Abstract
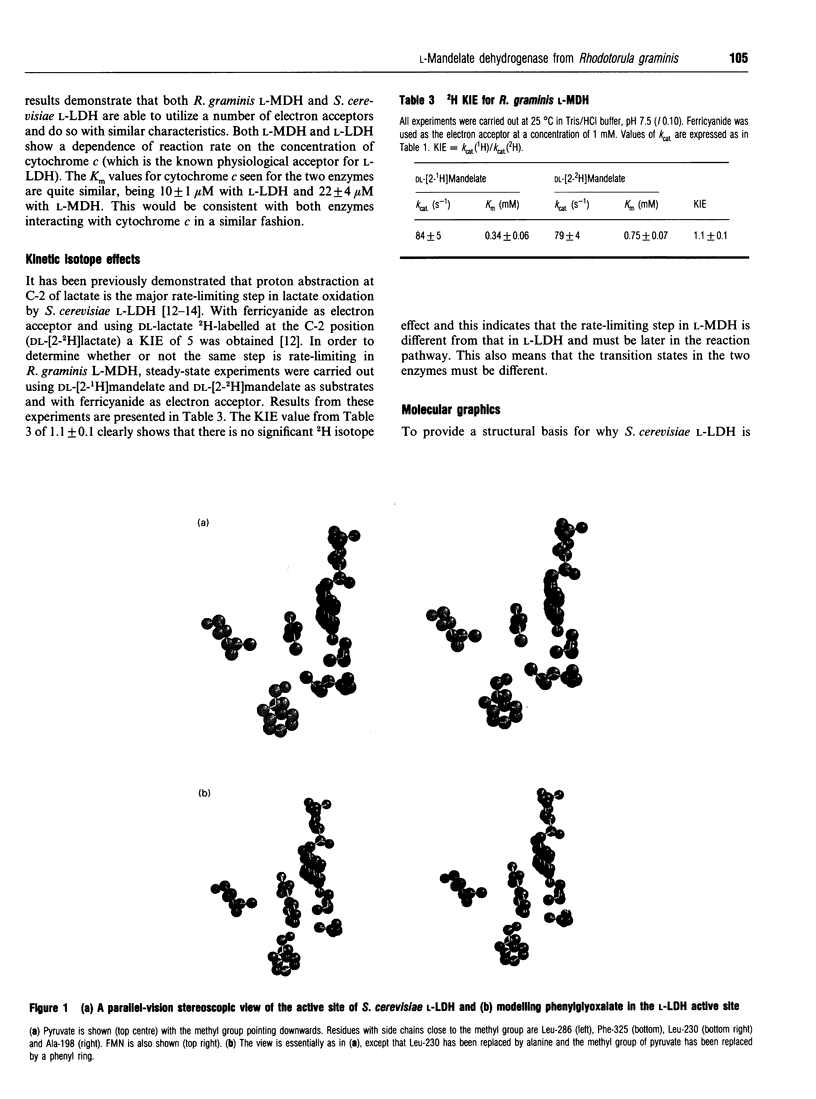
L-Lactate dehydrogenase (L-LDH) from Saccharomyces cerevisiae and L-mandelate dehydrogenase (L-MDH) from Rhodotorula graminis are both flavocytochromes b2. The kinetic properties of these enzymes have been compared using steady-state kinetic methods. The most striking difference between the two enzymes is found by comparing their substrate specificities. L-LDH and L-MDH have mutually exclusive primary substrates, i.e. the substrate for one enzyme is a potent competitive inhibitor for the other. Molecular-modelling studies on the known three-dimensional structure of S. cerevisiae L-LDH suggest that this enzyme is unable to catalyse the oxidation of L-mandelate because productive binding is impeded by steric interference, particularly between the side chain of Leu-230 and the phenyl ring of mandelate. Another major difference between L-LDH and L-MDH lies in the rate-determining step. For S. cerevisiae L-LDH, the major rate-determining step is proton abstraction at C-2 of lactate, as previously shown by the 2H kinetic-isotope effect. However, in R. graminis L-MDH the kinetic-isotope effect seen with DL-[2-2H]mandelate is only 1.1 +/- 0.1, clearly showing that proton abstraction at C-2 of mandelate is not rate-limiting. The fact that the rate-determining step is different indicates that the transition states in each of these enzymes must also be different.
Full text
PDF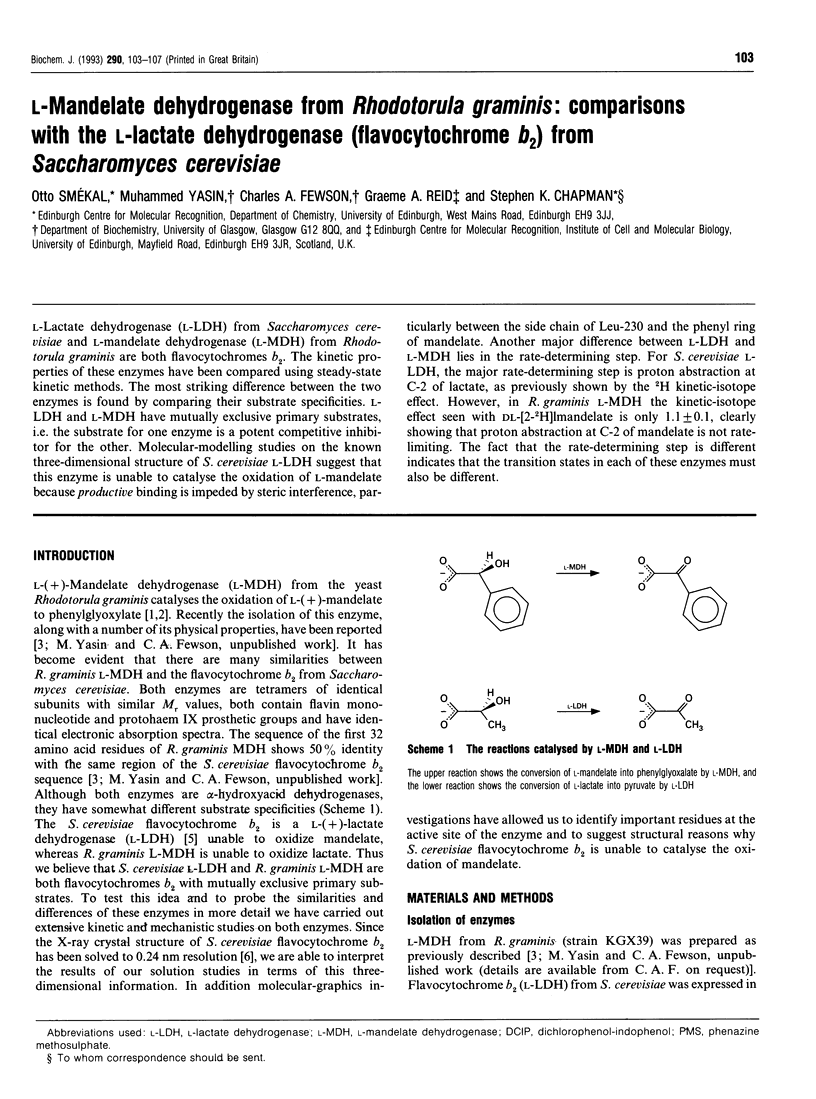


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Black M. T., White S. A., Reid G. A., Chapman S. K. High-level expression of fully active yeast flavocytochrome b2 in Escherichia coli. Biochem J. 1989 Feb 15;258(1):255–259. doi: 10.1042/bj2580255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois J., Chapman S. K., Mathews F. S., Reid G. A., Lederer F. Substitution of Tyr254 with Phe at the active site of flavocytochrome b2: consequences on catalysis of lactate dehydrogenation. Biochemistry. 1990 Jul 10;29(27):6393–6400. doi: 10.1021/bi00479a008. [DOI] [PubMed] [Google Scholar]
- Fewson C. A. Microbial metabolism of mandelate: a microcosm of diversity. FEMS Microbiol Rev. 1988 Apr-Jun;4(2):85–110. doi: 10.1111/j.1574-6968.1988.tb02737.x. [DOI] [PubMed] [Google Scholar]
- Hazzard J. T., Cusanovich M. A., Tainer J. A., Getzoff E. D., Tollin G. Kinetic studies of reduction of a 1:1 cytochrome c-flavodoxin complex by free flavin semiquinones and rubredoxin. Biochemistry. 1986 Jun 3;25(11):3318–3328. doi: 10.1021/bi00359a035. [DOI] [PubMed] [Google Scholar]
- Labeyrie F., Baudras A., Lederer F. Flavocytochrome b 2 or L-lactate cytochrome c reductase from yeast. Methods Enzymol. 1978;53:238–256. doi: 10.1016/s0076-6879(78)53030-9. [DOI] [PubMed] [Google Scholar]
- Lederer F. On the first steps of lactate oxidation by bakers' yeast L-(plus)-lactate dehydrogenase (cytochrome b2). Eur J Biochem. 1974 Jul 15;46(2):393–399. doi: 10.1111/j.1432-1033.1974.tb03632.x. [DOI] [PubMed] [Google Scholar]
- Miles C. S., Rouvière-Fourmy N., Lederer F., Mathews F. S., Reid G. A., Black M. T., Chapman S. K. Tyr-143 facilitates interdomain electron transfer in flavocytochrome b2. Biochem J. 1992 Jul 1;285(Pt 1):187–192. doi: 10.1042/bj2850187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompon D., Iwatsubo M., Lederer F. Flavocytochrome b2 (Baker's yeast). Deuterium isotope effect studied by rapid-kinetic methods as a probe for the mechanism of electron transfer. Eur J Biochem. 1980 Mar;104(2):479–488. doi: 10.1111/j.1432-1033.1980.tb04450.x. [DOI] [PubMed] [Google Scholar]
- Reid G. A., White S., Black M. T., Lederer F., Mathews F. S., Chapman S. K. Probing the active site of flavocytochrome b2 by site-directed mutagenesis. Eur J Biochem. 1988 Dec 15;178(2):329–333. doi: 10.1111/j.1432-1033.1988.tb14454.x. [DOI] [PubMed] [Google Scholar]
- Tsou A. Y., Ransom S. C., Gerlt J. A., Buechter D. D., Babbitt P. C., Kenyon G. L. Mandelate pathway of Pseudomonas putida: sequence relationships involving mandelate racemase, (S)-mandelate dehydrogenase, and benzoylformate decarboxylase and expression of benzoylformate decarboxylase in Escherichia coli. Biochemistry. 1990 Oct 23;29(42):9856–9862. doi: 10.1021/bi00494a015. [DOI] [PubMed] [Google Scholar]
- Xia Z. X., Mathews F. S. Molecular structure of flavocytochrome b2 at 2.4 A resolution. J Mol Biol. 1990 Apr 20;212(4):837–863. doi: 10.1016/0022-2836(90)90240-M. [DOI] [PubMed] [Google Scholar]



