Abstract
Transcranial magnetic stimulation traces the functional and structural connections that modulate amygdala activity, enabling advanced brain stimulation treatments for numerous psychiatric disorders.
The amygdala, an almond-shaped collection of nuclei nestled in each hemisphere of the brain, processes emotional responses and facilitates decision-making. It is one of the core neural circuits associated with depression, anxiety, and posttraumatic stress disorder (1). The amygdala has long been implicated in treatment studies of these disorders, from early pharmacologic interventions to more recent neuromodulatory approaches using transcranial magnetic stimulation (TMS). TMS induces rapidly fluctuating magnetic fields from a location on the scalp, delivered either in single pulses as a neurophysiological tool or in a more repetitive fashion as a therapeutic intervention for major depression and other neuropsychiatric conditions (2).
Current TMS technology only reaches several centimeters below the cortical surface, limiting the direct impact of the stimulation and putting deeper brain structures like the amygdala out of reach. Therefore, observations from existing TMS studies on noncortical parts of the brain have been indirect, often gleaned from investigations that combine TMS with resting-state functional connectivity or anatomical lesions (3) to infer its impact on brain structures located downstream from the cortical stimulation site.
These indirect inferences have motivated researchers to consider how to better target the amygdala, in the hope that improved engagement of this structure through its connections to the frontal lobe will lead to improved treatment outcomes. Building on this conceptual framework, direct and causal evidence is required to develop noninvasive interventions for amygdala modulation. Once identified, these treatments can be tested and adapted to meet the clinical needs of patients who do not respond to more traditional interventions.
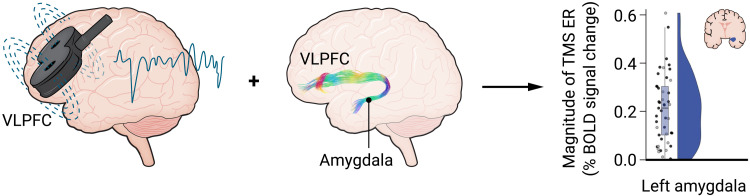
Enter the study by Sydnor et al. in this issue of Science Advances (4). This work elegantly maps out how we might improve amygdala targeting, with causal evidence that combines TMS with multimodal neuroimaging approaches. Like any good map, the details guide the way. The research team used interleaved magnetic resonance imaging (MRI) and single-pulse TMS—a technically advanced approach that allows causal inference between observed changes in brain metabolic activity and a magnetic perturbation. In a cohort of healthy individuals, they demonstrated that TMS pulses to the ventrolateral prefrontal cortex (VLPFC) modulated amygdala activity. Furthermore, the magnitude of this signal transduction was relatively larger in the amygdala compared to several other deep brain areas, and the density of white matter tracts connecting these structures (measured via diffusion MRI–based streamlines) was related to the observed functional effects. This signal propagates along a direct pathway between the VLPFC and the amygdala (Fig. 1).
Fig. 1. Magnetic stimulation applied to the scalp over the VLPFC engages an evolutionarily conserved pathway to elicit changes in amygdala activity.
ER, evoked response; BOLD, blood oxygen level–dependent. Credit: Ashley Mastin/Science Advances
This work demonstrates a causal indication that TMS to the VLPFC modulates the amygdala through a direct pathway, providing a coherent road map for the field to develop novel precision, circuit-based therapeutics. It is one thing to think we know how a circuit works; it is another entirely to demonstrate that the roads we wish to follow do, in fact, exist.
Like any good scout, Sydnor et al. were mindful that the trails they made needed to be useful for those that follow them. To this end, the paper used a rigorous control site—a region in the dorsolateral prefrontal cortex (DLPFC) defined by anticorrelated functional connectivity with the subgenual portion of the anterior cingulate cortex, located below the tip of the corpus callosum. This alternate route has been implicated in predicting a clinical response to TMS directed at the DLPFC for depression and posttraumatic stress disorder (5, 6).
Sydnor and colleagues showed that their finding of larger TMS-evoked changes in the amygdala were associated with higher fiber density in a VLPFC-amygdala pathway, which was not observed when delivering TMS to the subgenual-defined DLPFC control pathway, exquisitely underscoring the specificity of the observed effect. Perhaps unsurprisingly, single-pulse TMS to the left VLPFC yielded changes to both the left and right amygdala, reminding us of the interconnectedness of the circuit and the variety of ways to engage brain regions of interest.
The team also found variability in the TMS response, demonstrating that population averages in neuroimaging can obscure unique individual differences. While most of the participants exhibited a negative or suppressive activity response with single-pulse TMS in a substantial proportion of participants, the same stimulation elicited a positive response in others. Future research is warranted to identify the personal or behavioral characteristics associated with this variability to design basic neurophysiological experiments that manipulate the amygdala in a desired direction and to develop individualized treatment protocols in clinical populations.
As with any map, some routes can lead one astray. Sydnor et al. exclusively studied healthy participants, which is a useful starting point. However, observations in this population are often easier to acquire and more straightforward to interpret than in clinical samples. Yet, if the goal is to develop treatment interventions, the critical next step is to pursue this line of inquiry in the very patients we seek to treat. Individual variability may be more extreme in clinical populations, and identifying sources of this variability is particularly important in this context, as clinicians would not want to deliver a treatment that inadvertently worsens an already pathologically activated amygdala. In addition, the study lacked a sham control that mimics the somatosensory experience of TMS pulse delivery in the MRI scanner due to limitations in commercial hardware.
Future studies comparing the clinical effects of TMS delivered to the DLPFC-subgenual circuit compared to the VLPFC-amygdala circuit will help advance this area of study. Such an assessment will finally test whether improved and selective frontolimbic targeting can yield superior clinical outcomes and, if so, in which disorders. Alternatively, one of these circuits may be better suited for treating emotional dysregulation across many psychiatric disorders, which, to date, has been primarily tested by stimulating the DLPFC (7). Test-retest reliability of these measures will be important to characterize before performing target engagement studies. The issue of a sham control remains ever present in the neuropsychiatric use of TMS—single-pulse TMS in the scanner is not tolerated by all patients due to temporary headache or localized pain. Although it was not a limiting factor for the healthy participants in the present study, discomfort due to the procedure might affect some patients’ underlying amygdala activity and contribute to individual differences in tolerance and/or directionality of effects. The Sydnor study also relied on single-pulse TMS; whether the repetitive TMS more commonly used in therapeutic protocols scales appropriately remains to be demonstrated.
These caveats notwithstanding, this innovative work provides an important map to the future of precision TMS studies to target and induce top-down modulation of the amygdala. The field still needs to demonstrate that improved target engagement will yield treatments with superior outcomes. Yet, there is good reason for optimism; following this map, the amygdala appears to be in our reach, and with it the next generation of rationally designed studies of precision brain stimulation.
REFERENCES
- 1.Janak P. H., Tye K. M., From circuits to behaviour in the amygdala. Nature 517, 284–292 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.George M. S., Whither TMS: A one-trick pony or the beginning of a neuroscientific revolution? Am. J. Psychiatry 176, 904–910 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fox M. D., Buckner R. L., Liu H., Chakravarty M. M., Lozano A. M., Pascual-Leone A., Resting-state networks link invasive and noninvasive brain stimulation across diverse psychiatric and neurological diseases. Proc. Natl. Acad. Sci. U.S.A. 111, E4367–E4375 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sydnor V. J., Cieslak M., Duprat R., Deluisi J., Flounders M. W., Long H., Scully M., Balderston N. L., Sheline Y. I., Bassett D. S., Satterthwaite T. D., Oathes D. J., Cortical-subcortical structural connections support transcranial magnetic stimulation engagement of the amygdala. Sci. Adv. 8, eabn5803 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weigand A., Horn A., Caballero R., Cooke D., Stern A. P., Taylor S. F., Press D., Pascual-Leone A., Fox M. D., Prospective validation that subgenual connectivity predicts antidepressant efficacy of transcranial magnetic stimulation sites. Biol. Psychiatry 84, 28–37 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Philip N. S., Barredo J., van ‘t Wout-Frank M., Tyrka A. R., Price L. H., Carpenter L. L., Network mechanisms of clinical response to transcranial magnetic stimulation in posttraumatic stress disorder and major depressive disorder. Biol. Psychiatry 83, 263–272 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neacsiu A. D., Beynel L., Graner J. L., Szabo S. T., Appelbaum L. G., Smoski M. J., LaBar K. S., Enhancing cognitive restructuring with concurrent fMRI-guided neurostimulation for emotional dysregulation—A randomized controlled trial. J. Affect. Disord. 301, 378–389 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]