Abstract
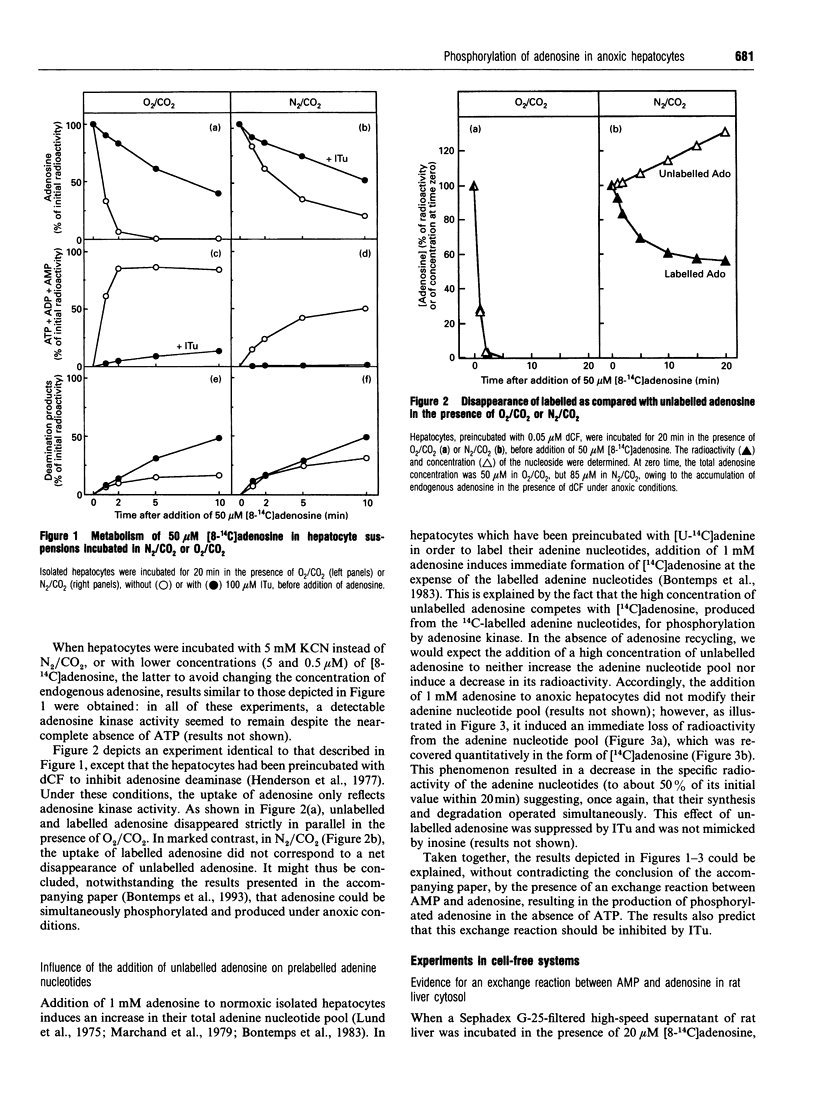
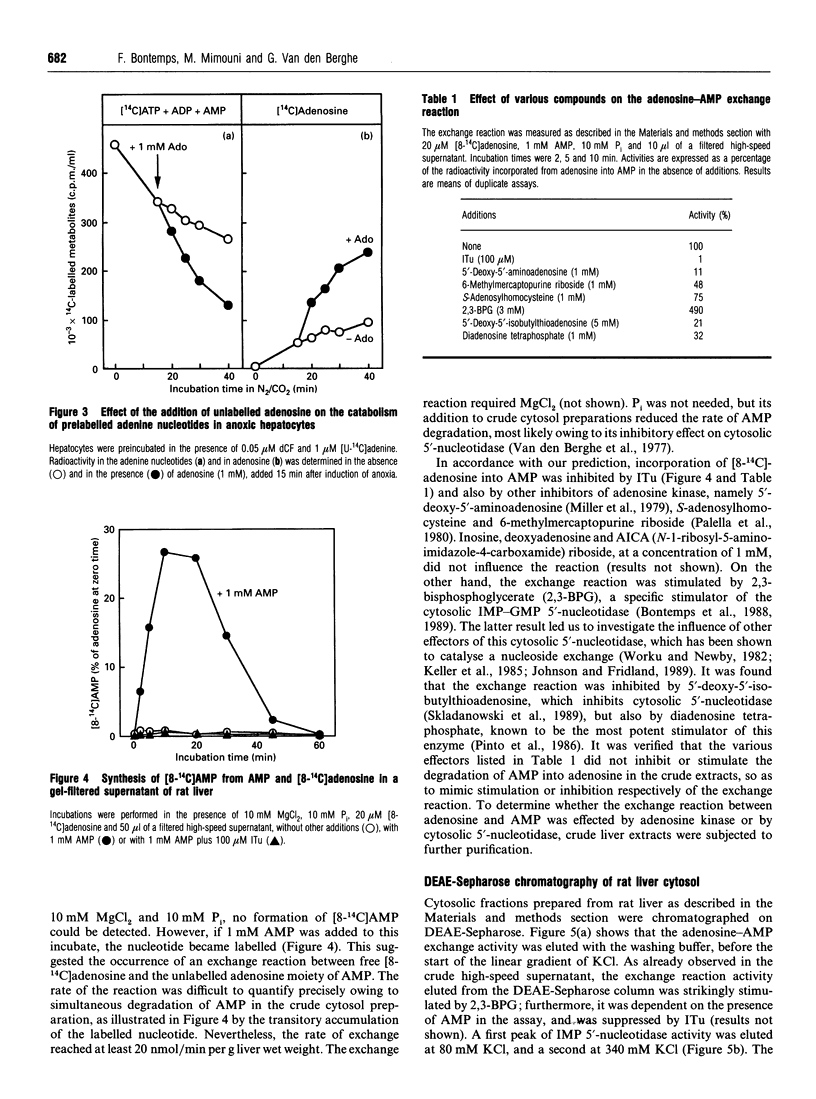
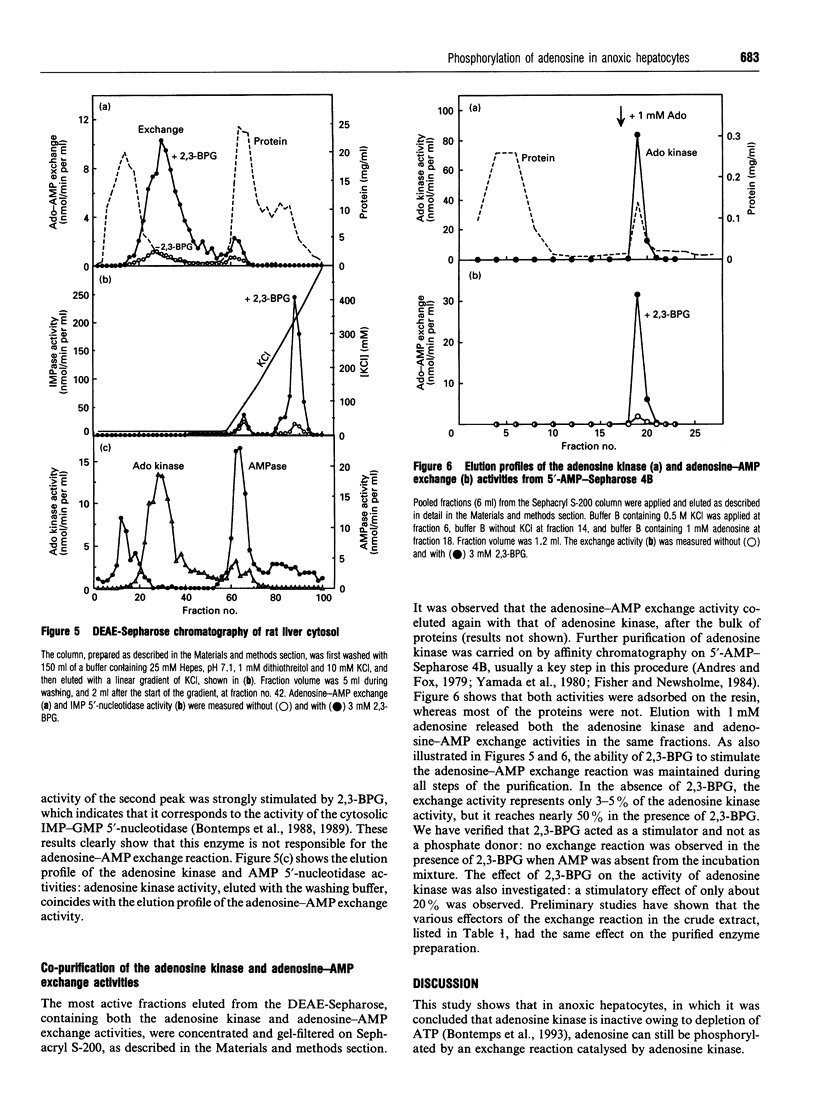
The elevation of adenosine levels induced by anoxia in isolated rat hepatocytes has been shown to result mainly from an arrest of the recycling of the nucleoside by adenosine kinase [Bontemps, Vincent and Van den Berghe (1993) Biochem. J. 290, 671-677]. To assess the activity of the latter enzyme in intact hepatocytes, incorporation of radioactive adenosine into the cells' adenine nucleotides was measured. Unexpectedly, despite the near-absence of ATP in anoxic cells, 40% of 50 microM [8-14C]adenosine was still incorporated into adenylates over 5 min. Moreover, whereas unlabelled and labelled adenosine were utilized in parallel in normoxic cells, uptake of [8-14C]adenosine did not correspond to a net disappearance of adenosine in anoxic cells. Addition of 1 mM unlabelled adenosine to anoxic hepatocytes in which the adenine nucleotides had been prelabelled with [U-14C]adenine induced an immediate loss of their radioactivity. The latter was recovered in the form of adenosine, but the size of the adenylate pool was not modified. Taken together, these results suggest the occurrence of an exchange reaction between AMP and adenosine. Incubation of Sephadex G-25-filtered high-speed supernatants of rat liver with 20 microM [8-14C]adenosine, 10 mM MgCl2 and 1 mM AMP resulted in the labelling of AMP in the total absence of ATP. This labelling was influenced by effectors of both adenosine kinase and cytosolic IMP-GMP 5'-nucleotidase; the latter is known to catalyse an exchange reaction [Worku and Newby (1982) Biochem. J. 205, 503-510]. Chromatography of cytosolic fractions of rat liver on DEAE-Sepharose, followed by Sephacryl S-200 and AMP-Sepharose, demonstrated that the exchange reaction between adenosine and AMP co-purified with adenosine kinase. It is concluded that incorporation of labelled adenosine into adenine nucleotides should not be considered to be proof of adenosine kinase activity in anoxia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres C. M., Fox I. H. Purification and properties of human placental adenosine kinase. J Biol Chem. 1979 Nov 25;254(22):11388–11393. [PubMed] [Google Scholar]
- Bontemps F., Van den Berghe G., Hers H. G. 5'-Nucleotidase activities in human erythrocytes. Identification of a purine 5'-nucleotidase stimulated by ATP and glycerate 2,3-bisphosphate. Biochem J. 1988 Mar 15;250(3):687–696. doi: 10.1042/bj2500687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontemps F., Van den Berghe G., Hers H. G. Evidence for a substrate cycle between AMP and adenosine in isolated hepatocytes. Proc Natl Acad Sci U S A. 1983 May;80(10):2829–2833. doi: 10.1073/pnas.80.10.2829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bontemps F., Vincent M. F., Van den Bergh F., van Waeg G., Van den Berghe G. Stimulation by glycerate 2,3-bisphosphate: a common property of cytosolic IMP-GMP 5'-nucleotidase in rat and human tissues. Biochim Biophys Acta. 1989 Jul 27;997(1-2):131–134. doi: 10.1016/0167-4838(89)90144-1. [DOI] [PubMed] [Google Scholar]
- Bontemps F., Vincent M. F., Van den Berghe G. Mechanisms of elevation of adenosine levels in anoxic hepatocytes. Biochem J. 1993 Mar 15;290(Pt 3):671–677. doi: 10.1042/bj2900671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chang C. H., Cha S., Brockman R. W., Bennett L. L., Jr Kinetic studies of adenosine kinase from L1210 cells: a model enzyme with a two-site ping-pong mechanism. Biochemistry. 1983 Feb 1;22(3):600–611. doi: 10.1021/bi00272a012. [DOI] [PubMed] [Google Scholar]
- Fisher M. N., Newsholme E. A. Properties of rat heart adenosine kinase. Biochem J. 1984 Jul 15;221(2):521–528. doi: 10.1042/bj2210521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwick R. A., Brown P. R. The performance of microparticle chemically-bonded anion-exchange resins in the analysis of nucleotides. J Chromatogr. 1975 Oct 29;112:650–662. doi: 10.1016/s0021-9673(00)99994-1. [DOI] [PubMed] [Google Scholar]
- Hawkins C. F., Bagnara A. S. Adenosine kinase from human erythrocytes: kinetic studies and characterization of adenosine binding sites. Biochemistry. 1987 Apr 7;26(7):1982–1987. doi: 10.1021/bi00381a030. [DOI] [PubMed] [Google Scholar]
- Henderson J. F., Brox L., Zombor G., Hunting D., Lomax C. A. Specificity of adenosine deaminase inhibitors. Biochem Pharmacol. 1977 Nov 1;26(21):1967–1972. doi: 10.1016/0006-2952(77)90003-x. [DOI] [PubMed] [Google Scholar]
- Henderson J. F., Mikoshiba A., Chu S. Y., Caldwell I. C. Kinetic studies of adenosine kinase from Ehrlich ascites tumor cells. J Biol Chem. 1972 Apr 10;247(7):1972–1975. [PubMed] [Google Scholar]
- Henderson J. F., Paterson A. R., Caldwell I. C., Paul B., Chan M. C., Lau K. F. Inhibitors of nucleoside and nucleotide metabolism. Cancer Chemother Rep 2. 1972 Nov;3(1):71–85. [PubMed] [Google Scholar]
- Johnson M. A., Fridland A. Phosphorylation of 2',3'-dideoxyinosine by cytosolic 5'-nucleotidase of human lymphoid cells. Mol Pharmacol. 1989 Aug;36(2):291–295. [PubMed] [Google Scholar]
- Keller P. M., McKee S. A., Fyfe J. A. Cytoplasmic 5'-nucleotidase catalyzes acyclovir phosphorylation. J Biol Chem. 1985 Jul 25;260(15):8664–8667. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lund P., Cornell N. W., Krebs H. A. Effect of adenosine on the adenine nucleotide content and metabolism of hepatocytes. Biochem J. 1975 Dec;152(3):593–599. doi: 10.1042/bj1520593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchand J. C., Lavoinne A., Giroz M., Matray F. The influence of adenosine on intermediary metabolism of isolated hepatocytes. Biochimie. 1979;61(11-12):1273–1282. doi: 10.1016/s0300-9084(80)80286-0. [DOI] [PubMed] [Google Scholar]
- Miller R. L., Adamczyk D. L., Miller W. H., Koszalka G. W., Rideout J. L., Beacham L. M., 3rd, Chao E. Y., Haggerty J. J., Krenitsky T. A., Elion G. B. Adenosine kinase from rabbit liver. II. Substrate and inhibitor specificity. J Biol Chem. 1979 Apr 10;254(7):2346–2352. [PubMed] [Google Scholar]
- Palella T. D., Andres C. M., Fox I. H. Human placental adenosine kinase. Kinetic mechanism and inhibition. J Biol Chem. 1980 Jun 10;255(11):5264–5269. [PubMed] [Google Scholar]
- Pinto R. M., Canales J., Günther Sillero M. A., Sillero A. Diadenosine tetraphosphate activates cytosol 5'-nucleotidase. Biochem Biophys Res Commun. 1986 Jul 16;138(1):261–267. doi: 10.1016/0006-291x(86)90274-3. [DOI] [PubMed] [Google Scholar]
- Rotllan P., Miras Portugal M. T. Adenosine kinase from bovine adrenal medulla. Eur J Biochem. 1985 Sep 2;151(2):365–371. doi: 10.1111/j.1432-1033.1985.tb09110.x. [DOI] [PubMed] [Google Scholar]
- Skladanowski A. C., Sala G. B., Newby A. C. Inhibition of IMP-specific cytosolic 5'-nucleotidase and adenosine formation in rat polymorphonuclear leucocytes by 5'-deoxy-5'-isobutylthio derivatives of adenosine and inosine. Biochem J. 1989 Aug 15;262(1):203–208. doi: 10.1042/bj2620203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauler A., Gil J., Bartrons R., Carreras J. Levels of glycerate 2,3-P2, 2,3-bisphosphoglycerate synthase and 2,3-bisphosphoglycerate phosphatase activities in rat tissues. A method to quantify blood contamination of tissue extracts. Comp Biochem Physiol B. 1987;86(1):11–13. doi: 10.1016/0305-0491(87)90167-2. [DOI] [PubMed] [Google Scholar]
- Van den Berghe G., Bontemps F., Hers H. G. Purine catabolism in isolated rat hepatocytes. Influence of coformycin. Biochem J. 1980 Jun 15;188(3):913–920. doi: 10.1042/bj1880913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worku Y., Newby A. C. Nucleoside exchange catalysed by the cytoplasmic 5'-nucleotidase. Biochem J. 1982 Sep 1;205(3):503–510. doi: 10.1042/bj2050503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada Y., Goto H., Ogasawara N. Purification and properties of adenosine kinase from rat brain. Biochim Biophys Acta. 1980 Dec 4;616(2):199–207. doi: 10.1016/0005-2744(80)90138-2. [DOI] [PubMed] [Google Scholar]
- van den Berghe G., van Pottelsberghe C., Hers H. G. A kinetic study of the soluble 5'-nucleotidase of rat liver. Biochem J. 1977 Mar 15;162(3):611–616. doi: 10.1042/bj1620611. [DOI] [PMC free article] [PubMed] [Google Scholar]


