Abstract
The expression of angiotensin-I converting enzyme (ACE; EC 3.4.15.1) in human circulating mononuclear cells was studied. T-lymphocytes contained the highest level of enzyme, approx. 28 times more per cell than monocytes. No activity was detected in B-lymphocytes. ACE was present mainly in the microsomal fraction, where it was found to be the major membrane-bound bradykinin-inactivating enzyme. An mRNA for ACE was detected and characterized after reverse transcription and amplification by PCR in T-lymphocytes and several T-cell leukaemia cell lines. We have previously observed that the interindividual variability in the levels of ACE in plasma is, in part, genetically determined and influenced by an insertion/deletion polymorphism of the ACE gene. To investigate the mechanisms involved in the regulation of ACE biosynthesis, the ACE levels of T-lymphocytes from 35 healthy subjects having different ACE genotypes were studied. These levels varied widely between individuals but were highly reproducible and influenced by the polymorphism of the ACE gene. T-lymphocyte levels of ACE were significantly higher in subjects who were homozygote for the deletion than in the other subjects. These results show that ACE is expressed in T-lymphocytes and indicate that the level of ACE expression in cells synthesizing the enzyme is genetically determined.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alhenc-Gelas F., Marchetti J., Allegrini J., Corvol P., Menard J. Measurement of urinary kallikrein activity. Species differences in kinin production. Biochim Biophys Acta. 1981 Nov 5;677(3-4):477–488. doi: 10.1016/0304-4165(81)90262-2. [DOI] [PubMed] [Google Scholar]
- Alhenc-Gelas F., Richard J., Courbon D., Warnet J. M., Corvol P. Distribution of plasma angiotensin I-converting enzyme levels in healthy men: relationship to environmental and hormonal parameters. J Lab Clin Med. 1991 Jan;117(1):33–39. [PubMed] [Google Scholar]
- Alhenc-Gelas F., Weare J. A., Johnson R. L., Jr, Erdös E. G. Measurement of human converting enzyme level by direct radioimmunoassay. J Lab Clin Med. 1983 Jan;101(1):83–96. [PubMed] [Google Scholar]
- Bar-Shavit Z., Goldman R., Stabinsky Y., Gottlieb P., Fridkin M., Teichberg V. I., Blumberg S. Enhancement of phagocytosis - a newly found activity of substance P residing in its N-terminal tetrapeptide sequence. Biochem Biophys Res Commun. 1980 Jun 30;94(4):1445–1451. doi: 10.1016/0006-291x(80)90581-1. [DOI] [PubMed] [Google Scholar]
- Bauvois B. Murine thymocytes possess specific cell surface-associated exoaminopeptidase activities: preferential expression by immature CD4-CD8- subpopulation. Eur J Immunol. 1990 Mar;20(3):459–468. doi: 10.1002/eji.1830200302. [DOI] [PubMed] [Google Scholar]
- Beaumont A., Brouet J. C., Roques B. P. Neutral endopeptidase 24.11 and angiotensin converting enzyme like activity in CALLA positive and CALLA negative lymphoid cells. Biochem Biophys Res Commun. 1989 May 15;160(3):1323–1329. doi: 10.1016/s0006-291x(89)80148-2. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Caldwell P. R., Seegal B. C., Hsu K. C., Das M., Soffer R. L. Angiotensin-converting enzyme: vascular endothelial localization. Science. 1976 Mar 12;191(4231):1050–1051. doi: 10.1126/science.175444. [DOI] [PubMed] [Google Scholar]
- Cambien F., Alhenc-Gelas F., Herbeth B., Andre J. L., Rakotovao R., Gonzales M. F., Allegrini J., Bloch C. Familial resemblance of plasma angiotensin-converting enzyme level: the Nancy Study. Am J Hum Genet. 1988 Nov;43(5):774–780. [PMC free article] [PubMed] [Google Scholar]
- Cambien F., Poirier O., Lecerf L., Evans A., Cambou J. P., Arveiler D., Luc G., Bard J. M., Bara L., Ricard S. Deletion polymorphism in the gene for angiotensin-converting enzyme is a potent risk factor for myocardial infarction. Nature. 1992 Oct 15;359(6396):641–644. doi: 10.1038/359641a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Connelly J. C., Skidgel R. A., Schulz W. W., Johnson A. R., Erdös E. G. Neutral endopeptidase 24.11 in human neutrophils: cleavage of chemotactic peptide. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8737–8741. doi: 10.1073/pnas.82.24.8737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defendini R., Zimmerman E. A., Weare J. A., Alhenc-Gelas F., Erdös E. G. Angiotensin-converting enzyme in epithelial and neuroepithelial cells. Neuroendocrinology. 1983 Jul;37(1):32–40. doi: 10.1159/000123512. [DOI] [PubMed] [Google Scholar]
- Ehlers M. R., Chen Y. N., Riordan J. F. Spontaneous solubilization of membrane-bound human testis angiotensin-converting enzyme expressed in Chinese hamster ovary cells. Proc Natl Acad Sci U S A. 1991 Feb 1;88(3):1009–1013. doi: 10.1073/pnas.88.3.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlers M. R., Fox E. A., Strydom D. J., Riordan J. F. Molecular cloning of human testicular angiotensin-converting enzyme: the testis isozyme is identical to the C-terminal half of endothelial angiotensin-converting enzyme. Proc Natl Acad Sci U S A. 1989 Oct;86(20):7741–7745. doi: 10.1073/pnas.86.20.7741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Friedland J., Setton C., Silverstein E. Induction of angiotensin converting enzyme in human monocytes in culture. Biochem Biophys Res Commun. 1978 Aug 14;83(3):843–849. doi: 10.1016/0006-291x(78)91471-7. [DOI] [PubMed] [Google Scholar]
- Hegen M., Niedobitek G., Klein C. E., Stein H., Fleischer B. The T cell triggering molecule Tp103 is associated with dipeptidyl aminopeptidase IV activity. J Immunol. 1990 Apr 15;144(8):2908–2914. [PubMed] [Google Scholar]
- Hooper N. M., Keen J., Pappin D. J., Turner A. J. Pig kidney angiotensin converting enzyme. Purification and characterization of amphipathic and hydrophilic forms of the enzyme establishes C-terminal anchorage to the plasma membrane. Biochem J. 1987 Oct 1;247(1):85–93. doi: 10.1042/bj2470085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson A. R., Erdös E. G. Metabolism of vasoactive peptides by human endothelial cells in culture. Angiotensin I converting enzyme (kininase II) and angiotensinase. J Clin Invest. 1977 Apr;59(4):684–695. doi: 10.1172/JCI108687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R. S., Kusari J., Roy S. N., Soffer R. L., Sen G. C. Structure of testicular angiotensin-converting enzyme. A segmental mosaic isozyme. J Biol Chem. 1989 Oct 5;264(28):16754–16758. [PubMed] [Google Scholar]
- Lattion A. L., Soubrier F., Allegrini J., Hubert C., Corvol P., Alhenc-Gelas F. The testicular transcript of the angiotensin I-converting enzyme encodes for the ancestral, non-duplicated form of the enzyme. FEBS Lett. 1989 Jul 31;252(1-2):99–104. doi: 10.1016/0014-5793(89)80897-x. [DOI] [PubMed] [Google Scholar]
- Lea T., Smeland E., Funderud S., Vartdal F., Davies C., Beiske K., Ugelstad J. Characterization of human mononuclear cells after positive selection with immunomagnetic particles. Scand J Immunol. 1986 Apr;23(4):509–519. doi: 10.1111/j.1365-3083.1986.tb03083.x. [DOI] [PubMed] [Google Scholar]
- Letarte M., Vera S., Tran R., Addis J. B., Onizuka R. J., Quackenbush E. J., Jongeneel C. V., McInnes R. R. Common acute lymphocytic leukemia antigen is identical to neutral endopeptidase. J Exp Med. 1988 Oct 1;168(4):1247–1253. doi: 10.1084/jem.168.4.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman J. Elevation of serum angiotensin-converting-enzyme (ACE) level in sarcoidosis. Am J Med. 1975 Sep;59(3):365–372. doi: 10.1016/0002-9343(75)90395-2. [DOI] [PubMed] [Google Scholar]
- Lopez M., Fechtenbaum J., David B., Martinache C., Chokri M., Canepa S., De Gramont A., Louvet C., Gorin I., Mortel O. Adoptive immunotherapy with activated macrophages grown in vitro from blood monocytes in cancer patients: a pilot study. J Immunother (1991) 1992 Apr;11(3):209–217. doi: 10.1097/00002371-199204000-00008. [DOI] [PubMed] [Google Scholar]
- Lotz M., Vaughan J. H., Carson D. A. Effect of neuropeptides on production of inflammatory cytokines by human monocytes. Science. 1988 Sep 2;241(4870):1218–1221. doi: 10.1126/science.2457950. [DOI] [PubMed] [Google Scholar]
- Mendelsohn F. A., Lloyd C. J., Kachel C., Funder J. W. Induction by glucocorticoids of angiotensin converting enzyme production from bovine endothelial cells in culture and rat lung in vivo. J Clin Invest. 1982 Sep;70(3):684–692. doi: 10.1172/JCI110663. [DOI] [PMC free article] [PubMed] [Google Scholar]
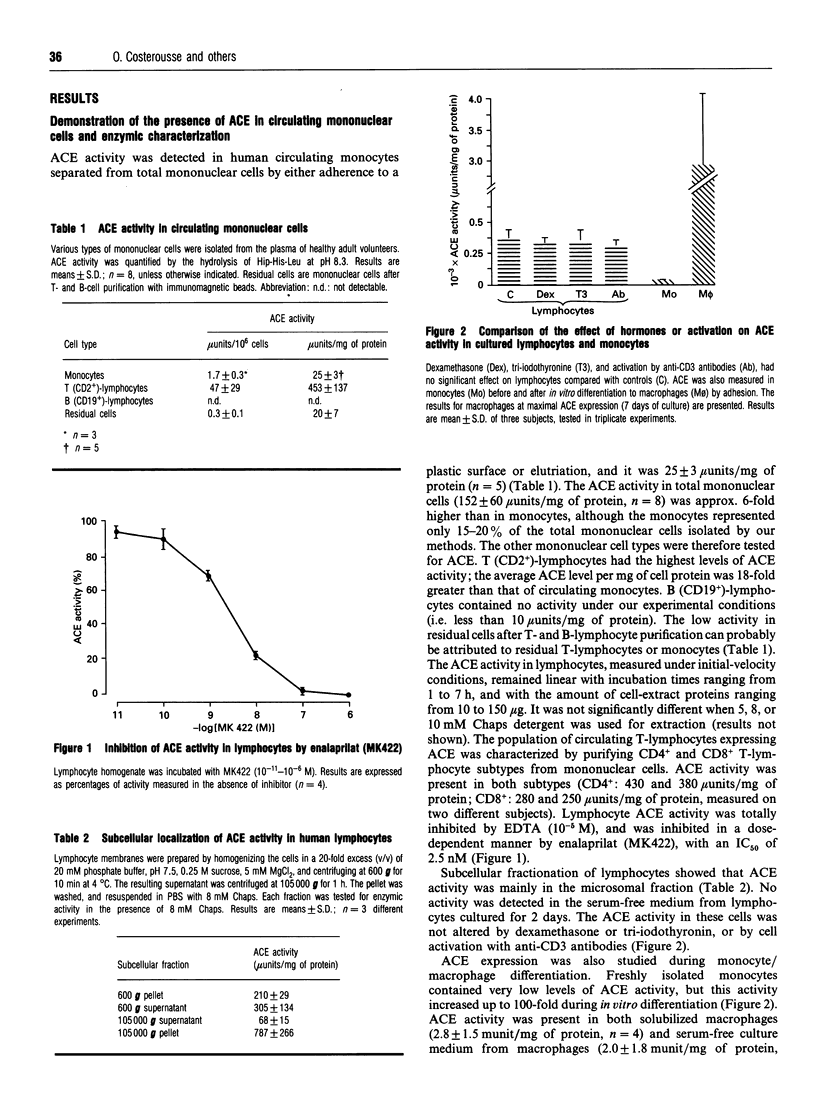
- Meuer S. C., Acuto O., Hercend T., Schlossman S. F., Reinherz E. L. The human T-cell receptor. Annu Rev Immunol. 1984;2:23–50. doi: 10.1146/annurev.iy.02.040184.000323. [DOI] [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Neitzel H. A routine method for the establishment of permanent growing lymphoblastoid cell lines. Hum Genet. 1986 Aug;73(4):320–326. doi: 10.1007/BF00279094. [DOI] [PubMed] [Google Scholar]
- Payan D. G. Neuropeptides and inflammation: the role of substance P. Annu Rev Med. 1989;40:341–352. doi: 10.1146/annurev.me.40.020189.002013. [DOI] [PubMed] [Google Scholar]
- Pernow B. Role of tachykinins in neurogenic inflammation. J Immunol. 1985 Aug;135(2 Suppl):812s–815s. [PubMed] [Google Scholar]
- Proud D., Kaplan A. P. Kinin formation: mechanisms and role in inflammatory disorders. Annu Rev Immunol. 1988;6:49–83. doi: 10.1146/annurev.iy.06.040188.000405. [DOI] [PubMed] [Google Scholar]
- Rigat B., Hubert C., Alhenc-Gelas F., Cambien F., Corvol P., Soubrier F. An insertion/deletion polymorphism in the angiotensin I-converting enzyme gene accounting for half the variance of serum enzyme levels. J Clin Invest. 1990 Oct;86(4):1343–1346. doi: 10.1172/JCI114844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SKEGGS L. T., Jr, KAHN J. R., SHUMWAY N. P. The preparation and function of the hypertensin-converting enzyme. J Exp Med. 1956 Mar 1;103(3):295–299. doi: 10.1084/jem.103.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson R. J., Shepperdson R. T., Vatter A. E., Talmage D. W. Isolation and enumeration of peripheral blood monocytes. J Immunol. 1977 Apr;118(4):1409–1414. [PubMed] [Google Scholar]
- Sangster R. N., Minowada J., Suciu-Foca N., Minden M., Mak T. W. Rearrangement and expression of the alpha, beta, and gamma chain T cell receptor genes in human thymic leukemia cells and functional T cells. J Exp Med. 1986 Jun 1;163(6):1491–1508. doi: 10.1084/jem.163.6.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider U., Schwenk H. U., Bornkamm G. Characterization of EBV-genome negative "null" and "T" cell lines derived from children with acute lymphoblastic leukemia and leukemic transformed non-Hodgkin lymphoma. Int J Cancer. 1977 May 15;19(5):621–626. doi: 10.1002/ijc.2910190505. [DOI] [PubMed] [Google Scholar]
- Soubrier F., Alhenc-Gelas F., Hubert C., Allegrini J., John M., Tregear G., Corvol P. Two putative active centers in human angiotensin I-converting enzyme revealed by molecular cloning. Proc Natl Acad Sci U S A. 1988 Dec;85(24):9386–9390. doi: 10.1073/pnas.85.24.9386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vivier I., Marguet D., Naquet P., Bonicel J., Black D., Li C. X., Bernard A. M., Gorvel J. P., Pierres M. Evidence that thymocyte-activating molecule is mouse CD26 (dipeptidyl peptidase IV). J Immunol. 1991 Jul 15;147(2):447–454. [PubMed] [Google Scholar]
- Vuk-Pavlović Z., Kreofsky T. J., Rohrbach M. S. Characteristics of monocyte angiotensin-converting enzyme (ACE) induction by dexamethasone. J Leukoc Biol. 1989 Jun;45(6):503–509. doi: 10.1002/jlb.45.6.503. [DOI] [PubMed] [Google Scholar]
- Ward P. E., Erdös E. G., Gedney C. D., Dowben R. M., Reynolds R. C. Isolation of membrane-bound renal enzymes that metabolize kinins and angiotensins. Biochem J. 1976 Sep 1;157(3):643–650. doi: 10.1042/bj1570643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L., Alhenc-Gelas F., Soubrier F., Michaud A., Corvol P., Clauser E. Expression and characterization of recombinant human angiotensin I-converting enzyme. Evidence for a C-terminal transmembrane anchor and for a proteolytic processing of the secreted recombinant and plasma enzymes. J Biol Chem. 1991 Mar 25;266(9):5540–5546. [PubMed] [Google Scholar]
- Weinstock J. V., Blum A. M., Kassab J. T. Angiotensin II is chemotactic for a T-cell subset which can express migration inhibition factor activity in murine schistosomiasis mansoni. Cell Immunol. 1987 Jun;107(1):180–187. doi: 10.1016/0008-8749(87)90278-4. [DOI] [PubMed] [Google Scholar]
- Williams T. A., Hooper N. M., Turner A. J. Characterization of neuronal and endothelial forms of angiotensin converting enzyme in pig brain. J Neurochem. 1991 Jul;57(1):193–199. doi: 10.1111/j.1471-4159.1991.tb02115.x. [DOI] [PubMed] [Google Scholar]
- Wu Q., Li L., Cooper M. D., Pierres M., Gorvel J. P. Aminopeptidase A activity of the murine B-lymphocyte differentiation antigen BP-1/6C3. Proc Natl Acad Sci U S A. 1991 Jan 15;88(2):676–680. doi: 10.1073/pnas.88.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H. Y., Erdös E. G., Levin Y. A dipeptidyl carboxypeptidase that converts angiotensin I and inactivates bradykinin. Biochim Biophys Acta. 1970 Aug 21;214(2):374–376. doi: 10.1016/0005-2795(70)90017-6. [DOI] [PubMed] [Google Scholar]