Abstract
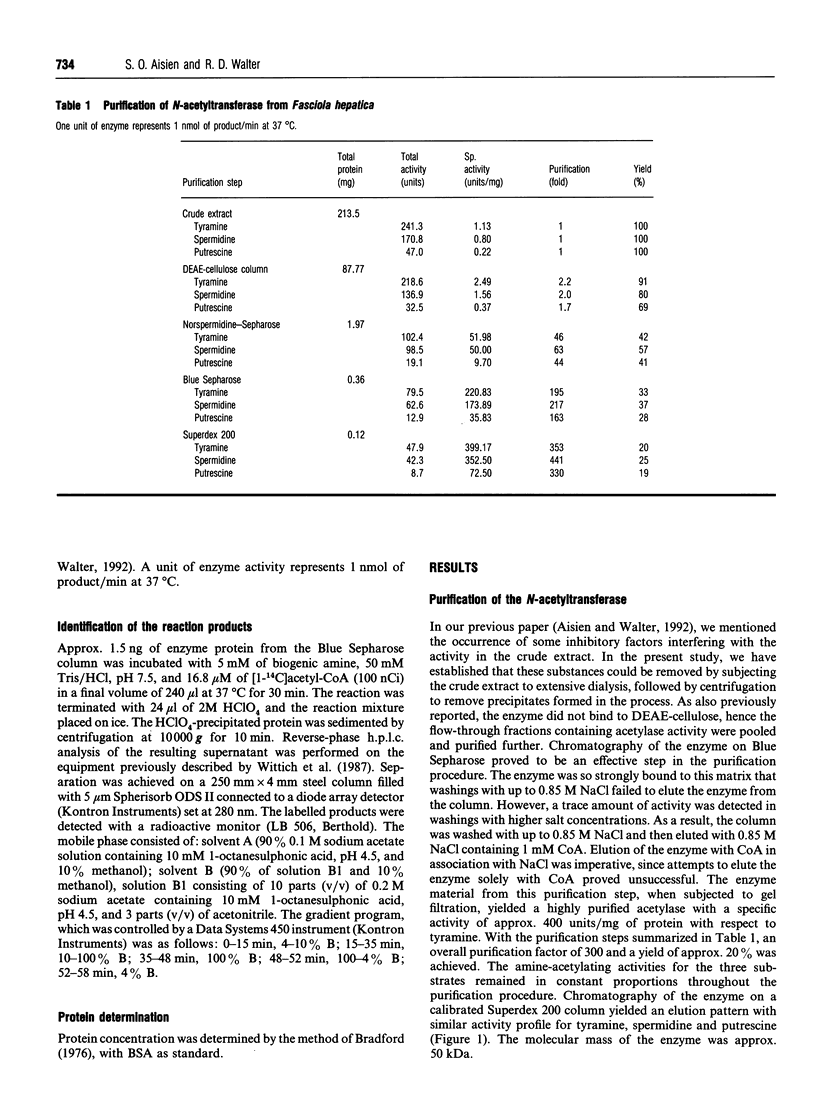
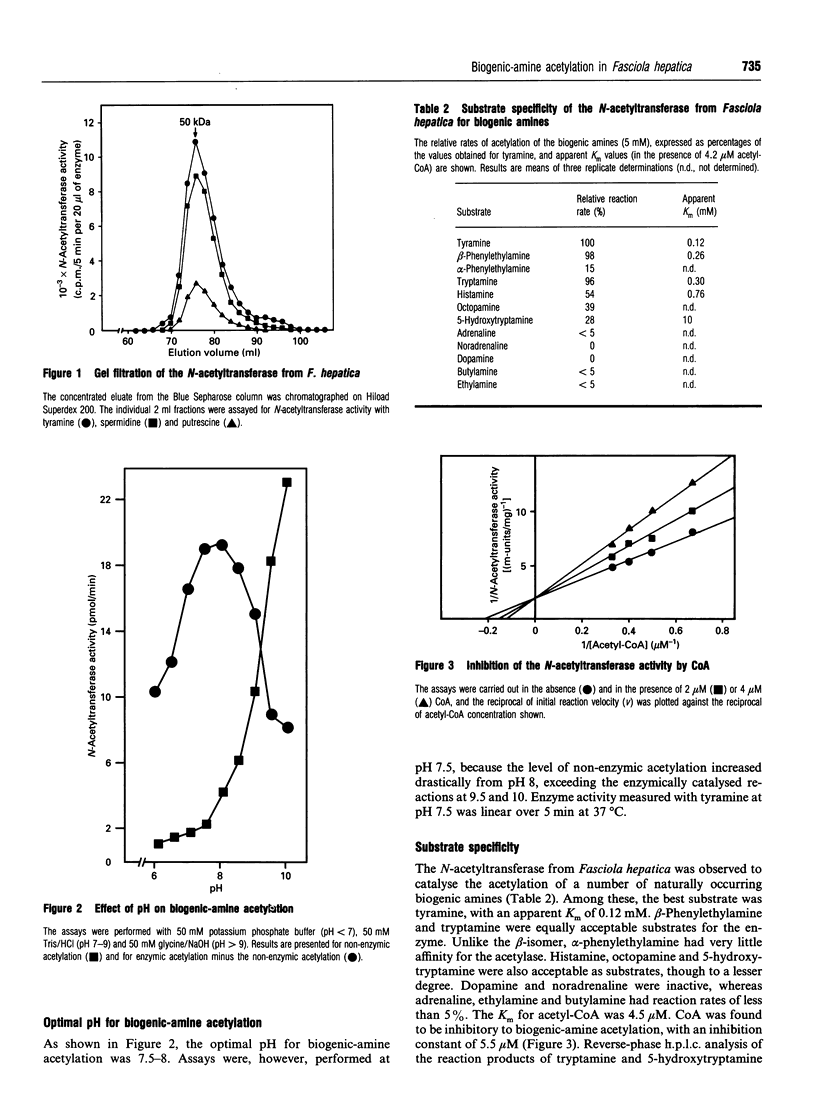
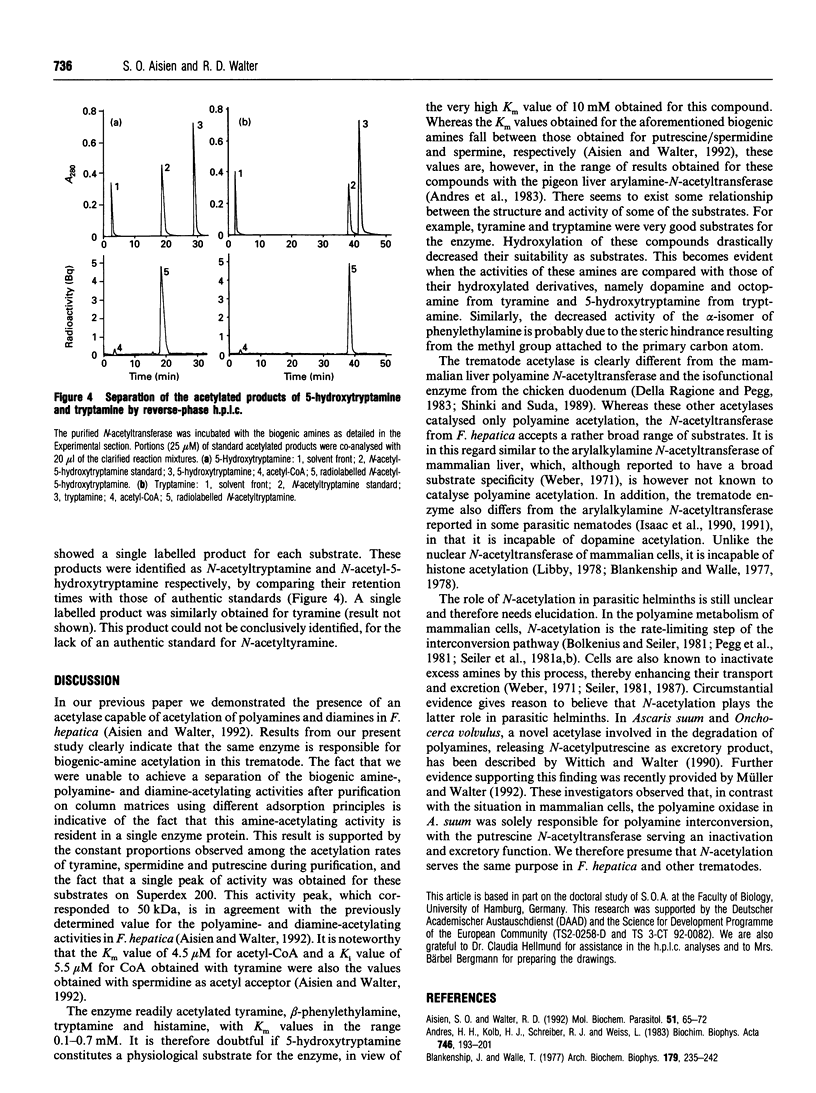
The previously described polyamine N-acetyltransferase from Fasciola hepatica has been observed to have an additional function, the acetylation of biogenic amines. The activities for biogenic amines, diamines and polyamines were in a constant ratio throughout the purification process. Biogenic amines found to be substrates for the enzyme included tyramine, tryptamine, beta-phenylethylamine and histamine, with Km values of 0.12 mM, 0.26 mM, 0.30 mM and 0.76 mM respectively. Octopamine, 5-hydroxytryptamine and alpha-phenylethylamine were also acceptable as substrates, though to a lesser degree. The optimum pH for biogenic-amine acetylation was 7.5, and CoA was inhibitory to the process, with a Ki of 5.5 microM. N-Acetylation appears to play a major role in the amine metabolism of this trematode. We presume that acetylation represents the process by which the parasite inactivates excess amines.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisien S. O., Walter R. D. Polyamine N-acetyltransferase from Fasciola hepatica. Mol Biochem Parasitol. 1992 Mar;51(1):65–72. doi: 10.1016/0166-6851(92)90201-t. [DOI] [PubMed] [Google Scholar]
- Andres H. H., Kolb H. J., Schreiber R. J., Weiss L. Characterization of the active site, substrate specificity and kinetic properties of acetyl-CoA:arylamine N-acetyltransferase from pigeon liver. Biochim Biophys Acta. 1983 Aug 16;746(3):193–201. doi: 10.1016/0167-4838(83)90074-2. [DOI] [PubMed] [Google Scholar]
- Blankenship J., Walle T. Acetylation of spermidine and spermine by rat liver and kidney chromatin. Arch Biochem Biophys. 1977 Feb;179(1):235–242. doi: 10.1016/0003-9861(77)90108-4. [DOI] [PubMed] [Google Scholar]
- Bolkenius F. N., Seiler N. Acetylderivatives as intermediates in polyamine catabolism. Int J Biochem. 1981;13(3):287–292. doi: 10.1016/0020-711x(81)90080-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Della Ragione F., Pegg A. E. Studies of the specificity and kinetics of rat liver spermidine/spermine N1-acetyltransferase. Biochem J. 1983 Sep 1;213(3):701–706. doi: 10.1042/bj2130701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaac R. E., Eaves L., Muimo R., Lamango N. N-acetylation of biogenic amines in Ascaridia galli. Parasitology. 1991 Jun;102(Pt 3):445–450. doi: 10.1017/s0031182000064428. [DOI] [PubMed] [Google Scholar]
- Isaac R. E., Muimo R., MacGregor A. N. N-acetylation of serotonin, octopamine and dopamine by adult Brugia pahangi. Mol Biochem Parasitol. 1990 Dec;43(2):193–198. doi: 10.1016/0166-6851(90)90144-b. [DOI] [PubMed] [Google Scholar]
- Libby P. R. Calf liver nuclear N-acetyltransferases. Purification and properties of two enzymes with both spermidine acetyltransferase and histone acetyltransferase activities. J Biol Chem. 1978 Jan 10;253(1):233–237. [PubMed] [Google Scholar]
- Müller S., Walter R. D. Purification and characterization of polyamine oxidase from Ascaris suum. Biochem J. 1992 Apr 1;283(Pt 1):75–80. doi: 10.1042/bj2830075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegg A. E., Matsui I., Seely J. E., Pritchard M. L., Pösö H. Formation of putrescine in rat liver. Med Biol. 1981 Dec;59(5-6):327–333. [PubMed] [Google Scholar]
- Seiler N., Bolkenius F. N., Knödgen B., Haegele K. The determination of N1-acetylspermine in mouse liver. Biochim Biophys Acta. 1981 Aug 5;676(1):1–7. doi: 10.1016/0304-4165(81)90002-7. [DOI] [PubMed] [Google Scholar]
- Seiler N., Bolkenius F. N., Rennert O. M. Interconversion, catabolism and elimination of the polyamines. Med Biol. 1981 Dec;59(5-6):334–346. [PubMed] [Google Scholar]
- Seiler N. Functions of polyamine acetylation. Can J Physiol Pharmacol. 1987 Oct;65(10):2024–2035. doi: 10.1139/y87-317. [DOI] [PubMed] [Google Scholar]
- Shinki T., Suda T. Purification and characterization of spermidine N1-acetyltransferase from chick duodenum. Eur J Biochem. 1989 Aug 1;183(2):285–290. doi: 10.1111/j.1432-1033.1989.tb14926.x. [DOI] [PubMed] [Google Scholar]
- WEISSBACH H., REDFIELD B. G., AXELROD J. The exzymic acetylation of serotonin and other naturally occurring amines. Biochim Biophys Acta. 1961 Nov 25;54:190–192. doi: 10.1016/0006-3002(61)90954-4. [DOI] [PubMed] [Google Scholar]
- Wittich R. M., Kilian H. D., Walter R. D. Polyamine metabolism in filarial worms. Mol Biochem Parasitol. 1987 Jun;24(2):155–162. doi: 10.1016/0166-6851(87)90102-2. [DOI] [PubMed] [Google Scholar]
- Wittich R. M., Walter R. D. A novel type of putrescine (diamine)-acetylating enzyme from the nematode Ascaris suum. Biochem J. 1989 May 15;260(1):265–269. doi: 10.1042/bj2600265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittich R. M., Walter R. D. Putrescine N-acetyltransferase in Onchocerca volvulus and Ascaris suum, an enzyme which is involved in polyamine degradation and release of N-acetylputrescine. Mol Biochem Parasitol. 1990 Jan 1;38(1):13–17. doi: 10.1016/0166-6851(90)90199-v. [DOI] [PubMed] [Google Scholar]


