Abstract
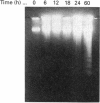
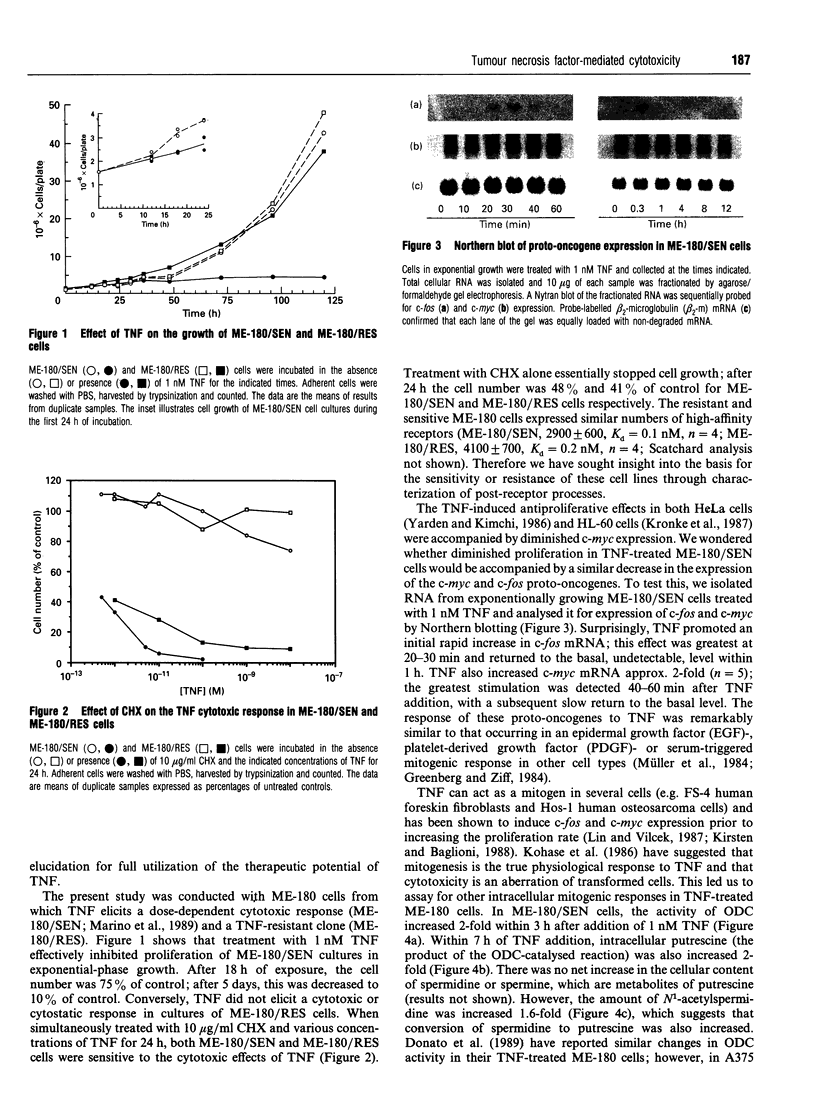
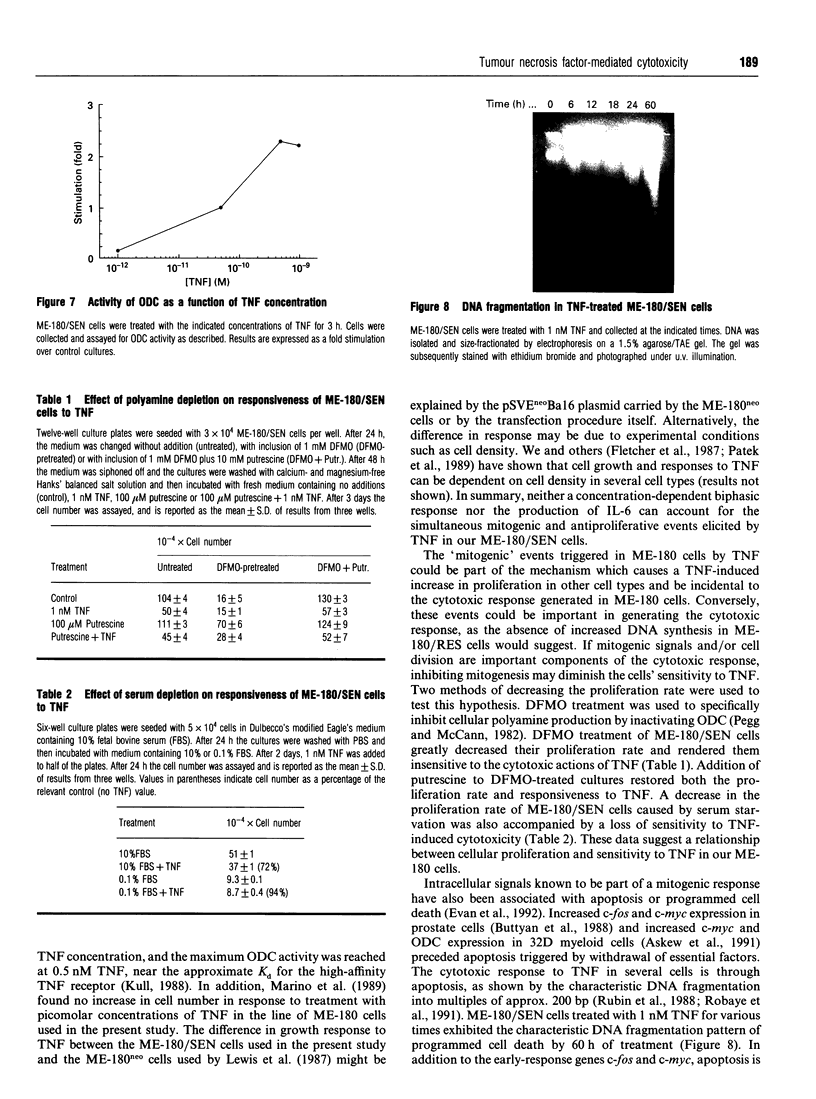
Tumour necrosis factor-alpha (TNF) induced a cytotoxic response in ME-180 human cervical carcinoma cells in vitro. This cytotoxic response was accompanied by a temporal series of intracellular signals that are commonly triggered by a mitogenic stimulus: increased c-fos (20-30 min) and c-myc (40-60 min) expression, increased activity of ornithine decarboxylase (3 h), increased intracellular polyamine content (7 h) and increased thymidine incorporation into DNA (14 h). A cytotoxic response independent of these mitogenic signals could not be explained by an induction of interleukin-6, which is an autocrine cytotoxic agent in some cell types; nor by a biphasic, dose-dependent response in which low concentrations of TNF are mitogenic and higher concentrations are cytotoxic. Conversely, a dependent role of these mitogenic signals was suggested by the absence of a TNF-promoted increase in thymidine incorporation into DNA in an ME-180 clone that is resistant to TNF-induced cytotoxicity. A decrease in the proliferation rate of TNF-sensitive cells induced by either alpha-difluoromethylornithine treatment (resulting in polyamine depletion) or serum starvation rendered the cells insensitive to TNF-induced cytotoxicity, further suggesting a role for mitogenic signals and cell division in TNF-mediated cytotoxicity. However, inhibiting proliferation with cycloheximide resulted in increased sensitivity to TNF, implying that mitogenesis itself was not essential for a cytotoxic response. TNF induced DNA fragmentation in sensitive cells, suggesting that cytotoxicity occurred via apoptosis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alitalo K., Schwab M., Lin C. C., Varmus H. E., Bishop J. M. Homogeneously staining chromosomal regions contain amplified copies of an abundantly expressed cellular oncogene (c-myc) in malignant neuroendocrine cells from a human colon carcinoma. Proc Natl Acad Sci U S A. 1983 Mar;80(6):1707–1711. doi: 10.1073/pnas.80.6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askew D. S., Ashmun R. A., Simmons B. C., Cleveland J. L. Constitutive c-myc expression in an IL-3-dependent myeloid cell line suppresses cell cycle arrest and accelerates apoptosis. Oncogene. 1991 Oct;6(10):1915–1922. [PubMed] [Google Scholar]
- Baloch Z., Cohen S., Coffman F. D. Synergistic interactions between tumor necrosis factor and inhibitors of DNA topoisomerase I and II. J Immunol. 1990 Nov 1;145(9):2908–2913. [PubMed] [Google Scholar]
- Beaven M. A., Wilcox G., Terpstra G. K. A microprocedure for the measurement of 14CO2 release from [14C]carboxyl-labeled amino acids. Anal Biochem. 1978 Feb;84(2):638–641. doi: 10.1016/0003-2697(78)90089-1. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buttyan R., Olsson C. A., Pintar J., Chang C., Bandyk M., Ng P. Y., Sawczuk I. S. Induction of the TRPM-2 gene in cells undergoing programmed death. Mol Cell Biol. 1989 Aug;9(8):3473–3481. doi: 10.1128/mcb.9.8.3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttyan R., Zakeri Z., Lockshin R., Wolgemuth D. Cascade induction of c-fos, c-myc, and heat shock 70K transcripts during regression of the rat ventral prostate gland. Mol Endocrinol. 1988 Jul;2(7):650–657. doi: 10.1210/mend-2-7-650. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Coffman F. D., Haviland D. L., Green L. M., Ware C. F. Cytotoxicity by tumor necrosis factor is linked with the cell cycle but does not require DNA synthesis. Growth Factors. 1989;1(4):357–364. doi: 10.3109/08977198909000259. [DOI] [PubMed] [Google Scholar]
- Curran T., Peters G., Van Beveren C., Teich N. M., Verma I. M. FBJ murine osteosarcoma virus: identification and molecular cloning of biologically active proviral DNA. J Virol. 1982 Nov;44(2):674–682. doi: 10.1128/jvi.44.2.674-682.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darzynkiewicz Z., Williamson B., Carswell E. A., Old L. J. Cell cycle-specific effects of tumor necrosis factor. Cancer Res. 1984 Jan;44(1):83–90. [PubMed] [Google Scholar]
- Donato N. J., Ince C., Rosenblum M. G., Gallick G. E. Early events in the antiproliferative action of tumor necrosis factor are similar to the early events in epidermal growth factor growth stimulation. J Cell Biochem. 1989 Nov;41(3):139–157. doi: 10.1002/jcb.240410305. [DOI] [PubMed] [Google Scholar]
- Endo Y., Matsushima K., Onozaki K., Oppenheim J. J. Role of ornithine decarboxylase in the regulation of cell growth by IL-1 and tumor necrosis factor. J Immunol. 1988 Oct 1;141(7):2342–2348. [PubMed] [Google Scholar]
- Evan G. I., Wyllie A. H., Gilbert C. S., Littlewood T. D., Land H., Brooks M., Waters C. M., Penn L. Z., Hancock D. C. Induction of apoptosis in fibroblasts by c-myc protein. Cell. 1992 Apr 3;69(1):119–128. doi: 10.1016/0092-8674(92)90123-t. [DOI] [PubMed] [Google Scholar]
- Fletcher W. H., Shiu W. W., Ishida T. A., Haviland D. L., Ware C. F. Resistance to the cytolytic action of lymphotoxin and tumor necrosis factor coincides with the presence of gap junctions uniting target cells. J Immunol. 1987 Aug 1;139(3):956–962. [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Kirstein M., Baglioni C. Tumor necrosis factor stimulates proliferation of human osteosarcoma cells and accumulation of c-myc messenger RNA. J Cell Physiol. 1988 Mar;134(3):479–484. doi: 10.1002/jcp.1041340321. [DOI] [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Krönke M., Schlüter C., Pfizenmaier K. Tumor necrosis factor inhibits MYC expression in HL-60 cells at the level of mRNA transcription. Proc Natl Acad Sci U S A. 1987 Jan;84(2):469–473. doi: 10.1073/pnas.84.2.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kull F. C., Jr The TNF receptor in TNF-mediated cytotoxicity. Nat Immun Cell Growth Regul. 1988;7(5-6):254–265. [PubMed] [Google Scholar]
- Kunkel S. L., Remick D. G., Strieter R. M., Larrick J. W. Mechanisms that regulate the production and effects of tumor necrosis factor-alpha. Crit Rev Immunol. 1989;9(2):93–117. [PubMed] [Google Scholar]
- Kyprianou N., English H. F., Davidson N. E., Isaacs J. T. Programmed cell death during regression of the MCF-7 human breast cancer following estrogen ablation. Cancer Res. 1991 Jan 1;51(1):162–166. [PubMed] [Google Scholar]
- Lewis G. D., Aggarwal B. B., Eessalu T. E., Sugarman B. J., Shepard H. M. Modulation of the growth of transformed cells by human tumor necrosis factor-alpha and interferon-gamma. Cancer Res. 1987 Oct 15;47(20):5382–5385. [PubMed] [Google Scholar]
- Lin J. X., Vilcek J. Tumor necrosis factor and interleukin-1 cause a rapid and transient stimulation of c-fos and c-myc mRNA levels in human fibroblasts. J Biol Chem. 1987 Sep 5;262(25):11908–11911. [PubMed] [Google Scholar]
- Marino M. W., Pfeffer L. M., Guidon P. T., Jr, Donner D. B. Tumor necrosis factor induces phosphorylation of a 28-kDa mRNA cap-binding protein in human cervical carcinoma cells. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8417–8421. doi: 10.1073/pnas.86.21.8417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May L. T., Sehgal P. B., LaForge K. S., Inouye M. Expression of the native alpha and beta interferon genes in human cells. Virology. 1983 Aug;129(1):116–126. doi: 10.1016/0042-6822(83)90400-2. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Old L. J. Tumor necrosis factor (TNF). Science. 1985 Nov 8;230(4726):630–632. doi: 10.1126/science.2413547. [DOI] [PubMed] [Google Scholar]
- Patek P. Q., Lin Y., Case P. G. Cell lines cultured at high density are resistant to lysis by tumor necrosis factor and natural cytotoxic cells. Proc Soc Exp Biol Med. 1989 Mar;190(3):234–239. doi: 10.3181/00379727-190-42854. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., McCann P. P. Polyamine metabolism and function. Am J Physiol. 1982 Nov;243(5):C212–C221. doi: 10.1152/ajpcell.1982.243.5.C212. [DOI] [PubMed] [Google Scholar]
- Robaye B., Mosselmans R., Fiers W., Dumont J. E., Galand P. Tumor necrosis factor induces apoptosis (programmed cell death) in normal endothelial cells in vitro. Am J Pathol. 1991 Feb;138(2):447–453. [PMC free article] [PubMed] [Google Scholar]
- Rubin B. Y., Smith L. J., Hellermann G. R., Lunn R. M., Richardson N. K., Anderson S. L. Correlation between the anticellular and DNA fragmenting activities of tumor necrosis factor. Cancer Res. 1988 Nov 1;48(21):6006–6010. [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Peptide growth factors are multifunctional. Nature. 1988 Mar 17;332(6161):217–219. doi: 10.1038/332217a0. [DOI] [PubMed] [Google Scholar]
- Sugarman B. J., Aggarwal B. B., Hass P. E., Figari I. S., Palladino M. A., Jr, Shepard H. M. Recombinant human tumor necrosis factor-alpha: effects on proliferation of normal and transformed cells in vitro. Science. 1985 Nov 22;230(4728):943–945. doi: 10.1126/science.3933111. [DOI] [PubMed] [Google Scholar]
- Suggs S. V., Wallace R. B., Hirose T., Kawashima E. H., Itakura K. Use of synthetic oligonucleotides as hybridization probes: isolation of cloned cDNA sequences for human beta 2-microglobulin. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6613–6617. doi: 10.1073/pnas.78.11.6613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G. T. Programmed cell death: apoptosis and oncogenesis. Cell. 1991 Jun 28;65(7):1097–1098. doi: 10.1016/0092-8674(91)90002-g. [DOI] [PubMed] [Google Scholar]
- Yarden A., Kimchi A. Tumor necrosis factor reduces c-myc expression and cooperates with interferon-gamma in HeLa cells. Science. 1986 Dec 12;234(4782):1419–1421. doi: 10.1126/science.3097823. [DOI] [PubMed] [Google Scholar]