Abstract
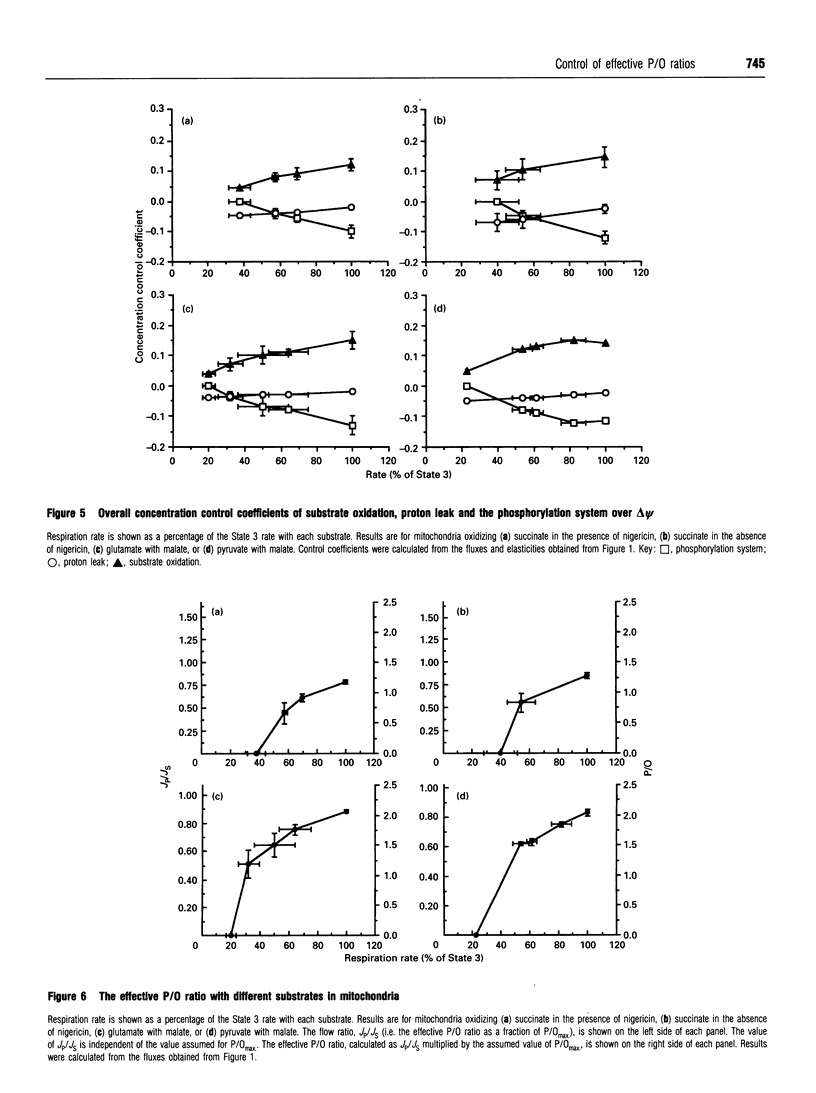
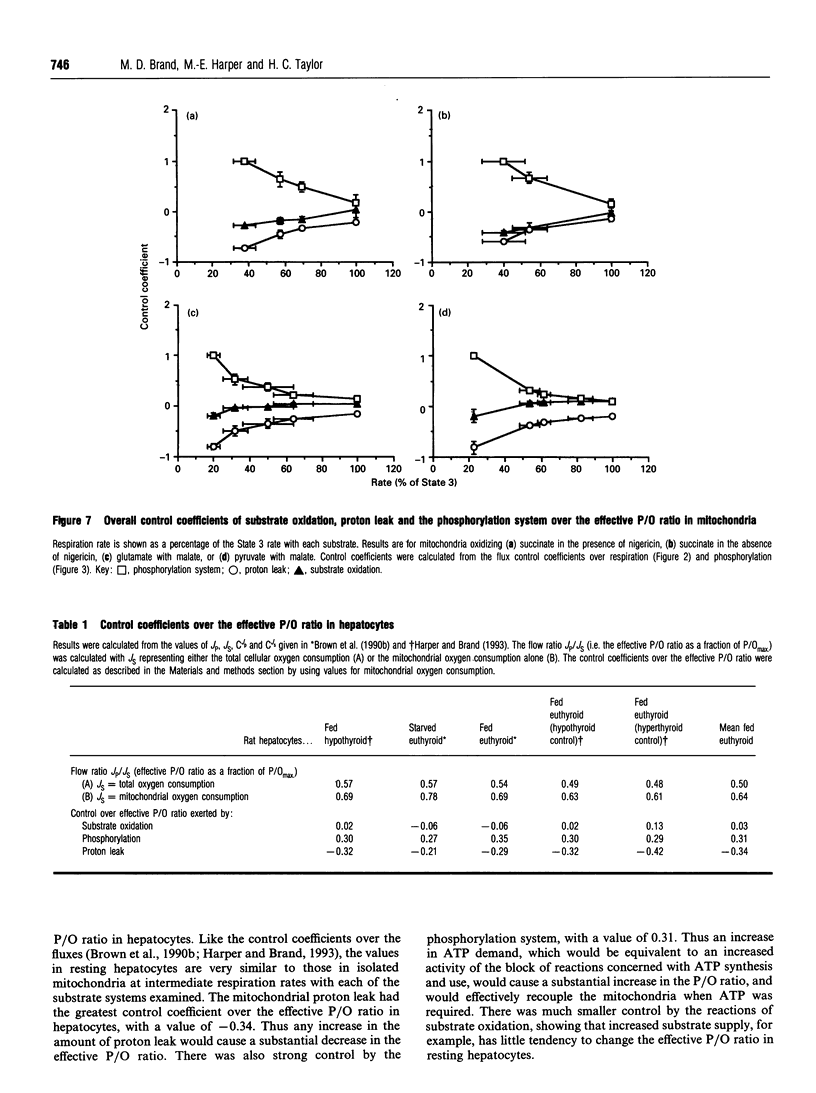
The control exerted by substrate oxidation reactions, by ATP turnover and by the proton leak over the oxygen consumption rate, the phosphorylation rate, the proton leak rate and the protonmotive force (delta p) in isolated rat liver mitochondria under a range of conditions between non-phosphorylating (State 4) and maximum phosphorylation (State 3) was investigated by using the top-down approach of metabolic control analysis. The experiments were carried out with saturating concentrations of the substrates succinate, glutamate with malate, or pyruvate with malate. The distribution of control was very similar with each of the three substrates. The effective P/O ratio (i.e. not corrected for leak reactions) was also measured; it varied from zero in State 4 to 80-90% of the maximum theoretical P/O ratio in State 3. Under most conditions control over the effective P/O ratio was shared between proton leak (which had negative control) and the phosphorylating subsystem (which had roughly equal positive control); near State 4, substrate oxidation reactions also acquired some control over this ratio. In resting hepatocytes the effective P/O ratio was only 50% of its maximum theoretical value, corresponding to an effective P/O ratio of only 1.3 for complete oxidation of glucose. The effective P/O ratio for intracellular mitochondrial oxygen consumption was 64% of the maximum value. The control coefficient of the mitochondrial proton leak over the effective P/O ratio in cells was -0.34; the control coefficient of phosphorylation reactions over this ratio was 0.31 and the control coefficient of substrate oxidation reactions over the ratio was 0.03, showing how the coupling efficiency in cells can respond sensitively to agents that change the proton leak or the ATP demand, but not to those that change substrate oxidation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brand M. D., Couture P., Else P. L., Withers K. W., Hulbert A. J. Evolution of energy metabolism. Proton permeability of the inner membrane of liver mitochondria is greater in a mammal than in a reptile. Biochem J. 1991 Apr 1;275(Pt 1):81–86. doi: 10.1042/bj2750081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand M. D., D'Alessandri L., Reis H. M., Hafner R. P. Stimulation of the electron transport chain in mitochondria isolated from rats treated with mannoheptulose or glucagon. Arch Biochem Biophys. 1990 Dec;283(2):278–284. doi: 10.1016/0003-9861(90)90643-d. [DOI] [PubMed] [Google Scholar]
- Brand M. D., Hafner R. P., Brown G. C. Control of respiration in non-phosphorylating mitochondria is shared between the proton leak and the respiratory chain. Biochem J. 1988 Oct 15;255(2):535–539. [PMC free article] [PubMed] [Google Scholar]
- Brand M. D., Murphy M. P. Control of electron flux through the respiratory chain in mitochondria and cells. Biol Rev Camb Philos Soc. 1987 May;62(2):141–193. doi: 10.1111/j.1469-185x.1987.tb01265.x. [DOI] [PubMed] [Google Scholar]
- Brand M. D. The proton leak across the mitochondrial inner membrane. Biochim Biophys Acta. 1990 Jul 25;1018(2-3):128–133. doi: 10.1016/0005-2728(90)90232-s. [DOI] [PubMed] [Google Scholar]
- Brown G. C., Brand M. D. Proton/electron stoichiometry of mitochondrial complex I estimated from the equilibrium thermodynamic force ratio. Biochem J. 1988 Jun 1;252(2):473–479. doi: 10.1042/bj2520473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. C., Brand M. D. Thermodynamic control of electron flux through mitochondrial cytochrome bc1 complex. Biochem J. 1985 Jan 15;225(2):399–405. doi: 10.1042/bj2250399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G. C., Hafner R. P., Brand M. D. A 'top-down' approach to the determination of control coefficients in metabolic control theory. Eur J Biochem. 1990 Mar 10;188(2):321–325. doi: 10.1111/j.1432-1033.1990.tb15406.x. [DOI] [PubMed] [Google Scholar]
- Brown G. C., Lakin-Thomas P. L., Brand M. D. Control of respiration and oxidative phosphorylation in isolated rat liver cells. Eur J Biochem. 1990 Sep 11;192(2):355–362. doi: 10.1111/j.1432-1033.1990.tb19234.x. [DOI] [PubMed] [Google Scholar]
- Duszyński J., Bogucka K., Wojtczak L. Homeostasis of the protonmotive force in phosphorylating mitochondria. Biochim Biophys Acta. 1984 Dec 18;767(3):540–547. doi: 10.1016/0005-2728(84)90053-7. [DOI] [PubMed] [Google Scholar]
- Fell D. A. Metabolic control analysis: a survey of its theoretical and experimental development. Biochem J. 1992 Sep 1;286(Pt 2):313–330. doi: 10.1042/bj2860313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groen A. K., Wanders R. J., Westerhoff H. V., van der Meer R., Tager J. M. Quantification of the contribution of various steps to the control of mitochondrial respiration. J Biol Chem. 1982 Mar 25;257(6):2754–2757. [PubMed] [Google Scholar]
- Hafner R. P., Brand M. D. Effect of protonmotive force on the relative proton stoichiometries of the mitochondrial proton pumps. Biochem J. 1991 Apr 1;275(Pt 1):75–80. doi: 10.1042/bj2750075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafner R. P., Brown G. C., Brand M. D. Analysis of the control of respiration rate, phosphorylation rate, proton leak rate and protonmotive force in isolated mitochondria using the 'top-down' approach of metabolic control theory. Eur J Biochem. 1990 Mar 10;188(2):313–319. doi: 10.1111/j.1432-1033.1990.tb15405.x. [DOI] [PubMed] [Google Scholar]
- Hafner R. P., Nobes C. D., McGown A. D., Brand M. D. Altered relationship between protonmotive force and respiration rate in non-phosphorylating liver mitochondria isolated from rats of different thyroid hormone status. Eur J Biochem. 1988 Dec 15;178(2):511–518. doi: 10.1111/j.1432-1033.1988.tb14477.x. [DOI] [PubMed] [Google Scholar]
- Heinrich R., Rapoport T. A. A linear steady-state treatment of enzymatic chains. General properties, control and effector strength. Eur J Biochem. 1974 Feb 15;42(1):89–95. doi: 10.1111/j.1432-1033.1974.tb03318.x. [DOI] [PubMed] [Google Scholar]
- Hinkle P. C., Kumar M. A., Resetar A., Harris D. L. Mechanistic stoichiometry of mitochondrial oxidative phosphorylation. Biochemistry. 1991 Apr 9;30(14):3576–3582. doi: 10.1021/bi00228a031. [DOI] [PubMed] [Google Scholar]
- Hinkle P. C., Yu M. L. The phosphorus/oxygen ratio of mitochondrial oxidative phosphorylation. J Biol Chem. 1979 Apr 10;254(7):2450–2455. [PubMed] [Google Scholar]
- Kacser H., Burns J. A. MOlecular democracy: who shares the controls? Biochem Soc Trans. 1979 Oct;7(5):1149–1160. doi: 10.1042/bst0071149. [DOI] [PubMed] [Google Scholar]
- Kesseler A., Diolez P., Brinkmann K., Brand M. D. Characterisation of the control of respiration in potato tuber mitochondria using the top-down approach of metabolic control analysis. Eur J Biochem. 1992 Dec 15;210(3):775–784. doi: 10.1111/j.1432-1033.1992.tb17480.x. [DOI] [PubMed] [Google Scholar]
- Kholodenko B. N., Millazhane V. Iu, Borutaite V. I., Ivanovene L. I., Iuodkaite R. A., Katiliute Z. P., Toleikis A. N. Reguliatsiia skorosti dykhaniia v mitokhondriiiakh serdtsa. Sravnenie okisleniia suktsinata i NAD-zavisimykh substratov. Biokhimiia. 1991 Aug;56(8):1420–1428. [PubMed] [Google Scholar]
- Moreno-Sánchez R., Devars S., López-Gómez F., Uribe A., Corona N. Distribution of control of oxidative phosphorylation in mitochondria oxidizing NAD-linked substrates. Biochim Biophys Acta. 1991 Nov 7;1060(3):284–292. doi: 10.1016/s0005-2728(05)80318-4. [DOI] [PubMed] [Google Scholar]
- Nobes C. D., Brown G. C., Olive P. N., Brand M. D. Non-ohmic proton conductance of the mitochondrial inner membrane in hepatocytes. J Biol Chem. 1990 Aug 5;265(22):12903–12909. [PubMed] [Google Scholar]
- Nobes C. D., Hay W. W., Jr, Brand M. D. The mechanism of stimulation of respiration by fatty acids in isolated hepatocytes. J Biol Chem. 1990 Aug 5;265(22):12910–12915. [PubMed] [Google Scholar]
- Stoner C. D. Determination of the P/2e- stoichiometries at the individual coupling sites in mitochondrial oxidative phosphorylation. Evidence for maximum values of 1.0, 0.5, and 1.0 at sites 1, 2, and 3. J Biol Chem. 1987 Aug 5;262(22):10445–10453. [PubMed] [Google Scholar]
- Tager J. M., Wanders R. J., Groen A. K., Kunz W., Bohnensack R., Küster U., Letko G., Böhme G., Duszynski J., Wojtczak L. Control of mitochondrial respiration. FEBS Lett. 1983 Jan 10;151(1):1–9. doi: 10.1016/0014-5793(83)80330-5. [DOI] [PubMed] [Google Scholar]


