res Care. 2023;11:e003469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee DH, Keum N, Hu FB, Orav EJ, Rimm EB, Willett WC, et al. Comparison of the association of predicted fat mass, body mass index, and other obesity indicators with type 2 diabetes risk: two large prospective studies in US men and women. Eur J Epidemiol 2018;33:1113–23. [DOI] [PubMed] [Google Scholar]
- 13.Hardoon SL, Morris RW, Thomas MC, Wannamethee SG, Lennon LT, Whincup PH. Is the recent rise in type 2 diabetes incidence from 1984 to 2007 explained by the trend in increasing BMI?: evidence from a prospective study of British men. Diabetes Care. 2010;33:1494–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maskarinec G, Grandinetti A, Matsuura G, Sharma S, Mau M, Henderson BE, et al. Diabetes prevalence and body mass index differ by ethnicity: the Multiethnic Cohort. Ethn Dis 2009;19:49–55. [PMC free article] [PubMed] [Google Scholar]
- 15.Maskarinec G, Raquinio P, Kristal BS, Franke AA, Buchthal SD, Ernst TM, et al. Body fat distribution, glucose metabolism, and diabetes status among older adults: the multiethnic cohort adiposity phenotype study. J Epidemiol 2022;32:314–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wander PL, Boyko EJ, Leonetti DL, McNeely MJ, Kahn SE, Fujimoto WY. Change in visceral adiposity independently predicts a greater risk of developing type 2 diabetes over 10 years in Japanese Americans. Diabetes Care. 2013;36:289–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Feng X, Wang J, Wu S, Wang Z, Wei Y, Li L, et al. Correlation analysis of anthropometric indices and type 2 diabetes mellitus in residents aged 60 years and older. Front Public Health. 2023;11:1122509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fujimoto WY, Boyko EJ, Hayashi T, Kahn SE, Leonetti DL, McNeely MJ, et al. Risk factors for Type 2 diabetes: lessons learned from Japanese Americans in Seattle. J Diabetes Investig 2012;3:212–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pischon T, Boeing H, Hoffmann K, Bergmann M, Schulze MB, Overvad K, et al. General and abdominal adiposity and risk of death in Europe. N Engl J Med 2008;359:2105–20. [DOI] [PubMed] [Google Scholar]
- 20.Nimptsch K, Konigorski S, Pischon T. Diagnosis of obesity and use of obesity biomarkers in science and clinical medicine. Metabolism. 2019;92:61–70. [DOI] [PubMed] [Google Scholar]
- 21.Lim U, Monroe KR, Buchthal S, Fan B, Cheng I, Kristal BS, et al. Propensity for intra-abdominal and hepatic adiposity varies among ethnic groups. Gastroenterology. 2019;156:966–75.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang N, Sun Y, Zhang H, Chen C, Wang Y, Zhang J, et al. Total and regional fat-to-muscle mass ratio measured by bioelectrical impedance and risk of incident type 2 diabetes. J Cachexia Sarcopenia Muscle. 2021;12:2154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Snijder MB, Dekker JM, Visser M, Bouter LM, Stehouwer CD, Yudkin JS, et al. Trunk fat and leg fat have independent and opposite associations with fasting and postload glucose levels: the Hoorn study. Diabetes Care. 2004;27:372–7. [DOI] [PubMed] [Google Scholar]
- 24.Vasan SK, Osmond C, Canoy D, Christodoulides C, Neville MJ, Di Gravio C, et al. Comparison of regional fat measurements by dual-energy X-ray absorptiometry and conventional anthropometry and their association with markers of diabetes and cardiovascular disease risk. Int J Obes 2018;42:850–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kouda K, Fujita Y, Ohara K, Tachiki T, Tamaki J, Yura A, et al. Associations between trunk-to-peripheral fat ratio and cardiometabolic risk factors in elderly Japanese men: baseline data from the Fujiwara-kyo Osteoporosis Risk in Men (FORMEN) study. Environ Health Prev Med 2021;26:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhong P, Tan S, Zhu Z, Zhu Z, Liang Y, Huang W, et al. Normal-weight central obesity and risk of cardiovascular and microvascular events in adults with prediabetes or diabetes: Chinese and British cohorts. Diabetes Metab Res Rev 2023;39:e3707. [DOI] [PubMed] [Google Scholar]
- 27.Kolonel LN, Henderson BE, Hankin JH, Nomura AMY, Wilkens LR, Pike MC, et al. A multiethnic cohort in Hawaii and Los Angeles: baseline characteristics. Am J Epidemiol 2000;151:346–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stram DO, Hankin JH, Wilkens LR, Pike MC, Monroe KR, Park S, et al. Calibration of the dietary questionnaire for a multiethnic cohort in Hawaii and Los Angeles. Am J Epidemiol 2000;151:358–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.U.S. Department of Agriculture and U.S. Department of Health and Human Services. Dietary guidelines for Americans 2020–2025 Washington, DC2020 updated 12/2020. 9th:Available from: https://www.dietaryguidelines.gov/. [Google Scholar]
- 30.Xia MF, Lin HD, Chen LY, Wu L, Ma H, Li Q, et al. Association of visceral adiposity and its longitudinal increase with the risk of diabetes in Chinese adults: A prospective cohort study. Diabetes Metab Res Rev 2018;34:e3048. [DOI] [PubMed] [Google Scholar]
- 31.Shi L, Shu XO, Li H, Cai H, Liu Q, Zheng W, et al. Physical activity, smoking, and alcohol consumption in association with incidence of type 2 diabetes among middle-aged and elderly Chinese men. PLoS One. 2013;8:e77919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maddatu J, Anderson-Baucum E, Evans-Molina C. Smoking and the risk of type 2 diabetes. Transl Res 2017;184:101–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li XH, Yu FF, Zhou YH, He J. Association between alcohol consumption and the risk of incident type 2 diabetes: a systematic review and dose-response meta-analysis. Am J Clin Nutr 2016;103:818–29. [DOI] [PubMed] [Google Scholar]
- 34.Burchfiel CM, Sharp DS, Curb JD, Rodriguez BL, Hwang LJ, Marcus EB, et al. Physical activity and incidence of diabetes: the Honolulu Heart Program. Am J Epidemiol 1995;141:360–8. [DOI] [PubMed] [Google Scholar]
- 35.Aune D, Norat T, Leitzmann M, Tonstad S, Vatten LJ. Physical activity and the risk of type 2 diabetes: a systematic review and dose-response meta-analysis. Eur J Epidemiol 2015;30:529–42. [DOI] [PubMed] [Google Scholar]
- 36.International Diabetes Federation (IDF). Diabetes risk factors: IDF; 2018. [Available from: https://idf.org/about-diabetes/type-2-diabetes/. [Google Scholar]
- 37.Jacobs S, Boushey CJ, Franke AA, Shvetsov YB, Monroe KR, Haiman CA, et al. A priori-defined diet quality indices, biomarkers and risk for type 2 diabetes in five ethnic groups: the Multiethnic Cohort. Br J Nutr 2017;118:312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boonpor J, Petermann-Rocha F, Parra-Soto S, Pell JP, Gray SR, Celis-Morales C, et al. Types of diet, obesity, and incident type 2 diabetes: Findings from the UK Biobank prospective cohort study. Diabetes Obes Metab 2022;24:1351–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Branski LK, Norbury WB, Herndon DN, Chinkes DL, Cochran A, Suman O, et al. Measurement of body composition in burned children: is there a gold standard? JPEN J Parenter Enteral Nutr 2010;34:55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.American Diabetes A Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:S62–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chung ST, Courville AB, Onuzuruike AU, Galvan-De La Cruz M, Mabundo LS, DuBose CW, et al. Gluconeogenesis and risk for fasting hyperglycemia in Black and White women. JCI Insight. 2018;3:e121495. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this study cannot be made publicly available because they contain patient identifying information. Data are available from the Multiethnic Cohort study (http://www.uhcancercenter.org/research/the-multiethnic-cohort-study-mec/data-sharing-mec) for researchers who meet the criteria for access to data.


