Abstract
As the opposite ends of the orodigestive tract, the oral cavity and the intestine share anatomical, microbial, and immunological ties that have bidirectional health implications. A growing body of evidence suggests an interconnection between oral pathologies and inflammatory bowel disease [IBD], implying a shift from the traditional concept of independent diseases to a complex, reciprocal cycle. This review outlines the evidence supporting an ‘oral–gut’ axis, marked by a higher prevalence of periodontitis and other oral conditions in IBD patients and vice versa. We present an in-depth examination of the interconnection between oral pathologies and IBD, highlighting the shared microbiological and immunological pathways, and proposing a ‘multi-hit’ hypothesis in the pathogenesis of periodontitis-mediated intestinal inflammation. Furthermore, the review underscores the critical need for a collaborative approach between dentists and gastroenterologists to provide holistic oral–systemic healthcare.
Keywords: Periodontitis, inflammatory bowel disease [IBD], oral bacteria, oral–gut axis, gum–gut axis, immune response, dysbiosis, ulcerative colitis, Crohn’s disease
1. Introduction
Oral pathologies can profoundly impact general health; however, oral health and general health are often incorrectly perceived as separate entities.1,2 Over the past four decades, several studies have highlighted the connection between the oral cavity and the rest of the body, linking gingival and periodontal inflammation with more than 50 systemic conditions, including inflammatory bowel diseases [IBD].1–7 A bidirectional relationship exists between periodontitis and IBD, where microbial and inflammatory changes originating in either the oral cavity or intestinal tissues can influence each other.3,8–33 The growing body of literature associating periodontitis with gastrointestinal [GI] disorders such as IBD and colorectal cancer has seen a remarkable surge in recent years, with over 100 relevant articles published since 2020. This review aims to augment current knowledge by providing an in-depth analysis of the underlying mechanisms contributing to the bidirectional relationship between oral pathologies and IBD, with a particular emphasis on periodontitis. We utilize the ‘oral–gut axis’ as a framework to investigate the reciprocal relationship between the oral cavity and the GI tract. This narrative review encapsulates historical studies on oral manifestations in IBD, and explores recent advances, current knowledge gaps, and potential future directions. Furthermore, the review underscores the critical need for a collaborative approach between dentists, gastroenterologists, immunologists, and infectious disease experts/microbiologists to provide holistic oral–systemic healthcare.
2. Periodontitis
Periodontitis is a chronic inflammatory disease that affects the periodontium, encompassing tooth-supporting structures such as the gingiva, alveolar bone, and periodontal ligament.7,34–36 It is ranked as the 11th most prevalent condition worldwide, affecting ~50% of adults >30 years of age and ~70.1% of adults >65 years of age.37–41 The hallmark symptoms of periodontitis are gingival inflammation, gingival recession, deep periodontal pockets, clinical attachment loss, and tooth mobility.7,34–36 If left untreated, periodontitis results in tooth loss, and is the leading cause of tooth loss in adults worldwide.36,37,42,43
Periodontitis is primarily initiated by the accumulation of bacterial plaque on tooth surfaces or within the gingival sulcus, which subsequently triggers an inflammatory response.35,44 The host’s hyper-immunoinflammatory response, in turn, leads to the destruction of tooth-supporting structures.45–47 The bacterial plaque/calculus associated with the initiation of periodontitis specifically contains bacteria, such as Porphyromonas gingivalis, Tannerella forsythia, Treponema denticola, Aggregatibacter actinomycetemcomitans, Prevotella intermedia, Fusobacterium nucleatum, and Campylobacter rectus.44–46,48–52 Periodontitis is a polymicrobial disease primarily driven by bacteria; however, fungi, protozoa, and viruses might also contribute to its pathogenesis.35,53,54
Several risk factors have been associated with periodontitis, including poor oral hygiene, smoking, diabetes, advanced age, genetics, and certain medications.7,55–57 The prognosis depends on disease severity, patient compliance with treatment, and control of risk factors.58 Through dissemination of oral pathogens and promotion of low-grade systemic inflammation, periodontitis may contribute to other systemic diseases such as IBD, cardiovascular disease, diabetes, and pre-term birth.27,59–63
3. Inflammatory Bowel Disease
IBD, encompassing Crohn’s disease [CD] and ulcerative colitis [UC], refers to a group of chronic, idiopathic, and recurring inflammatory disorders that affect the GI tract.64–66 The clinical manifestations of IBD vary depending on the location and severity of inflammation within the GI tract. CD is characterized by patchy inflammation that can affect the full thickness of the bowel wall throughout the small and large intestines.66–69 There may be areas of healthy tissue between inflamed sections [skip lesions]. In contrast, UC presents with continuous, uniform inflammation limited to the mucosal layer, extending from the rectum to throughout the colon.66–70 Common symptoms include abdominal pain, cramping, persistent diarrhoea, nausea, vomiting, weight loss, fatigue, and fever.64–66,71 UC is more likely to cause bloody diarrhoea, urgency to defecate, and tenesmus.70–73 Approximately 25–40% of UC and CD patients may exhibit extraintestinal manifestations such as arthritis, erythema nodosum, pyoderma gangrenosum, oral lesions [discussed in detail in later sections], uveitis, hepatic steatosis, primary sclerosing cholangitis, and metabolic bone disease.74–91 Both UC and CD patients are at a higher risk of developing colorectal cancer, although the risk is greater in UC than in CD.92,93 IBD patients experience a diminished quality of life and have a lower life expectancy compared to the general population.94,95
Between 1990 and 2017, the global incidence of IBD surged by 31%, affecting around 6.8 million people worldwide and becoming a major public health concern.96–99 The incidence of IBD varies, with highest rates observed in North America, Western Europe, and Australia.98,99 The prevalence of IBD in the USA is estimated to be over 1.6 million, with 780 000 diagnosed with CD and 907 000 with UC.97,100,101
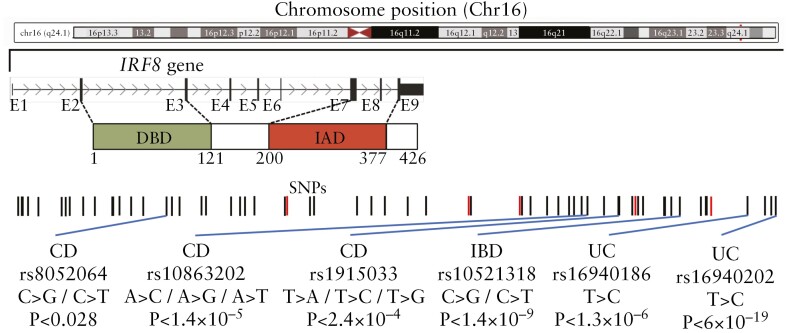
The aetiology of IBD remains largely unclear, but it is believed to result from a complex interplay between genetic, environmental and, microbial factors, and the host immune responses.102–107 Over 200 genetic loci related to immune regulation, intestinal barrier function, and other biological pathways have been associated with IBD, suggesting a strong genetic influence.102,108–113 Notable genetic loci include NOD2 [one of the first genetic loci identified as a risk factor for CD], IL23R, ATG16L1, IRGM, PTGER4, TNFSF15, and IRF8.102,108–113 Previously, our group and others identified several single nucleotide polymorphisms [SNPs] located 1.7–60 kb downstream of IRF8 as risk factors for UC and CD111,112,114–124 [Figure 1]. IRF8 SNPs are significantly associated with CD in Ashkenazi Jews, who have a 4- to 7-fold higher incidence of CD than non-Ashkenazi European Jews.120,125 IRF8 is expressed in the intestinal epithelial cells [IECs] in a gradient pattern, with highest expression in the differentiated cell zones and low levels in the proliferating and stem cell zones.126,127 IRF8 functions as a suppressor of colonic inflammation, and its deficiency in IECs disrupts the epithelial barrier, contributing to colitis-related colon tumorigenesis in both humans and mice.126,128,129 Beyond IECs, IRF8 is crucial for the development and maturation of dendritic cells and macrophages,130–134 key to immune surveillance in the gut. IRF8 deficiency causes conventional type 1 dendritic cells (cDC1) cells to acquire cDC2-like characteristics, impacting their antigen presentation and immune responses to bacterial antigens.135,136 Additionally, IRF8 regulates Class I and Class II MHC machinery, balancing pro-inflammatory and anti-inflammatory cytokine production.134,137,138 IRF8 also regulates pathogen-associated molecular pattern [PAMP] receptors,137,138 crucial for microbial recognition and gut defence. Consequently, IRF8 deficiency could disrupt gut immune homeostasis, leading to impaired responses to intestinal microbiota and exacerbating the dysbiosis and abnormal immune reactions associated with IBD. In mice, IRF8 deficiency promotes colitis mediated by Th17 and Tfh cells128,129 and impairs gastric innate immunity against infection.139 Dysregulation of IRF/IFN-I signalling is extensively involved in IBD pathogenesis and targeting this pathway represents a promising intervention strategy for IBD.140 Additionally, we have shown that IRF8 is also critical for periodontal homeostasis.141–143 Collectively, these findings highlight the importance of IRF8 in intestinal health and establish the critical value of Irf8-deficient mice in providing novel insights into human GI diseases.
Figure 1.
Schematic of IRF8 gene structure with previously reported IBD single nucleotide polymorphisms [SNPs], genotypic changes, and p values.
Some of the identified risk factors for IBD include: [a] family history—individuals with a first-degree relative with IBD face a higher risk of developing the condition144,145; [b] age—IBD is more commonly diagnosed in individuals under the age of 30 years, although it can occur at any age. Historically believed to be a disease of children and young adults [20–30 years], it is now recognized that 10–15% of patients develop IBD after the age of 60 years [older adults].146–150 The cause of older-onset IBD is unclear, but it is thought to be less associated with genetics and more influenced by age-related changes in the immune system and gut microbiome146,151–154. [c] Ethnicity—IBD is more common in Caucasians and people of Ashkenazi Jewish descent120,125,144,155,156; and [d] smoking—cigarette smoking increases the risk of CD and may exacerbate its progression, while it appears to have a protective effect against UC.157,158
The course of IBD varies, with some experiencing periods of remission while others endure frequent relapses. IBD may lead to complications such as strictures, fistulas, or colorectal cancer.64–71 Disease severity and treatment response significantly influence prognosis. Currently, there is no cure for IBD, and the primary management focuses on resolving inflammation, preventing flare-ups and other complications, and improving quality of life.159–161
4. Oral Manifestations of IBD
Extraintestinal manifestations of IBD, which can affect nearly all organ systems, are common in both UC and CD.74–79 Approximately 10–30% of IBD patients exhibit oral manifestations of the disease, which can precede, coincide with, or follow the onset of GI symptoms.80–82,87,88,91 Commonly observed oral manifestations include aphthous ulcers, mucosal tags, cobblestoned oral mucosa, pyostomatitis vegetans, gingivitis, periodontitis, angular cheilitis, and oral lichen planus.63,80–91,162–164 Additionally, some individuals may present with halitosis, atrophic glossitis, burning mouth syndrome, and xerostomia.80–91 Some of these lesions are more common in CD versus UC, and more frequently noted in children versus adults.164
Although some IBD patients exhibit oral manifestations, the correlation is not universal. Epidemiological studies reporting a higher incidence of oral lesions in IBD patients often lack proper control groups, making it difficult to ascertain if the incidence of oral lesions is indeed increased in IBD patients beyond the general population. For example, aphthous ulcers affect 20–25% of the general population165,166 and it is unclear if IBD patients experience a higher incidence of aphthous ulcers than the general population. Additionally, it is difficult to determine precisely which oral manifestations are related to IBD, and which are related to IBD therapy or other aetiologies such as nutritional deficiencies and malabsorption. It is logical to hypothesize that some of these lesions are in fact direct consequences of the disease or a secondary reaction to IBD treatment. Furthermore, oral granulomas can also result from exposure to dental materials, including retained amalgams or endodontic sealers.167 Therefore, potential confounders must be meticulously excluded prior to establishing a causative link between oral lesions and IBD.
The aetiology of oral manifestations in IBD is complex and not fully understood; however, it is believed to involve a combination of genetic predisposition, immune dysregulation, and alterations in the oral microbiome.80–91 Several studies underscore the role of cytokine activity in both the GI and oral regions. Elevated levels of IL-6, IL-8, IL-1β, TNF-α, and MCP-1 have been noted in saliva and gingival tissues of patients with active IBD versus non-active IBD or healthy controls.20,168–170 Higher levels of CXCL-9 and -10 have been identified in the buccal mucosal of children with CD compared to healthy children and adult CD patients.171 Mutations in IL-10RA and IL-10RB, which disrupt the IL-10/STAT3 cascade, have been associated with paediatric IBD.172,173 Patients deficient in IL-10 and IL-10R exhibit recurrent aphthous ulcers and subtle immunological abnormalities, including decreased CD4+/CD8+ T-cell ratios, dysregulated serum immunoglobulin levels, and dysregulated NK, B, or T cells.174,175 Higher concentrations of activated matrix metalloproteinase-8 [MMP-8] have been noted in the gingival crevicular fluid [GCF] of IBD patients compared to healthy controls.176 Also, decreased IL-4 levels and elevated serum IL-18 levels have been noted in UC patients, with the latter positively correlated with IL-1β in the GCF from deeper pockets.177 Likewise, a significant elevation in IL-1β concentration has been noted in GCF, alongside increased TNF-α and reduced IL-10 levels in the saliva of UC patients diagnosed with periodontitis.178 Despite these findings, the contribution of these inflammatory cytokines and immune cells to the development of oral lesions in IBD patients remains unclear, necessitating further investigations.
Conversely, IBD may immunologically trigger oral manifestations, partially due to the recognition of common epitopes throughout the body. The extraintestinal manifestations of IBD could stem from a broad adaptive immune response triggered by local intestinal dysbiosis, leading to recognition of these epithelial epitopes in other organs and the oral cavity, ultimately causing extraintestinal pathologies.78,179–181 A molecular similarity between gut microbiota antigens and non-microbial epitopes present on cells in the oral cavity could potentially lead to immune cross-reactivity.182,183 Furthermore, in IBD, local immune responses to gut dysbiosis may initiate systemic T cell-mediated responses and cytokine production,184,185 which could potentially lead to induction of oral lesions. In response to antigenic stimulation, T cells can migrate to the oral mucosa, where CD8+ T lymphocytes, accompanied by infiltrating macrophages and neutrophils, may cause epithelial damage and ulceration commonly observed in pyostomatitis vegetans.186 Overexpression of pro-inflammatory cytokines such as IL-6, IL-8, and TNF-α in pyostomatitis vegetans can potentially lead to the recruitment of inflammatory cells to UC lesions, thus synergistically contributing to the pro-inflammatory pathogenesis of pyostomatitis vegetans and IBD.186 Collectively, these studies suggest that immune system dysregulation may be an important link bridging the oral cavity and the intestines.
Several studies have suggested that oral microbial dysbiosis, and secondary inflammatory responses within the gut, might potentially contribute to oral manifestations in IBD.78,87,187,188 Remarkably, the periodontal inflammation in IBD does not appear to correlate with plaque accumulation, giving rise to the notion that systemic inflammation associated with IBD could induce alterations in the oral microbiome, consequently intensifying oral inflammation.189,190 An overabundance of specific oral bacteria, including Streptococcus, Prevotella, Veillonella, and Haemophilus, in the oral cavity is linked to inflammatory responses triggered by reduced salivary lysozyme and increased IL-1β levels, which may be associated with gut microbial dysbiosis.20 These changes are connected to heightened oxidative stress and virulence [e.g. metabolism of terpenoids, polyketides, carbohydrates, and lipids, and biosynthesis of secondary metabolites], enzyme activity [e.g. protein kinases], bacterial aggression [e.g. bacterial toxins and invasion of epithelial cells], and apoptosis in the oral region, implying a connection between oral microbial dysbiosis and IBD.15 Altered salivary lysozyme levels could be related to gut imbalance and subsequent periodontitis development.191 Furthermore, intestinal dysbiosis has been linked to the chronic inflammatory state and activation of gut-associated lymphoid tissue [GALT],192,193 which could potentially lead to extraintestinal pathologies.194 The proven effectiveness of adjuvant probiotic therapy in treating oral ulcers supports the hypothesis that oral ulcers in IBD might result from a combination of intestinal dysbiosis and other factors, such as oral mucosa microtraumas.195 Collectively, these findings indicate a possible correlation between oral and gut microbial dysbiosis and the occurrence of oral manifestations in IBD.
5. Historical Perspective of Oral–Gut Interconnection
Over the years, numerous case reports have contributed to our understanding of oral and gut interconnection. The first report of oral involvement in IBD, by Dudeney and Todd in 1969, described a CD patient who developed granulomatous lesions in the left buccal mucosa 16 years after the initial diagnosis.196 In 1972, Bottomely et al. documented a case of a teenage girl with CD who exhibited gingival hyperplasia across maxillary anterior teeth, with 5–6-mm ‘pseudopockets’ indicative of a severe hyperplastic phenotype that worsened periodically.197 In 1972, Croft et al. retrospectively analysed 332 CD patients and found that 6.1% had developed oral ulcers at some point during their illness.198 In 1975, a systematic study by Asquith et al. involving 100 CD patients, 100 UC patients, and 100 healthy controls matched for age, sex, and denture status reported that 9% of CD and 2% of UC patients developed oral lesions with macroscopic and histological features similar to those in the GI tract.187 Additionally, they noted salivary IgA production was reduced in CD patients with active bowel disease.187 In 1982, Lamster et al. analysed polymorphonuclear leukocytes from 30 patients with active or inactive IBD and reported that those with active IBD had higher levels of circulating immune complex activity and their peripheral neutrophils exhibited greater metabolic activity compared to inactive IBD or healthy controls.199 Additionally, a higher prevalence of oral pathologies was observed among subjects with active IBD. In 1986, Van Dyke et al. evaluated 20 IBD patients with and without periodontal disease and found that the periodontal flora of IBD patients predominantly consisted of small, motile, gram-negative rods, closely related to the genus Wolinella.200 All 10 IBD subjects with periodontal disease exhibited serum-mediated defects in neutrophil chemotaxis, while neutrophil phagocytosis was normal. Additionally, PGE2 levels in GCF from IBD patients with periodontal disease were 4-fold higher than in adult periodontitis patients without IBD.200
Building upon these initial case reports, cross-sectional studies were designed to explore the positive correlation between IBD and periodontitis-afflicted individuals. In 1991, Flemmig et al. examined the periodontal status of 107 IBD patients and identified a 12% higher prevalence of periodontitis but a 0.6-mm lower clinical attachment loss in IBD patients when compared to the general US adult population.201 In 2006, Grössner-Schreiber et al. examined 62 IBD patients and 59 healthy controls and found that IBD patients exhibited a significantly higher incidence of caries, but no distinct differences in periodontal findings.202 In contrast, in 2008 Brito et al. examined 99 CD patients, 80 UC patients, and 74 healthy controls and found a significantly higher prevalence of periodontitis in UC [90%] and CD [82%] patients when compared to healthy controls [68%].162 Altogether, these studies provide some of the earliest evidence for the oral–gut axis, highlighting the potential connection between periodontitis and IBD. Over the next several years, similar studies have been published expanding our knowledge about periodontitis–IBD interconnection. Table 1 summarizes recent studies demonstrating associations between periodontitis and IBD.
Table 1.
Studies demonstrating an association between periodontitis and IBD
| Study [reference number] | IBD [n] | Non-IBD [n] | Periodontitis-IBD associations | 95% CI |
|---|---|---|---|---|
| Grossner-Schreiber et al. 2006202 | IBD [62] | [59] | IBD [RR = 2.47] CAL ≥ 5 mm |
1.02–5.99 |
| Habashneh et al. 2012203 | CD [59] UC [101] |
[100] | CD [OR = 4.9] UC [OR = 7.0] |
1.8–13.2 2.8–17.5 |
| Vavricka et al. 2013163 | CD [69] UC [44] |
[113] | CD [OR = 3.91] UC [OR = 3.94] |
1.78–8.57 1.64–9.46 |
| Chi et al. 2018204 | CD [6657] | [26 628] | [HR = 1.36] | 1.25–1.48 |
| Yu et al. 2018205 | CD [7] UC [20] |
[108] | IBD [HR = 1.82] CD [HR = 3.95] UC [HR = 1.39] |
1.09–3.03 1.59–9.82 0.69–2.46 |
| Zhang et al. 2020206 | CD [265] UC [124] |
[265] | CD [OR = 4.46] UC [OR = 4.66] |
2.50–7.95 2.49–8.71 |
| Bertl et al. 2022207 | IBD [1108] | [3429] | CD [OR = 1.74] UC [OR = 2.57] |
1.36–2.24 1.99–3.32 |
| Wang et al. 2023208 | IBD [12 882] | [21 770] | IBD [OR = 1.06] | 1.01–1.12 |
| Baima et al. 202363 | CD [117] UC [60] |
[180] | IBD [OR = 4.48] CD [OR = 4.01] UC [OR = 4.43] |
2.30–8.73 1.92–8.38 1.66–11.81 |
IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis; OR, odds ratio; HR, hazard ratio; RR, relative risk; CI, confidence interval; CAL, clinical attachment loss.
Currently, limited evidence suggests that IBD treatment can improve periodontal disease outcomes, and vice versa, hinting that both diseases might share microbiological and immunoinflammatory pathways. IBD patients treated with anti-TNF-α biologics experienced rapid healing of apical periodontitis compared to controls.209 Corticosteroids, which are often used in IBD management, may confer a protective effect against periodontitis.204 In UC patients responsive to treatment with biologics, salivary levels of IgA and MPO increased significantly, suggesting that successful UC therapy may also boost oral defence mechanisms.210 Furthermore, biologics used to treat other chronic inflammatory diseases appear to restrict periodontitis progression and enhance the healing response to periodontal treatment.211 Conversely, periodontitis treatment with mesenchymal stem cell-derived exosomes has been shown to attenuate experimental colitis in murine models.212 Clinical observations suggest that periodontal therapy can reduce intestinal levels of Enterobacteriaceae and Porphyromonadaceae, enriching Lachnospiraceae, in patients with gingivitis or mild/moderate periodontitis.213 Similarly, our preliminary data [unpublished] indicate that in cases of advanced periodontitis [stage III/stage IV], periodontal therapy markedly decreases the intestinal abundance of Bacteroides, Faecalibacterium, and Lachnospiraceae. Furthermore, de Oliveira et al. demonstrated that in periodontitis patients who are systemically healthy, subgingival instrumentation caused an increase in intestinal levels of Actinobacteria, with a concurrent decrease in Bacteroidetes and Verrucomicrobia populations.214 These results underscore a relationship between the oral cavity and the gut microbiota that can be modulated by changing the oral milieu with periodontal therapy. However, the full therapeutic implications of these changes on IBD and the usefulness of assaying oral secretions as a potential biomarker for IBD requires further investigation. Taken together, these findings indicate that the immune-modulating effect of certain drugs may not only improve IBD outcomes, but also reduce inflammation within periodontal tissues. Moreover, periodontitis treatment could influence gut microbiota. Nonetheless, it is crucial to note that medications used in IBD management that result in immunosuppression, such as corticosteroids, thiopurines, anti-TNF-α, Janus kinase inhibitors, and other biologics, could increase the risk of opportunistic infections in the oral cavity.89
6. A Multi-hit Model of Periodontitis-Mediated IBD Pathogenesis
Every day, the average adult generates and ingests ~1.5 L of saliva, which contains ~1.5 × 1012 oral bacteria.28,215–218 More than 99% of these bacteria are inactivated as they pass through the stomach.218,219 In healthy individuals, several defence mechanisms prevent ingested oral bacteria from colonizing the gut and promoting IBD. These include: [a] the physical barrier provided by the mucus layer and IECs,220 [b] bacterial clearance by mucus,221 [c] competition between resident gut microbes and exogenous microbes invading from the mouth,222,223 and [d] neutralization of oral bacteria by gastric acid and pepsin.224,225 However, when these defence mechanisms are compromised by factors such as genetics, systemic disorders, medications, lifestyle, or ageing, oral microbes can potentially infiltrate the intestine and trigger immune responses, leading to IBD development.3,8,223,226–232
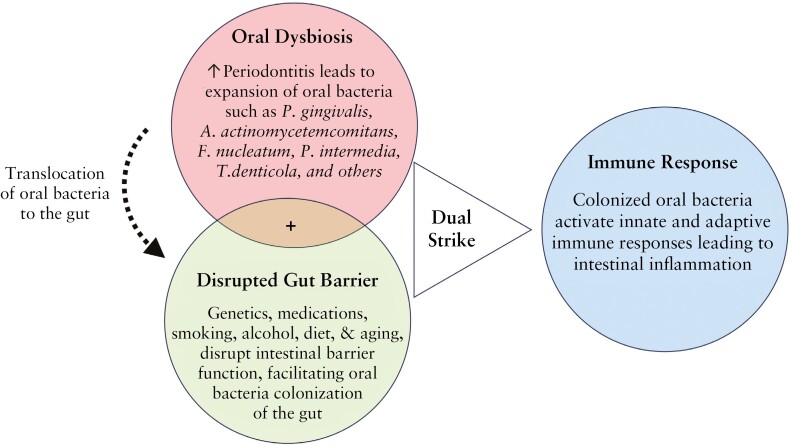
The mucus layer rich in antimicrobial peptides and secretory immunoglobulins prevents direct contact between oral pathogens and the intestinal epithelium, creating a hostile environment for potential invaders.233,234 Conditions such as cystic fibrosis, stress, infection, an unhealthy diet, and ageing can disrupt the mucus layer, facilitating bacterial penetration.153,235–237 Beneath the mucus, the IEC barrier, fortified by occludins, claudins, and other junction proteins, provides another layer of protection.238 Disruption of the IEC barrier can result in a ‘leaky’ gut, permitting microorganisms and intestinal contents to infiltrate the mucosal barrier and initiate inflammation.239 Further, the resident commensal gut microbiota resist exogenous oral pathogen colonization through nutrient competition and niche occupation.222,240,241 These microbes ferment dietary fibres, producing short-chain fatty acids [SCFAs] that serve as an energy source for colonocytes and exhibit anti-inflammatory properties.242 However, a shift in microbial equilibrium, or dysbiosis, frequently triggered by antibiotic overuse or dietary changes, can exacerbate IBD risk.243 Chemical barriers, including gastric acid and pepsin in the stomach, help thwart opportunistic pathogens.224,225 However, hypochlorhydria or prolonged use of proton pump inhibitors disrupt the production of gastric acid and pepsin, which may predispose individuals to bacterial overgrowth and infections such as from Clostridium difficile.244 Collectively, the compromised mucus layer and epithelial barriers, coupled with a dysbiotic gut microbiota and heightened immune responses, can increase the risk for IBD. Based on these findings, we propose a multi-hit model to illustrate how periodontitis and related oral bacteria may enhance the susceptibility to IBD [Figure 2].
Figure 2.
One hit, not enough wit—a multi-hit model. [Red circle] The onset of periodontitis leads to an expansion of pathogenic oral bacteria. These bacteria can translocate to the intestine, either via ingestion or through the circulatory system. Normally, ingested oral bacteria are inactivated as they pass through the stomach. [Green circle] Several factors, such as genetic disorders, medications, diet, smoking, alcohol, ageing, and stress, may disrupt intestinal barrier function and shift the gut microbiota in the long-term. This disruption can reduce colonization resistance, facilitating the establishment of oral bacteria in the gut. [Blue circle] Once established in the gut, oral bacteria activate both innate and adaptive immune responses leading to intestinal inflammation. Oral dysbiosis or disrupted gut barrier alone are insufficient to predispose the host to IBD. Both a sufficient load of oral pathogens and disrupted barrier function are necessary for oral pathogens to successfully colonize the gut, which then activates immune responses leading to intestinal inflammation.
6.1. Stage 1: Initiation of oral dysbiosis
In a host exhibiting homeostatic oral and gut compartments, oral bacteria neither proliferate nor infiltrate the gut, thus maintaining a state of health. The onset of periodontitis causes oral dysbiosis, leading to the expansion of virulent oral bacteria.245 There is an enrichment of gram-negative bacteria that can thrive under anaerobic conditions, such as those found in the gut. The oral microbiome is subject to constant environmental perturbations, such as those from food, drinks, smoking, and oral hygiene practices, which affect the pH, temperature, oxygen levels, and nutrient profiles within the mouth.46,246 The oral microbiota exhibits evolutionary adaptations to environmental perturbations,247 which are reminiscent of the survival strategies employed by GI microbiota. In a biofilm state, Po. gingivalis can endure acidic conditions, showing resilience to simulated gastric fluids.248 Detection of genetically identical strains of F. nucleatum and Campylobacter concisus in both the oral cavity and the intestines of IBD and colorectal cancer patients indicates microbial adaptations that promote oral-to-gut transmission.14,249
6.2. Stage 2: Oral bacteria translocation and intestinal colonization
The next stage involves oral bacteria translocation to the intestine, either via ingestion or through the circulatory system [bacteraemia]. Normally, more than 99% of swallowed oral microorganisms are inactivated as they pass through the stomach.218,219 However, several conditions such as gastric ulcers, gastro-oesophageal reflux disease, genetic disorders, an unhealthy diet, ageing, stress, and the use of tobacco, alcohol, antibiotics, non-steroidal anti-inflammatory drugs, and/or proton-pump inhibitors may disrupt the intestinal barrier function and perturb the gut microbiota ecosystem, thus disrupting colonization resistance and facilitating the establishment of oral bacteria in the gut.153,235–237,243,244,250–252 A 10-fold increase in ileal Fusobacteriaceae was noted following antibiotic usage.18 Similarly, increased intestinal colonization of oral bacteria was noted in individuals who were using proton pump inhibitors.253,254 Additionally, periodontitis can lead to bacteraemia, with Po. gingivalis, F. nucleatum, Tr. denticola, and Pr. intermedia capable of evading immune surveillance and proliferating within the immune cells,35,255 and potentially exacerbating IBD development.
6.3. Stage 3: Induction of intestinal inflammation
In homeostasis, gut-resident microbes promote development of bacterium-specific tolerogenic responses against commensals that do not elicit intestinal inflammation.256,257 However, when certain exogenous oral pathogens colonize the gut, they can opportunistically elicit pathogenic immune responses. After epithelial disruption, ingested oral bacteria can interact with gut-associated immune cells stimulating production of pro-inflammatory cytokines such as IL-17, IL-1β, and IFN-γ.8,17 Furthermore, oral pathogen-reactive Th17 cells that arise de novo in the oral cavity can migrate to the inflamed gut, where they are activated by ectopically colonized oral pathogens, and subsequently contribute to gut inflammation.8 Thus, periodontitis can exacerbate gut inflammation by supplying the gut with both colitogenic oral pathogens and pathogenic T cells.8
In summary, neither oral dysbiosis nor gut barrier disruption alone is sufficient to predispose the host to IBD. However, the simultaneous presence of oral dysbiosis [providing an adequate supply of oral pathogens] and impaired gut resistance [disrupted barrier function against oral pathogens] creates susceptible conditions for oral pathogens to colonize the gut, which then promotes intestinal inflammation by activating innate and adaptive immune responses.
7. Oral and Gut Microbiome
The oral microbiota ranks as the second-largest microbial community after the gut microbiota. It harbours more than 700 species and 1300 bacterial strains, along with a diverse array of ultrasmall Candidate Phyla Radiation bacteria, fungi, amoebae, flagellates, archaea, and viruses.50,53,216,258–265 In health, the oral microbiota is remarkably stable and dominated by commensal bacteria such as Streptococcus, Actinomyces, Haemophilus, and Neisseria, which maintain a dynamic equilibrium with the host, ensuring symbiosis and facilitating normal immune and metabolic functions within the oral cavity.35,44,46,260,266 However, when this balance is disrupted, it can lead to dysbiosis, resulting in periodontitis.35,44,46,51,260,266 Oral dysbiosis is characterized by the overgrowth of anaerobic bacteria such as Po. gingivalis, Ta. forsythia, Tr. denticola [collectively referred to as the ‘red complex’], and other species within the phyla Firmicutes, Proteobacteria, Spirochaetes, and Bacteroidetes.35,44,46,51,260,266 These dysbiotic bacteria produce virulence factors that promote tissue destruction, impair host immune defences, and disrupt the oral microbial community’s stability.44 While the dysbiotic oral microbiota enriched in virulence factors triggers periodontitis, it is the host’s hyperactive immunoinflammatory responses to the altered microflora that cause major destruction of tooth-supporting structures.45,47
Po. gingivalis is a keystone periodontal pathogen, which adheres to mucus membranes, the periodontal pocket epithelium, and other bacterial surfaces through adhesion factors such as fimbriae, haemagglutinins, proteases, and adhesins.267–269Po. gingivalis produces a range of virulence factors, including endotoxins (e.g. lipopolysaccharide [LPS]), proteases [e.g. gingipains], outer membrane vesicles, acid and alkaline phosphatases, and organic acids, that contribute to the degradation of host proteins and evasion of host immune defences.268–271 Consequently, this leads to clinical manifestations such as oedema, neutrophil infiltration, and haemorrhage. In addition to Po. gingivalis, other periodontal pathogens, such as A. actinomycetemcomitans, F. nucleatum, Ta. forsythia, and Pr. intermedia, play crucial roles in the pathogenesis of periodontitis.49,272–276A. actinomycetemcomitans is a facultative anaerobe and its key virulence factors include leukotoxin [LtxA], which selectively targets immune cells, cytolethal distending toxin [Cdt] that induces cell cycle arrest and apoptosis in multiple cell types, and adhesins that promote bacterial attachment to host tissues and other oral microbiota.274–278 Additionally, its LPS triggers inflammation, and the extracellular matrix-degrading enzymes lead to tissue destruction, together orchestrating the complex pathophysiological processes underlying periodontitis. F. nucleatum is an anaerobic bacterium known to promote biofilm formation and support the growth of other periodontal pathogens.272,273,279 It produces various virulence factors, including adhesins, haemagglutinins, and proteases, that contribute to tissue destruction and evasion of host defences.280,281Ta. forsythia, another important periodontal pathogen, produces virulence factors such as proteases [e.g. karilysin and mirolase] and surface structures such as the BspA protein, which facilitate tissue invasion and immune evasion.282,283 The combined actions of multiple periodontal pathogens and their virulence factors contribute to the molecular mechanisms underlying periodontitis.268–283
At opposite ends of the orodigestive tract, the oral cavity and the intestine share microbial pathogenesis mechanisms. In a healthy state, the gut microbiota is composed primarily of Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria, with smaller populations of Fusobacteria and Cyanobacteria contributing to the overall diversity.284–287 Additionally, archaea, fungi, and viruses colonize the human GI tract. This intricate microbial community maintains gut homeostasis by executing essential functions such as nutrient metabolism, immune modulation, and defence against pathogens.284–289 The host tissues offer a nutrient-rich environment, reciprocated by the gut microbiota through the production of SCFAs and essential vitamins, fostering a mutually beneficial symbiosis.287,289 However, in IBD, the gut microbiota undergoes ecological dysbiosis, characterized by reduced microbial diversity—a decrease in beneficial commensal bacteria [e.g. Firmicutes], and an increase in potentially pathogenic bacteria [e.g. Proteobacteria].284–287 This dysbiosis leads to reduced production of beneficial metabolites such as SCFAs and an increase in pro-inflammatory molecules, which can further exacerbate the intestinal inflammation and compromise the intestinal barrier function.284–287 As a result, the delicate balance between the host and the gut microbiota is disrupted, leading to a vicious cycle of inflammation and tissue damage that characterizes IBD.
Clinical and animal studies have demonstrated an emerging microbial signature in IBD, characterized by the expansion of oral bacteria. Table 2 summarizes various clinical studies demonstrating evidence of oral bacterial species enriched in the intestinal tissues of patients with IBD and colorectal cancer [another disease implicated in the oral–gut axis]. In an early study, Van Dyke et al. [1986] investigated differences in the oral microbiome among IBD and periodontitis patients, and healthy controls.200 In IBD patients, they observed a predominant presence of gram-negative rod-shaped bacteria, closely related to the genus Wolinella. Interestingly, periodontitis-free IBD patients displayed a decreased bacterial load within the gingival sulcus compared to their counterparts with periodontitis. These findings suggest distinct variations in the oral microbiome of IBD patients compared to periodontitis patients and healthy controls. Meurman et al. [1994] found higher salivary yeast, Lactobacilli, and Streptococcus mutans counts in individuals with active CD compared to inactive CD.302 Stein et al. [2010] detected higher frequencies of C. rectus [94.6%], A. actinomycetemcomitans [76.9%], Po. gingivalis [62.6%], Pr. intermedia [79.6%], and Ta. forsythia [64.6%] in subgingival plaque from 147 CD patients stratified for CARD15-gene mutations.303 Notably, this study did not include a control group for comparative analysis. Man et al. [2010] and Strauss et al. [2011] identified a higher abundance of C. concisus and F. nucleatum in faeces and intestinal biopsies of IBD patients, suggesting that viable C. concisus and F. nucleatum can migrate to the intestines and establish themselves in the mucosal environment.23,300 Docktor et al. [2012] investigated the oral microbiome of children and young adults with IBD by analysing tongue and buccal mucosal brushings.304 They found a significant decrease in Fusobacteria and Firmicutes in CD patients compared to healthy controls. In UC patients, a similar decrease in Fusobacteria was observed, while Spirochaetes, Synergistetes, and Bacteroidetes were increased compared to controls. Brito et al. [2013] investigated 45 untreated periodontitis patients with either CD or UC, and patients with untreated periodontitis as controls.27 They found CD patients harboured significantly higher concentrations of Bacteriodes ureolyticus, Campylobacter gracilis, Prevotella melaninogenica, Staphylococcus aureus, Staphylococcus anginosus, Staphylococcus intermedius, and Streptococcus mutans compared to UC and controls subjects. Said et al. [2014] analysed saliva from 21 CD, 14 UC patients, and 24 healthy controls, and found higher abundance of Bacteroidetes, Prevotella, and Veillonella in CD and UC patients compared to healthy controls, while Proteobacteria, Streptococcus, and Haemophilus were lower.20Neisseria and Gemella were also lower in CD patients versus healthy controls. Kelsen et al. [2015] characterized subgingival microbiota in paediatric patients with active or non-active CD and identified 17 genera more abundant in CD patients versus healthy controls, including Capnocytophaga, Rothia, and TM7.305 Both antibiotic exposure and disease state were linked to differences in bacterial community composition. Schmidt et al. [2018] examined subgingival plaque and found a significantly lower prevalence of Eubacterium nodatum and Eikenella corrodens in 59 IBD patients compared to 59 healthy controls.176 Xun et al. [2018] investigated oral microbial dysbiosis in saliva samples from 54 UC patients, 13 active or remissive CD patients, and 25 healthy adults. They found an enrichment of Streptococcaceae and Enterobacteriaceae in UC and Veillonellaceae in CD, and depletion of Lachnospiraceae and Porphyromonadaceae in UC, as well as Neisseriaceae and Haemophilus in CD patients.15 Alpha diversity was significantly lower in UC and CD patients. Recently, Kitamoto et al. [2020] showed that Klebsiella spp. from the oral cavity can translocate to the gut, where they activate the inflammasome in lamina propria macrophages, exacerbating gut inflammation.8
Table 2.
Oral bacteria enriched in the intestines of patients with IBD and colorectal cancer
| Study [reference number] | Study design | Subjects [n] | Samples | Methods | Key findings | |
|---|---|---|---|---|---|---|
|
Fusobacterium spp. [F. nucleatum] |
Strauss et al. 201123 | Case-control | UC [4], CD [17], intermediate colitis [1], healthy controls [32], IBS [2] | Colon biopsies | Isolation, culture, 16S rRNA sequencing | Fusobacterium spp. abundant in patients with GI disease [64%] vs healthy controls [26%]. Highly invasive strains of F. nucleatum enriched in IBD patients |
| Castellarin et al. 201211 | Cross-sectional | CRC [11] | CRC biopsies and adjacent normal tissues | Isolation, culture, RNA-sequencing, qPCR | Higher abundance of Fusobacterium in colorectal tumour specimens | |
| Dejea et al. 201412 | Cross-sectional | USA CRC/polyp [34], healthy controls [62], MAL CRC/polyp [22] | Intestinal biopsies from CRC and healthy controls | 16S rRNA sequencing | Fusobacterium predominant in non-biofilm CRC specimens and undetected in normal tissues | |
| Gevers et al. 201418 | Case-control | Paediatric CD [447], healthy controls [221] | Ileal and rectal biopsies, stool, serum | 16S rRNA sequencing | Fusobacteriaceae enriched in treatment-naïve CD patients and associated with disease progression. Oral species better represented in biopsies vs stool. Antibiotic use amplified the microbial dysbiosis associated with CD | |
| Drewes et al. 2017290 | Case-control | MAL1 CRC [21], polyp [1], healthy controls [34]; MAL2 CRC [23], healthy controls [23]; USA CRC [35], polyp [4], healthy controls [60] | Stool, intestinal biopsies from CRC, polyp, and healthy tissues | 16S rRNA sequencing | Oral pathogens, including F. nucleatum, Parvimonas micra, and Peptostreptococcus stomatis highly enriched in CRC tissues | |
| Pascal et al. 201724 | Case-control | UC [74], CD [87], healthy controls [111] | Stool | 16S rRNA sequencing | Fusobacterium enriched in CD | |
| Flemer et al. 201813 | Case-control | CRC [99], colorectal polyps [32], healthy controls [103] | Oral swab, colonic biopsies, stool | 16S rRNA sequencing | F. nucleatum enriched in CRC, but also noted in healthy controls | |
| Schirmer et al. 201822 | Observational | Paediatric UC [405] | Rectal biopsies, stool | 16S rRNA sequencing | Fusobacterium enriched and associated with disease progression, and twice higher in rectal biopsies vs stool | |
| Dinakaran et al. 201825 | Cross-sectional | Disease tissue from UC [13], CD [13], and adjacent healthy tissue [13] | Colonic biopsies | 16S rRNA sequencing | Fusobacterium enriched in diseased colonic biopsies vs adjacent healthy tissue | |
| Komiya et al. 201914 | Cross-sectional | CRC [14] | Saliva, colonic biopsies | Isolation, culture, PCR, 16S rRNA sequencing | Identical F. nucleatum strains found in saliva and colon tumours | |
| Vieira-Silva et al. 2019291 | Case-control | PSC [64], UC [13], CD [29], healthy [1120] | Stool | 16S rRNA sequencing | Fusobacterium abundance associated with severity of intestinal inflammation | |
| Liu et al. 2020292 | Case-control | UC [20], CD [71], healthy controls [43] | Stool | 16S rRNA sequencing | F. nucleatum enriched and associated with disease activity in UC and CD patients. F. nucleatum infection in DSS-treated mice exacerbated colitis by promoting expression of IL-1β, TNF-α, IFN-γ, IL-6, and IL-17, and fostering CD4+ T cell differentiation into Th1 and Th17 cells | |
|
Porphyromonas spp. [Po. gingivalis] |
Flemer et al. 2017293 | Case-control | CRC [59], colon polyps [21], healthy controls [56] | Stool, CRC biopsies and adjacent normal tissues | 16S rRNA sequencing | Microbiota differed in CRC vs controls. Alterations were noted throughout the colon and not restricted to cancer |
| Ahn et al. 2013294 | Case-control | CRC [47], healthy controls [94] | Stool | 16S rRNA sequencing | Fusobacterium and Porphyromonas were enriched in CRC, whereas Clostridia were decreased | |
| Yang et al. 2019295 | Case-control | CRC [50], healthy controls [50] | Stool | 16S rRNA sequencing, gas chromatography-mass spectrometry | CRC had less microbial diversity and enriched Fusobacteria, Parvimonas and Porphyromonas; 17 metabolites were specifically perturbed in CRC | |
| Wang et al. 2021296 | Cross-sectional | Healthy [22], CRC [23], colorectal adenoma [32] | Stool, CRC, and normal tissue biopsies | 16S rRNA sequencing, qPCR | Po. gingivalis enriched in stool and biopsies of CRC and associated with poor prognosis. In ApcMin/+ mice, Po. gingivalis promoted CRC by activating NLRP3 inflammasome | |
| Lee et al. 2022297 | Case-control | CD [11], control [8] | Stool | 16S rRNA sequencing | Porphyromonadaceae enriched in CD. Po. gingivalis infection in DSS-treated mice exacerbated colitis by promoting expression of TNF-α and IL-6 | |
| Aggregatibacter spp. | Dinakaran et al. 201825 | Cross-sectional | Disease tissue from UC [13], CD [13], and adjacent healthy tissue [13] | Colon biopsies | 16S rRNA sequencing | Aggregatibacter, Porphyromonas, Prevotella, Staphylococcus, Streptococcus, enriched in diseased colon biopsies vs adjacent healthy tissue |
| Prevotella spp. | Atarashi et al. 201717 | Case-control | UC [51], CD [7], PSC [27], GERD [18], alcoholic [16], healthy controls [150] | Saliva, stool | Isolation, culture, 16S rRNA sequencing, metagenomic analysis | Prevotella, Streptococcus, Neisseria, Rothia, and Gemella enriched in UC, PSC, GERD and alcoholic faeces |
| Dinakaran et al. 201825 | Cross-sectional | Disease tissue from UC [13], CD [13], and adjacent healthy tissue [13] | Colon biopsies | 16S rRNA sequencing | Prevotella enriched in diseased colon vs adjacent healthy tissue | |
| Prasoodanan et al. 202121 | Population-based | IBD [189] and healthy controls [200] | Stool | Whole metagenome sequencing | Higher abundance and diversity of Prevotella copri in Indian and non-Western vs Western populations | |
| Veillonellaceae spp. [Veillonella parvula] |
Gevers et al. 201418 | Case-control | Paediatric CD [447], healthy controls [221] | Ileal and rectal biopsies, stool, serum | 16S rRNA sequencing | Veillonellaceae enriched in CD and associated with disease progression. Oral species better represented in biopsies vs stool |
| Schirmer et al. 201822 | Observational | Paediatric UC [405] | Rectal biopsies, stool | 16S rRNA sequencing | V. parvula enriched and associated with disease progression, and higher in rectal biopsies vs stool | |
| Vieira-Silva et al. 2019291 | Case-control | PSC [64], UC [13], CD [29], healthy [1,120] | Stool | 16S rRNA sequencing | Veillonella abundance associated with severity of intestinal inflammation | |
|
Klebsiella spp. [Klebsiella pneumoniae] |
Atarashi et al. 201717 | Case-control | UC [51], CD [7], PSC [27], GERD [18], alcoholic [16], healthy controls [150] | Saliva, stool | Isolation, culture, 16S rRNA sequencing, metagenomic analysis | Klebsiella enriched in CD. Antibiotic-resistant Klebsiella tend to colonize when gut microbiota is dysbiotic and elicit severe inflammation in a genetically susceptible host by promoting Th1 cells |
| Lloyd-Price et al. 2019298 | Case-control | UC [38], CD [67], healthy controls [27] | Colon biopsies, stool, blood | 16S rRNA sequencing, metagenomic, metatranscriptomic, proteomic, metabolomic analysis | K. pneumoniae and Haemophilus parainfluenzae levels during dysbiosis associated with acylcarnitines and bile acid levels | |
| Imai et al. 2021299 | Case-control | UC [42], CD [18], healthy controls [45] | Saliva and stool | 16S rRNA sequencing | Enterobacteriaceae enriched in CD patients with periodontitis | |
| Streptococcus spp. | Pascal et al. 201724 | Case-control | UC [74], CD [87], healthy controls [111] | Stool | 16S rRNA sequencing | Higher abundance of Streptococcus noted in CD with post-operative recurrence compared to those who remained in remission |
| Atarashi et al. 201717 | Case-control | UC [51], CD [7], PSC [27], GERD [18], alcoholic [16], healthy controls [150] | Saliva, stool | Isolation, culture, 16S rRNA sequencing, metagenomic analysis | Streptococcus, enriched in faeces of UC, PSC, GERD, and alcoholic patients | |
| Vieira-Silva et al. 2019291 | Case-control | PSC [64], UC [13], CD [29], healthy [1120] | Stool | 16S rRNA sequencing | Streptococcus abundance associated with severity of intestinal inflammation | |
|
Campylobacter spp. [Campylobacter concisus] |
Man et al. 2010300 | Case-control | Pediatric CD [54], non-IBD [27], and healthy controls [33] | Stool | PCR | Higher prevalence of C. concisus noted in paediatric CD vs non-IBD and healthy controls |
| Kirk et al. 2016301 | Case-control | UC [16], CD [9], IPAA [27], and healthy controls [26] | Ileum and colon biopsies | Isolation, culture, and PCR | Higher abundance of C. concisus noted in IBD vs control. |
IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis; IBS, irritable bowel syndrome; CRC, colorectal cancer; PSC, primary sclerosing cholangitis; GERD, gastro-oesophageal reflux disease; GI, gastrointestinal; MAL1, Malaysian cohort 1; MAL2, Malaysian cohort 2; DSS, dextran sodium sulfate; IPAA, ileal pouch–anal anastomosis.
8. Shared Immunoinflammatory Responses
The pathogenesis of both periodontitis and IBD involves a complex interplay between host immunity and specific bacterial stimuli.306 Here, we postulate a mechanism through which oral bacteria could potentially contribute to periodontitis-mediated IBD progression, and elaborate on the mechanistic details of the roles of neutrophils, macrophages, dendritic cells [DCs], T cells, and B cells in the process. Oral pathogen-mediated immune responses that drive gut inflammation in IBD are concisely illustrated in Figure 3.
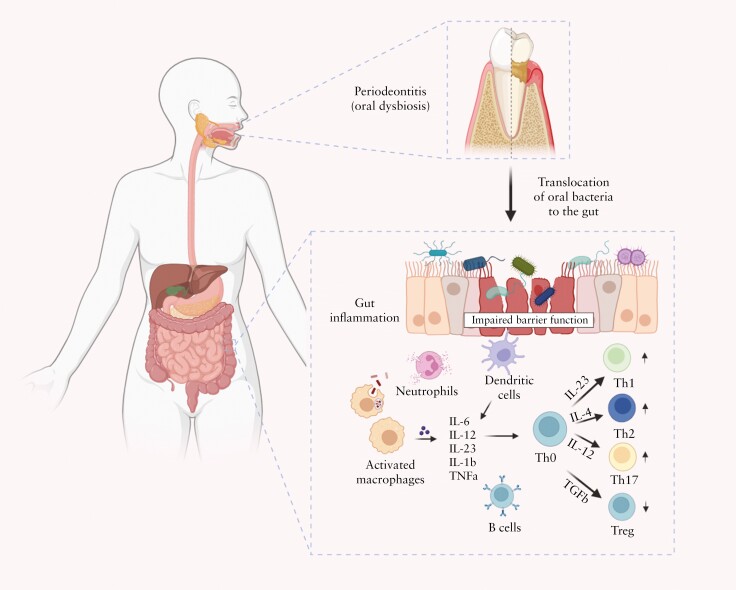
Figure 3.
Oral pathogen-mediated immune responses that drive gut inflammation in IBD. Periodontitis onset triggers expansion of pathogenic oral bacteria. Constant saliva swallowing enables these bacteria to translocate to the gut. A compromised intestinal barrier function, marked by diminished mucus and epithelial barrier integrity, facilitates the penetration of oral bacteria into the sub-epithelial regions. Neutrophils, the first responders, attempt to phagocytose the ingested bacteria and release antimicrobial substances. Additionally, the antigen-presenting cells [APCs] in the gut recognize the microbial invaders through pattern recognition receptors such as Toll-like receptors [TLRs]. Once activated, these cells release a cocktail of pro-inflammatory molecules: interleukin [IL]-6, IL-12, IL-23, IL-1β, tumour necrosis factor α [TNF-α], and chemokines. Consequently, these molecules guide the differentiation of naive T helper [Th0] cells into Th1, Th2, Th17, and Treg cells. Furthermore, B cells become activated by recognizing bacterial antigens and differentiate into plasma cells, which produce antibodies specific to the oral bacterial antigens. These antibodies neutralize or opsonize bacteria and can form immune complexes, intensifying inflammation. The concerted actions of innate and adaptive immune cells lead to initiation or exacerbation of the inflammatory process in IBD, highlighting the far-reaching effects of oral bacterial dysbiosis. Figure created with BioRender.com.
Both periodontitis307,308 and IBD309–311 have been associated with rheumatoid arthritis [RA]. Drawing parallels from their established links with RA, we can postulate the potential contribution of oral bacteria to the pathogenesis of IBD, particularly through processes involving citrullination and immune response cross-reactivity. In periodontitis, oral bacteria, such as Streptococcus, Porphyromonas, Actinomyces and Prevotella species undergo citrullination, a process catalysed by peptidylarginine deiminase [PAD] enzymes found in both microbial and human cells.307,308,312–314 This enzymatic action converts peptidylarginine into peptidylcitrulline, which fundamentally alters the protein structure and function.315–318 The altered structure is recognized by the T and B cell immune repertoire inducing epitope spreading and loss of immune tolerance.307 These citrullinated bacterial antigens have been implicated in triggering autoimmune responses.319–321 However, the potential role of gut or oral bacteria in enhancing the citrullination cascade in IBD remains unknown.322
We hypothesize that in IBD, citrullinated oral bacterial antigens, either translocated to the gut via ingestion or systemic circulation, could instigate a comparable autoimmune cascade seen in RA.307 Once in the gut, these citrullinated bacterial antigens could induce an immune response that initially targets the microbial proteins but subsequently exhibits cross-reactivity towards citrullinated human proteins in the gut.307,314,323,324 We hypothesize that this cross-reactivity induced by epitope spreading could contribute to the autoimmune nature of IBD, where the immune system, originally primed against bacterial antigens, erroneously targets the gut tissues due to molecular mimicry.325–327 Additionally, the immune response in IBD might involve the formation of anti-citrullinated protein antibodies [ACPAs],328–330 similar to those observed in RA,307,331,332 targeting the citrullinated antigens from oral bacteria. Neutrophils, forming extracellular traps [NETs] containing citrullinated proteins, could add to the antigenic load, contributing to the inflammation and tissue damage characteristic of IBD.333–335
Therefore, we hypothesize that the interplay of citrullination, immune cross-reactivity, epitope spreading, chronic inflammation, and gut dysbiosis, stemming from oral bacteria and their antigens in the context of periodontal disease, could play a pivotal in the onset and progression of IBD. The detailed roles of specific PAD isotypes, the exact nature of the citrullinated epitopes, and the specific types of immune cells and receptors involved in these processes are areas that require further investigation.
Neutrophils, as the first responders to bacterial invasion, play a pivotal role in the initial stages of periodontitis and IBD.35,336–340 In periodontitis, Po. gingivalis, through its virulence factors such as gingipains, promotes a hyperactive and sustained neutrophilic response, leading to elevated release of proteolytic enzymes and reactive oxygen species.35,336,341 This results in collateral tissue damage and subsequent alveolar bone loss, a hallmark of periodontitis. Moreover, Po. gingivalis can dysregulate neutrophil function, such as impairing NET formation and phagocytosis, leading to an imbalanced host response.35,336,341 In IBD, similar neutrophil dysfunctions are observed, where excessive and sustained neutrophilic infiltration in the intestinal mucosa contributes to chronic inflammation and tissue damage.338,339,342 Furthermore, neutrophil dysregulation, including abnormal recruitment and impaired clearance, is also seen in IBD.338,339,342Po. gingivalis may dysregulate neutrophil function, potentially exacerbating IBD pathogenesis. Systemically circulating neutrophils in individuals with periodontitis exhibit cytokine hyper-reactivity and impaired chemotaxis, which could potentially contribute to the oral–gut axis in the context of IBD.199,200,343 S100A8, S100A9, and S100A12 are small calcium-binding proteins abundantly expressed by neutrophils in acute inflammation and have been implicated in immune regulation in both periodontitis and IBD.344–346 Higher salivary expression of S100A12 has been found in UC patients with periodontitis.347 The role of S100 proteins in periodontitis–IBD pathogenesis needs to be further investigated.
Macrophages, key players in the immune response, are implicated in both periodontitis and IBD. In the context of periodontitis, Po. gingivalis can influence macrophage polarization, favouring a pro-inflammatory [M1] phenotype over an anti-inflammatory [M2] phenotype, thus promoting inflammation and tissue damage.348F. nucleatum also stimulates M1 macrophage polarization via the AKT2 pathway and fosters Th1 and Th17 cell expansion through STAT3 signalling.292,349 Similar macrophage polarization patterns are seen in IBD, contributing to a sustained inflammatory environment in the gut.350,351 Specifically, Enterobacteriaceae such as Klebsiella isolated from the oral cavity trigger IL-1β secretion from macrophages, intensifying intestinal inflammation by mediating IL-1 signalling.8 Intriguingly, Enterobacteriaceae isolated from the intestines do not evoke similar IL-1β secretion, suggesting a specific response to oral microbes.8
Dendritic cells [DCs] are antigen-presenting cells that bridge innate and adaptive immunity.352,353 DCs play a crucial role in maintaining oral tolerance to commensal bacteria and preventing unwanted inflammatory responses.354 However, certain periodontal pathogens, such as Po. gingivalis and A. actinomycetemcomitans, can dysregulate DC function and promote inflammation.355,356 In IBD, dysregulated DCs can initiate and perpetuate intestinal inflammation through an exaggerated T cell response.357,358 Saliva from CD and UC patients can cause intestinal inflammation in mice lacking IL-10 through Klebsiella-induced DC signalling and Th1 activation.17 In response to oral bacteria, IFN-γ+ CD4+ T cells accumulate in the intestinal mucosa of these mice, as observed in humans with CD.17
T cells are key orchestrators of immune responses in both periodontitis and IBD, with their activation and subsequent modulation of inflammation serving as common pathogenic threads. Po. gingivalis stimulates aberrant CD4+ T cell responses, tilting the balance towards a pro-inflammatory milieu. More specifically, Po. gingivalis incites an increase in Th17 cell responses, characterized by distinct production of IL-17 and simultaneous modulation of the Th17/Treg ratio.32,359 This results in an augmentation of Th17-related transcription factors and pro-inflammatory cytokines IL-17 and IL-6 via the TLR4 pathway, paralleled by downregulation of Foxp3, TGF-β, and IL-10.360 Further, Po. gingivalis elicits intestinal inflammation by altering the gut microbiota composition and disrupting epithelial barrier function through IL-9-producing CD4+ T cells.231 These shifts towards pro-inflammatory responses and a skewed T cell landscape could contribute to IBD progression.32,360F. nucleatum exacerbates this inflammatory cascade by compromising the integrity of the intestinal epithelial barrier, thereby increasing permeability and promoting secretion of inflammatory cytokines such as TNF-α, IFN-γ, IL-1β, IL-6, and IL-17, and in parallel inhibiting the production of the anti-inflammatory cytokine IL-10.292 Numerous studies have further delineated the shared inflammatory mechanisms between periodontitis and IBD by evaluating cytokine expression in affected tissues. For example, Menegat et al.361 and Figueredo et al.170,177 identified heightened levels of IL‐17A, IL‐17F, IL‐22, IL‐23, IL‐25, IL‐33, INF‐γ, and IL‐10 in gingival tissues of patients with IBD and chronic periodontitis. However, they found IL‐1β, IL‐4, IL‐10, and IL‐21 to be significantly elevated in patients with active IBD, suggesting that an inflammation score based on IL‐1β, IL‐6, IL‐21, and sCD40L expression was higher in gingival tissues of active IBD patients. Furthermore, IBD patients treated with anti-TNF-α biologics had more rapid healing of apical periodontitis compared to controls.209 Collectively, these studies underscore the intertwined relationship between periodontitis and IBD and the central role of T-cell-mediated immune responses, thereby providing a clearer understanding of the common pathogenic landscape and potential targets for future therapeutics.
B cells, essential constituents of adaptive immunity that play a key role in the crosstalk between periodontitis and IBD, mediate their effects primarily through the production of antibodies against specific pathogens. Po. gingivalis can stimulate B cells to produce antibodies, such as IgG and IgA, but also fosters an environment of sustained inflammation in the oral cavity.362,363 There is emerging evidence that B cell dysregulation and inappropriate antibody responses also contribute to IBD pathogenesis.364 Our group recently demonstrated a highly dysregulated B cell response in UC, highlighting a potential role of B cells in disease pathogenesis.365 We found expansion of naive B cells and IgG+ plasma cells with curtailed diversity and maturation within the mucosal B cell compartment of UC patients.365
9. Animal Studies Demonstrating Periodontitis–IBD Interconnection
Animal models have been instrumental in delineating the immunoinflammatory interplay between periodontitis and IBD, suggesting potential parallels in humans. Yamazaki et al. demonstrated that oral inoculation of Po. gingivalis disrupts mouse gut microbiota and compromises intestinal barrier integrity by diminishing the expression of tight junction proteins.229,366 This was associated with an upsurge in colonic inflammatory cytokines [IL-6, IL-12β, IFN-γ, and IL-17c] and higher serum endotoxin levels, suggesting a direct link between oral pathogens and systemic inflammation.229,366 Similarly, Liu et al. observed that Po. gingivalis inoculation in mice led to a reduction in gut microbial diversity and a marked increase in Th1 cells and associated cytokines [IFN-γ and TNF-α] in the gut and spleen.367 No changes were noted in tight junction proteins, and the authors posited that Po. gingivalis could disrupt their distribution rather than their overall expression. Zhao et al. showed that Po. gingivalis exacerbates Dextran sulphate sodium [DSS]-induced colitis in mice by promoting Th17 and IL-17 while reducing Treg and IL-10 production, an effect partially ascribed to a virulence factor of Po. gingivalis, peptidylarginine deiminase [PPAD].32 Sohn et al reported that oral administration of Po. gingivalis induces ileal inflammation and alters gut microbiota composition, notably reducing microbial alpha diversity despite the absence of Po. gingivalis in the lower GI tract.231 They documented a substantial increase in IL9+ CD4+ T cells within the small intestinal lamina propria, indicating that the inflammation might result from subsequent loss of gut microbial diversity. Qian et al. noted that transferring salivary microbiota from periodontitis patients into mice altered gut microbiota composition and exacerbated DSS-induced colitis via enhanced M2 polarization and Th2 cell induction.368 Atarashi et al. found that Klebsiella species from the oral cavity of CD patients can colonize mouse intestines and provoke Th1 cell responses via the TLR4 receptor, leading to IL-10 deficiency and further amplification of intestinal inflammation.17 Notably, Klebsiella also promoted the expression of interferon-inducible [IFI] genes, which may facilitate colonization and subsequent recruitment of Th1 cells, thereby promoting a Th1-skewed inflammatory response reminiscent of IBD-like colitis.17 Kitamoto et al. revealed that in a model combining ligature-induced periodontitis and DSS treatment in mice, oral pathogen-reactive Th17 cells arise de novo in the oral cavity and migrate to the inflamed gut, where they are activated by ectopically colonized oral pathogens, and subsequently contribute to gut inflammation.8 Conversely, Nagao et al. found that orally administered Po. gingivalis is internalized by Peyer’s patches and specifically activates intestinal Th17 cells influenced by the gut microbiota. These intestine-derived Th17 cells migrate from the gut to the mouth and exacerbate periodontitis.9 Collectively, these findings suggests that oral pathogens may contribute to the immunological and cytokine disturbances associated with human IBD by exploiting pathways similar to those documented in rodent models.
10. Current Knowledge Gaps and Future Directions
10.1. Clinical and animal studies
Although evidence supports the notion that periodontitis and IBD are interrelated, the specific mechanisms by which oral bacteria contribute to gut inflammation and vice versa are still unclear. The directionality and causality of this relationship are obscured by confounding factors. Tobacco use, a Western diet, and lifestyle are recognized risk factors for IBD, with some genes linked to mucosal barrier function and immune regulation potentially implicated.64,106,107,369 However, the precise role of these factors remains unclear. Much of the existing research linking periodontitis and IBD is cross-sectional, limiting our understanding of causal relationships.63,163,178,207,208,299,370 There is a need for longitudinal studies that can provide insights into the temporal sequence of these conditions, and whether periodontitis precedes, follows, or develops concurrently with IBD. To date, most studies have focused on bacterial dysbiosis, and the role of fungi and viruses has been neglected. It remains ambiguous whether dysbiosis acts as a primary cause of the disease or if it is simply a by-product of changes in the immune system, metabolism, or diet. Furthermore, the microbial dissimilarity between humans and mice raises questions about the applicability of murine findings to humans. The role of various immune cells and the impact of trained immunity in both conditions remains ill-defined. The influence of age on the association between periodontitis and IBD is not fully clear. Age is a significant risk factor in the pathogenesis of both conditions, and understanding its role could potentially inform prevention and treatment strategies. Further, it remains unanswered whether periodontitis treatment ameliorates IBD risk or severity, and if IBD management can reduce periodontitis occurrence. These significant knowledge gaps call for well-designed, robust, mechanistic clinical and animal studies to gain a comprehensive understanding of the interrelationships between periodontitis and IBD pathogenesis.
10.2. Concerns regarding the DSS-induced colitis model
While several animal models [at least 66 different kinds] have been employed to study IBD pathogenesis,371–374 we focus on the DSS-colitis model, which has been extensively used to investigate colitis pathogenesis and the potential causal link between periodontitis and IBD.8,32,292,360,368,371–377 However, this model has inherent limitations that may impact the interpretation and translatability of oral and gut findings. DSS administration induces acute colonic inflammation by direct chemical injury to the intestinal epithelium.371–374 The physical damage to the epithelial lining leads to luminal microbial ingress into the lamina propria, which stimulates innate and adaptive immune responses. This process differs from human IBD, which is a chronic condition characterized by alternating periods of inflammation and remission involving a complex interplay of genetic, environmental, and immunological factors.64,106,107,369 While DSS is administered to cause colonic inflammation, it can have off-target effects on periodontal tissues and oral microbiota. Oz and Ebersole [2010] found that 2% DSS alone causes periodontal inflammation and significant alveolar bone loss in BALB/c mice.378,379 Significant bone loss was detected as early as 7 weeks after initiation of DSS treatment, progressing to severe periodontitis by 18 weeks. The authors concluded that DSS impacts mucosal tissue in a more generalized and systemic manner, affecting both the intestine and the oral cavity. Similarly, Mello-Neto et al. reported that DSS-treated rats exhibited inflammatory cells extending into periodontal connective tissues, which contained significantly elevated expression of Th1/Th2-related cytokines such as IL-1α, IL-1β, IL-2, IL-6, IL-12, IL-13, GM-CSF, IFN-γ, and TNF-α.380 They concluded that DSS should be used with caution as it can lead to more widespread and indiscriminate lesions, and the increased pro-inflammatory cytokines in the gingival tissues caused by DSS alone might create an environment conducive to future alveolar bone loss. In another study, Rautava et al. showed that C57BL/6 male mice treated with 2% DSS for 1 week exhibited altered oral and gut microbiome composition, with a decrease in oral levels of Spirochetes, Betaproteobacteria, and Lactobacillus.381 The salivary microbiota exhibited the most significant change when compared to the microbiota of the tongue and buccal mucosa. However, they noted no visible oral inflammation, and it is unclear how much of the observed effect is attributed to gender imbalance in the study. Additionally, Metzger et al.382 and Hamdani et al.383 demonstrated that DSS treatment suppresses bone formation and increases bone resorption, resulting in reduced bone mass and altered bone microarchitecture. In DSS-treated mice, elevated TNF-α, IL-6, RANKL, OPG, and sclerostin corresponded with higher osteoclast surfaces and lower rates of bone formation. Collectively, these findings suggest that DSS promotes periodontal inflammation, alveolar bone loss, and altered oral microbiota.
Based on these findings, the question arises whether DSS is a confounder in studying periodontal–IBD interconnection. While seminal studies in DSS-treated mice showed that bacteria and immune cells from the oral cavity migrate to the gut and exacerbate colonic inflammation,8 the extent to which this inter-relationship is confounded by DSS treatment is uncertain. Previous studies investigating the periodontitis–IBD interconnection lacked evidence that DSS treatment alone did not affect periodontal tissues, the oral microbiome, and oral immune cells.8,32,292,360,368,375–377 This major limitation highlights the need for future research to focus on developing more accurate and representative animal models that better mimic the complex interactions between periodontitis, oral and gut bacteria, and IBD in humans. This may involve the use of alternative mouse models exhibiting increased susceptibility to both periodontitis and IBD without the need for chemical induction.
11. Conclusions
As our understanding of periodontitis and IBD pathogenesis advances, both disorders represent a complex interplay of genetic, environmental, microbial, and immunological factors. Central to this discussion is the ‘multi-hit’ model, suggesting that a sequence of events—dysbiosis of the oral microbiota, a disrupted intestinal barrier function, and an aberrant immune response—are crucial for the development of periodontitis-associated IBD. The oral cavity may serve as a potential reservoir for intestinal pathogens. Oral bacteria and their byproducts can reach the gut through ingestion or systemic circulation. Factors such as genetics, an unhealthy diet, ageing, stress, and the use of tobacco, alcohol, or medications may disrupt intestinal barrier function, allowing the oral bacteria to colonize the gut. Once colonized, these oral bacteria can elicit excessive immune responses that promote intestinal inflammation. While current studies provide valuable insights, there is a need for longitudinal studies to provide an in-depth understanding of the periodontitis–IBD interconnection. Despite the valuable insights gained from the DSS-colitis animal models, their limitations must be carefully considered when exploring the interconnection between periodontitis and IBD. Lastly, collaboration between dentists, gastroenterologists, immunologists, and infectious disease experts/microbiologists, alongside other healthcare professionals, is necessary to provide holistic oral–systemic healthcare. Optimal dental care could reduce the supply of pathogenic oral bacteria to the gut, potentially offering innovative methods to reduce the risk and severity of IBD.
Contributor Information
Himanshi Tanwar, Division of Periodontology, University of Maryland School of Dentistry, Baltimore, MD, USA.
Jeba Mercy Gnanasekaran, Division of Periodontology, University of Maryland School of Dentistry, Baltimore, MD, USA.
Devon Allison, Division of Periodontology, University of Maryland School of Dentistry, Baltimore, MD, USA.
Ling-shiang Chuang, Division of Gastroenterology, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Xuesong He, Department of Microbiology, The Forsyth Institute, Cambridge, MA, USA.
Mario Aimetti, Department of Surgical Sciences, C.I.R. Dental School, University of Turin, Turin, Italy.
Giacomo Baima, Department of Surgical Sciences, C.I.R. Dental School, University of Turin, Turin, Italy.
Massimo Costalonga, Department of Diagnostic and Biological Sciences, School of Dentistry, University of Minnesota, Minneapolis, MN, USA.
Raymond K Cross, Division of Gastroenterology & Hepatology, University of Maryland School of Medicine, Baltimore, MD, USA.
Cynthia Sears, Division of Infectious Diseases, Johns Hopkins University School of Medicine, Baltimore, MD, USA.
Saurabh Mehandru, Division of Gastroenterology, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Judy Cho, Division of Gastroenterology, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Jean-Frederic Colombel, Division of Gastroenterology, Department of Medicine, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Jean-Pierre Raufman, Division of Gastroenterology & Hepatology, University of Maryland School of Medicine, Baltimore, MD, USA.
Vivek Thumbigere-Math, Division of Periodontology, University of Maryland School of Dentistry, Baltimore, MD, USA; National Institute of Dental and Craniofacial Research, NIH, Bethesda, MD, USA.
Funding
This work was supported by the National Institutes of Health grants R03DE029258 and R56DK131277 to V.T.M.; University of Maryland School of Dentistry start-up funds and INSPIRE grants to V.T.M.; Merit Review Award BX004890 from the U.S. Department of Veterans Affairs Biomedical Laboratory Research and Development Program to J-P.R. [the contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government]; and The Bloomberg–Kimmel Institute for Immunotherapy, Johns Hopkins School of Medicine to C.L.S.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author Contributions
All authors have made substantial contributions to the following: [a] the conception and design of the study, or acquisition of data, or analysis and interpretation of data, [b] drafting the article or revising it critically for important intellectual content, and [c] final approval of the version to be submitted.
Data Availability
The data underlying this article are available in the article and in its online supplementary material.
References
- 1. D’Souza RN, Collins FS, Murthy VH.. Oral health for all - realizing the promise of science. N Engl J Med 2022;386:809–11. [DOI] [PubMed] [Google Scholar]
- 2. Somerman M, Mouradian WE.. Integrating oral and systemic health: innovations in transdisciplinary science, health care and policy. Frontiers in Dental Medicine 2021;1:674329. [Google Scholar]
- 3. Hajishengallis G, Chavakis T.. Local and systemic mechanisms linking periodontal disease and inflammatory comorbidities. Nat Rev Immunol 2021;21:426–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kim J, Amar S.. Periodontal disease and systemic conditions: a bidirectional relationship. Odontology 2006;94:10–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bui FQ, Almeida-da-Silva CLC, Huynh B, et al. Association between periodontal pathogens and systemic disease. Biomed J 2019;42:27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.In: Oral Health in America: Advances and Challenges: Executive Summary. Bethesda, MD: National Institutes of Health. 2021. [PubMed] [Google Scholar]
- 7. Pihlstrom BL, Michalowicz BS, Johnson NW.. Periodontal diseases. Lancet 2005;366:1809–20. [DOI] [PubMed] [Google Scholar]
- 8. Kitamoto S, Nagao-Kitamoto H, Jiao Y, et al. The intermucosal connection between the mouth and gut in commensal pathobiont-driven colitis. Cell 2020;182:447–462.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Nagao JI, Kishikawa S, Tanaka H, et al. Pathobiont-responsive Th17 cells in gut-mouth axis provoke inflammatory oral disease and are modulated by intestinal microbiome. Cell Rep 2022;40:111314. [DOI] [PubMed] [Google Scholar]
- 10. Ahn J, Segers S, Hayes RB.. Periodontal disease, Porphyromonas gingivalis serum antibody levels and orodigestive cancer mortality. Carcinogenesis 2012;33:1055–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Castellarin M, Warren RL, Freeman JD, et al. Fusobacterium nucleatum infection is prevalent in human colorectal carcinoma. Genome Res 2012;22:299–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Dejea CM, Wick EC, Hechenbleikner EM, et al. Microbiota organization is a distinct feature of proximal colorectal cancers. Proc Natl Acad Sci U S A 2014;111:18321–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Flemer B, Warren RD, Barrett MP, et al. The oral microbiota in colorectal cancer is distinctive and predictive. Gut 2018;67:1454–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Komiya Y, Shimomura Y, Higurashi T, et al. Patients with colorectal cancer have identical strains of Fusobacterium nucleatum in their colorectal cancer and oral cavity. Gut 2019;68:1335–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xun Z, Zhang Q, Xu T, Chen N, Chen F.. Dysbiosis and ecotypes of the salivary microbiome associated with inflammatory bowel diseases and the assistance in diagnosis of diseases using oral bacterial profiles. Front Microbiol 2018;9:1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yachida S, Mizutani S, Shiroma H, et al. Metagenomic and metabolomic analyses reveal distinct stage-specific phenotypes of the gut microbiota in colorectal cancer. Nat Med 2019;25:968–76. [DOI] [PubMed] [Google Scholar]
- 17. Atarashi K, Suda W, Luo C, et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science 2017;358:359–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gevers D, Kugathasan S, Denson LA, et al. The treatment-naive microbiome in new-onset Crohn’s disease. Cell Host Microbe 2014;15:382–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Qi Y, Zang SQ, Wei J, et al. High-throughput sequencing provides insights into oral microbiota dysbiosis in association with inflammatory bowel disease. Genomics 2021;113:664–76. [DOI] [PubMed] [Google Scholar]
- 20. Said HS, Suda W, Nakagome S, et al. Dysbiosis of salivary microbiota in inflammatory bowel disease and its association with oral immunological biomarkers. DNA Res 2014;21:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Prasoodanan PKV, Sharma AK, Mahajan S, et al. Western and non-western gut microbiomes reveal new roles of Prevotella in carbohydrate metabolism and mouth-gut axis. NPJ Biofilms Microbiomes 2021;7:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Schirmer M, Denson L, Vlamakis H, et al. Compositional and temporal changes in the gut microbiome of pediatric ulcerative colitis patients are linked to disease course. Cell Host Microbe 2018;24:600–610.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Strauss J, Kaplan GG, Beck PL, et al. Invasive potential of gut mucosa-derived Fusobacterium nucleatum positively correlates with IBD status of the host. Inflamm Bowel Dis 2011;17:1971–8. [DOI] [PubMed] [Google Scholar]
- 24. Pascal V, Pozuelo M, Borruel N, et al. A microbial signature for Crohn’s disease. Gut 2017;66:813–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dinakaran V, Mandape SN, Shuba K, et al. Identification of specific oral and gut pathogens in full thickness colon of colitis patients: implications for colon motility. Front Microbiol 2018;9:3220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Engel LD, Pasquinelli KL, Leone SA, Moncla BJ, Nielson KD, Rabinovitch PS.. Abnormal lymphocyte profiles and leukotriene B4 status in a patient with Crohn’s disease and severe periodontitis. J Periodontol 1988;59:841–7. [DOI] [PubMed] [Google Scholar]
- 27. Brito F, Zaltman C, Carvalho AT, et al. Subgingival microflora in inflammatory bowel disease patients with untreated periodontitis. Eur J Gastroenterol Hepatol 2013;25:239–45. [DOI] [PubMed] [Google Scholar]
- 28. Schmidt TS, Hayward MR, Coelho LP, et al. Extensive transmission of microbes along the gastrointestinal tract. Elife 2019;8:e42693:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Chen W, Liu F, Ling Z, Tong X, Xiang C.. Human intestinal lumen and mucosa-associated microbiota in patients with colorectal cancer. PLoS One 2012;7:e39743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Sun B, Liu B, Gao X, Xing K, Xie L, Guo T.. Metagenomic analysis of saliva reveals disease-associated microbiotas in patients with periodontitis and Crohn’s disease-associated periodontitis. Front Cell Infect Microbiol 2021;11:719411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ryan FJ, Ahern AM, Fitzgerald RS, et al. Colonic microbiota is associated with inflammation and host epigenomic alterations in inflammatory bowel disease. Nat Commun 2020;11:1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhao X, Liu J, Zhang C, et al. Porphyromonas gingivalis exacerbates ulcerative colitis via Porphyromonas gingivalis peptidylarginine deiminase. Int J Oral Sci 2021;13:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Abdelbary MMH, Hatting M, Bott A, et al. The oral–gut axis: salivary and fecal microbiome dysbiosis in patients with inflammatory bowel disease. Front Cell Infect Microbiol 2022;12:1010853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kinane DF, Attstrom R; European Workshop in Periodontology group B., European Workshop in Periodontology group B. Advances in the pathogenesis of periodontitis. Group B consensus report of the fifth European Workshop in Periodontology. J Clin Periodontol 2005;32:130–1. [DOI] [PubMed] [Google Scholar]
- 35. Hajishengallis G. Periodontitis: from microbial immune subversion to systemic inflammation. Nat Rev Immunol 2015;15:30–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kinane DF, Stathopoulou PG, Papapanou PN.. Periodontal diseases. Nat Rev Dis Primers 2017;3:17038. [DOI] [PubMed] [Google Scholar]
- 37. Kassebaum NJ, Smith AGC, Bernabe E, et al. ; GBD 2015 Oral Health Collaborators. Global, regional, and national prevalence, incidence, and disability-adjusted life years for oral conditions for 195 Countries, 1990–2015: a systematic analysis for the global burden of diseases, injuries, and risk factors. J Dent Res 2017;96:380–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nazir M, Al-Ansari A, Al-Khalifa K, Alhareky M, Gaffar B, Almas K.. Global prevalence of periodontal disease and lack of its surveillance. ScientificWorld Journal 2020;2020:2146160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Disease GBD, Injury I, Prevalence C.. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1211–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Eke PI, Dye BA, Wei L, et al. Update on prevalence of periodontitis in adults in the United States: NHANES 2009 - 2012. J Periodontol 2015;86:611–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Eke PI, Wei L, Borgnakke WS, et al. Periodontitis prevalence in adults >/= 65 years of age, in the USA. Periodontol 2000 2016;72:76–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Highfield J. Diagnosis and classification of periodontal disease. Aust Dent J 2009;54:S11–26. [DOI] [PubMed] [Google Scholar]
- 43. Petersen PE, Yamamoto T.. Improving the oral health of older people: the approach of the WHO Global Oral Health Programme. Community Dent Oral Epidemiol 2005;33:81–92. [DOI] [PubMed] [Google Scholar]
- 44. Hajishengallis G, Lamont RJ.. Beyond the red complex and into more complexity: the polymicrobial synergy and dysbiosis (PSD) model of periodontal disease etiology. Mol Oral Microbiol 2012;27:409–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Lamont RJ, Hajishengallis G.. Polymicrobial synergy and dysbiosis in inflammatory disease. Trends Mol Med 2015;21:172–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lamont RJ, Koo H, Hajishengallis G.. The oral microbiota: dynamic communities and host interactions. Nat Rev Microbiol 2018;16:745–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tsukasaki M, Komatsu N, Nagashima K, et al. Host defense against oral microbiota by bone-damaging T cells. Nat Commun 2018;9:701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Socransky SS, Haffajee AD.. Periodontal microbial ecology. Periodontol 2000 2005;38:135–87. [DOI] [PubMed] [Google Scholar]
- 49. Holt SC, Ebersole JL.. Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia: the “red complex”, a prototype polybacterial pathogenic consortium in periodontitis. Periodontol 2000 2005;38:72–122. [DOI] [PubMed] [Google Scholar]
- 50. Dewhirst FE, Chen T, Izard J, et al. The human oral microbiome. J Bacteriol 2010;192:5002–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Darveau RP. Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol 2010;8:481–90. [DOI] [PubMed] [Google Scholar]
- 52. Paster BJ, Boches SK, Galvin JL, et al. Bacterial diversity in human subgingival plaque. J Bacteriol 2001;183:3770–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Aas JA, Paster BJ, Stokes LN, Olsen I, Dewhirst FE.. Defining the normal bacterial flora of the oral cavity. J Clin Microbiol 2005;43:5721–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Curtis MA, Zenobia C, Darveau RP.. The relationship of the oral microbiotia to periodontal health and disease. Cell Host Microbe 2011;10:302–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kinane DF, Peterson M, Stathopoulou PG.. Environmental and other modifying factors of the periodontal diseases. Periodontol 2000 2006;40:107–19. [DOI] [PubMed] [Google Scholar]
- 56. Genco RJ, Borgnakke WS.. Risk factors for periodontal disease. Periodontol 2000 2013;62:59–94. [DOI] [PubMed] [Google Scholar]
- 57. Baima G, Romandini M, Citterio F, Romano F, Aimetti M.. Periodontitis and accelerated biological aging: a geroscience approach. J Dent Res 2022;101:125–32. [DOI] [PubMed] [Google Scholar]
- 58. Kwok V, Caton JG.. Commentary: prognosis revisited: a system for assigning periodontal prognosis. J Periodontol 2007;78:2063–71. [DOI] [PubMed] [Google Scholar]
- 59. Seymour GJ, Ford PJ, Cullinan MP, Leishman S, Yamazaki K.. Relationship between periodontal infections and systemic disease. Clin Microbiol Infect 2007;13:3–10. [DOI] [PubMed] [Google Scholar]
- 60. Chapple IL, Genco R; Working Group 2 of the Joint EFP/AAP Workshop. Diabetes and periodontal diseases: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol 2013;84:S106–112. [DOI] [PubMed] [Google Scholar]
- 61. Tonetti MS, Van Dyke TE; Working Group 1 of the Joint EFP/AAP Workshop. Periodontitis and atherosclerotic cardiovascular disease: consensus report of the Joint EFP/AAP Workshop on periodontitis and systemic diseases. J Periodontol 2013;84:S24–9. [DOI] [PubMed] [Google Scholar]
- 62. Sanz M, Kornman K; Working Group 3 of the Joint EFP/AAP Workshop. Periodontitis and adverse pregnancy outcomes: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J Periodontol 2013;84:S164–169. [DOI] [PubMed] [Google Scholar]
- 63. Baima G, Muwalla M, Testa G, et al. Periodontitis prevalence and severity in inflammatory bowel disease: a case-control study. J Periodontol 2023;94:313–22. [DOI] [PubMed] [Google Scholar]
- 64. Strober W, Fuss I, Mannon P.. The fundamental basis of inflammatory bowel disease. J Clin Invest 2007;117:514–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. North American Society for Pediatric Gastroenterology, Hepatology, Nutrition, and the Crohn’s and Colitis Foundation of America. Differentiating ulcerative colitis from Crohn disease in children and young adults: report of a working group of the North American Society for Pediatric Gastroenterology, Hepatology, and Nutrition and the Crohn’s and Colitis Foundation of America. J Pediatr Gastroenterol Nutr 2007;44:653–74. [DOI] [PubMed] [Google Scholar]
- 66. Hendrickson BA, Gokhale R, Cho JH.. Clinical aspects and pathophysiology of inflammatory bowel disease. Clin Microbiol Rev 2002;15:79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Magro F, Langner C, Driessen A, et al. ; European Society of Pathology (ESP). European consensus on the histopathology of inflammatory bowel disease. J Crohns Colitis 2013;7:827–51. [DOI] [PubMed] [Google Scholar]
- 68. Lockhart-Mummery H, Morson B.. Crohn’s disease (regional enteritis) of the large intestine and its distinction from ulcerative colitis. Gut 1960;1:87–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Geboes K. Histopathology of Crohn’s disease and ulcerative colitis. Inflammatory Bowel Disease 2003;4:210–28. [Google Scholar]
- 70. Ungaro R, Mehandru S, Allen PB, Peyrin-Biroulet L, Colombel JF.. Ulcerative colitis. Lancet 2017;389:1756–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Bernstein CN, Fried M, Krabshuis J, et al. World Gastroenterology Organization Practice Guidelines for the diagnosis and management of IBD in 2010. Inflamm Bowel Dis 2010;16:112–24. [DOI] [PubMed] [Google Scholar]
- 72. Kornbluth A, Sachar DB.. Ulcerative colitis practice guidelines in adults: American College of Gastroenterology, Practice Parameters Committee. ACG 2010;105:501–23. [DOI] [PubMed] [Google Scholar]
- 73. Ordas I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ.. Ulcerative colitis. Lancet 2012;380:1606–19. [DOI] [PubMed] [Google Scholar]
- 74. Levine JS, Burakoff R.. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Hepatol (N Y) 2011;7:235–41. [PMC free article] [PubMed] [Google Scholar]
- 75. Su CG, Judge TA, Lichtenstein GR.. Extraintestinal manifestations of inflammatory bowel disease. Gastroenterol Clin North Am 2002;31:307–27. [DOI] [PubMed] [Google Scholar]
- 76. Rothfuss KS, Stange EF, Herrlinger KR.. Extraintestinal manifestations and complications in inflammatory bowel diseases. World J Gastroenterol 2006;12:4819–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Harbord M, Annese V, Vavricka SR, et al. ; European Crohn’s and Colitis Organisation. The first European Evidence-based consensus on extra-intestinal manifestations in inflammatory bowel disease. J Crohns Colitis 2016;10:239–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Vavricka SR, Schoepfer A, Scharl M, Lakatos PL, Navarini A, Rogler G.. Extraintestinal manifestations of inflammatory bowel disease. Inflamm Bowel Dis 2015;21:1982–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Zippi M, Corrado C, Pica R, et al. Extraintestinal manifestations in a large series of Italian inflammatory bowel disease patients. World J Gastroenterol 2014;20:17463–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lauritano D, Boccalari E, Di Stasio D, et al. Prevalence of oral lesions and correlation with intestinal symptoms of inflammatory bowel disease: a systematic review. Diagnostics (Basel) 2019;9:77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Greuter T, Bertoldo F, Rechner R, et al. ; Swiss IBD Cohort Study Group. Extraintestinal manifestations of pediatric inflammatory bowel disease: prevalence, presentation, and anti-TNF treatment. J Pediatr Gastroenterol Nutr 2017;65:200–6. [DOI] [PubMed] [Google Scholar]
- 82. Koutsochristou V, Zellos A, Dimakou K, et al. Dental caries and periodontal disease in children and adolescents with inflammatory bowel disease: a case-control study. Inflamm Bowel Dis 2015;21:1839–46. [DOI] [PubMed] [Google Scholar]
- 83. Veloso FT, Carvalho J, Magro F.. Immune-related systemic manifestations of inflammatory bowel disease. A prospective study of 792 patients. J Clin Gastroenterol 1996;23:29–34. [DOI] [PubMed] [Google Scholar]
- 84. Lankarani KB, Sivandzadeh GR, Hassanpour S.. Oral manifestation in inflammatory bowel disease: a review. World J Gastroenterol 2013;19:8571–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Storwick GS, Prihoda MB, Fulton RJ, Wood WS.. Pyodermatitis-pyostomatitis vegetans: a specific marker for inflammatory bowel disease. J Am Acad Dermatol 1994;31:336–41. [DOI] [PubMed] [Google Scholar]
- 86. Harty S, Fleming P, Rowland M, et al. A prospective study of the oral manifestations of Crohn’s disease. Clin Gastroenterol Hepatol 2005;3:886–91. [DOI] [PubMed] [Google Scholar]
- 87. Katz J, Shenkman A, Stavropoulos F, Melzer E.. Oral signs and symptoms in relation to disease activity and site of involvement in patients with inflammatory bowel disease. Oral Dis 2003;9:34–40. [DOI] [PubMed] [Google Scholar]
- 88. Pittock S, Drumm B, Fleming P, et al. The oral cavity in Crohn’s disease. J Pediatr 2001;138:767–71. [DOI] [PubMed] [Google Scholar]
- 89. Muhvic-Urek M, Tomac-Stojmenovic M, Mijandrusic-Sincic B.. Oral pathology in inflammatory bowel disease. World J Gastroenterol 2016;22:5655–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Ojha J, Cohen DM, Islam NM, Stewart CM, Katz J, Bhattacharyya I.. Gingival involvement in Crohn disease. J Am Dent Assoc 2007;138:1574–81; quiz 1614. [DOI] [PubMed] [Google Scholar]
- 91. Eckel A, Lee D, Deutsch G, Maxin A, Oda D.. Oral manifestations as the first presenting sign of Crohn’s disease in a pediatric patient. J Clin Exp Dent 2017;9:e934–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Gillen CD, Walmsley RS, Prior P, Andrews HA, Allan RN.. Ulcerative colitis and Crohn’s disease: a comparison of the colorectal cancer risk in extensive colitis. Gut 1994;35:1590–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Choi PM, Zelig MP.. Similarity of colorectal cancer in Crohn’s disease and ulcerative colitis: implications for carcinogenesis and prevention. Gut 1994;35:950–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kuenzig ME, Manuel DG, Donelle J, Benchimol EI.. Life expectancy and health-adjusted life expectancy in people with inflammatory bowel disease. CMAJ 2020;192:E1394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Faye AS, Colombel JF.. Aging and IBD: a new challenge for clinicians and researchers. Inflamm Bowel Dis 2022;28:126–32. [DOI] [PubMed] [Google Scholar]
- 96. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nat Rev Gastroenterol Hepatol 2015;12:720–7. [DOI] [PubMed] [Google Scholar]
- 97. GBD Inflammatory Bowel Disease Collaborators. The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol 2020;5:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Ng SC, Shi HY, Hamidi N, et al. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet (London, England) 2017;390:2769–78. [DOI] [PubMed] [Google Scholar]
- 99. Kaplan GG. The global burden of IBD: from 2015 to 2025. Nature Reviews Gastroenterology & Hepatology 2015;12:720–7. [DOI] [PubMed] [Google Scholar]
- 100. Kappelman MD, Rifas-Shiman SL, Kleinman K, et al. The prevalence and geographic distribution of Crohn’s disease and ulcerative colitis in the United States. Clin Gastroenterol Hepatol 2007;5:1424–9. [DOI] [PubMed] [Google Scholar]
- 101. Shivashankar R, Tremaine WJ, Harmsen WS, Loftus EV. Jr. Incidence and prevalence of Crohn’s disease and ulcerative colitis in Olmsted County, Minnesota from 1970 through 2010. Clin Gastroenterol Hepatol 2017;15:857–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Loddo I, Romano C.. Inflammatory bowel disease: genetics, epigenetics, and pathogenesis. Front Immunol 2015;6:551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. de Souza HS, Fiocchi C.. Immunopathogenesis of IBD: current state of the art. Nat Rev Gastroenterol Hepatol 2016;13:13–27. [DOI] [PubMed] [Google Scholar]
- 104. Turpin W, Goethel A, Bedrani L, Croitoru Mdcm K.. Determinants of IBD heritability: genes, bugs, and more. Inflamm Bowel Dis 2018;24:1133–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Knights D, Lassen KG, Xavier RJ.. Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome. Gut 2013;62:1505–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Chang JT. Pathophysiology of inflammatory bowel diseases. N Engl J Med 2020;383:2652–64. [DOI] [PubMed] [Google Scholar]
- 107. Xavier RJ, Podolsky DK.. Unravelling the pathogenesis of inflammatory bowel disease. Nature 2007;448:427–34. [DOI] [PubMed] [Google Scholar]
- 108. Huang H, Fang M, Jostins L, et al. ; International Inflammatory Bowel Disease Genetics Consortium. Fine-mapping inflammatory bowel disease loci to single-variant resolution. Nature 2017;547:173–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Satsangi J, Parkes M, Louis E, et al. Two stage genome-wide search in inflammatory bowel disease provides evidence for susceptibility loci on chromosomes 3, 7 and 12. Nat Genet 1996;14:199–202. [DOI] [PubMed] [Google Scholar]
- 110. McGovern DP, Gardet A, Torkvist L, et al. ; NIDDK IBD Genetics Consortium. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat Genet 2010;42:332–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Anderson CA, Boucher G, Lees CW, et al. Meta-analysis identifies 29 additional ulcerative colitis risk loci, increasing the number of confirmed associations to 47. Nat Genet 2011;43:246–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Barrett JC, Hansoul S, Nicolae DL, et al. ; NIDDK IBD Genetics Consortium. Genome-wide association defines more than 30 distinct susceptibility loci for Crohn’s disease. Nat Genet 2008;40:955–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. de Lange KM, Moutsianas L, Lee JC, et al. Genome-wide association study implicates immune activation of multiple integrin genes in inflammatory bowel disease. Nat Genet 2017;49:256–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Ye BD, McGovern DP.. Genetic variation in IBD: progress, clues to pathogenesis and possible clinical utility. Expert Rev Clin Immunol 2016;12:1091–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Okamoto D, Kawai Y, Kakuta Y, et al. Genetic analysis of ulcerative colitis in Japanese individuals using population-specific SNP array. Inflamm Bowel Dis 2020;26:1177–87. [DOI] [PubMed] [Google Scholar]
- 116. Yang SK, Hong M, Zhao W, et al. Genome-wide association study of ulcerative colitis in Koreans suggests extensive overlapping of genetic susceptibility with Caucasians. Inflamm Bowel Dis 2013;19:954–66. [DOI] [PubMed] [Google Scholar]
- 117. Chen J, Tian W.. Explaining the disease phenotype of intergenic SNP through predicted long range regulation. Nucleic Acids Res 2016;44:8641–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Ramos PS, Criswell LA, Moser KL, et al. ; International Consortium on the Genetics of Systemic Erythematosus. A comprehensive analysis of shared loci between systemic lupus erythematosus (SLE) and sixteen autoimmune diseases reveals limited genetic overlap. PLoS Genet 2011;7:e1002406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Park SC, Jeen YT.. Genetic studies of inflammatory bowel disease-focusing on Asian patients. Cells 2019;8:404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Elding H, Lau W, Swallow DM, Maniatis N.. Dissecting the genetics of complex inheritance: linkage disequilibrium mapping provides insight into Crohn disease. Am J Hum Genet 2011;89:798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Jostins L, Ripke S, Weersma RK, et al. ; International IBD Genetics Consortium (IIBDGC). Host-microbe interactions have shaped the genetic architecture of inflammatory bowel disease. Nature 2012;491:119–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Raychaudhuri S, Plenge RM, Rossin EJ, et al. ; International Schizophrenia Consortium. Identifying relationships among genomic disease regions: predicting genes at pathogenic SNP associations and rare deletions. PLoS Genet 2009;5:e1000534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Elding H, Lau W, Swallow DM, Maniatis N.. Refinement in localization and identification of gene regions associated with Crohn disease. Am J Hum Genet 2013;92:107–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. De Jager PL, Jia X, Wang J, et al. ; International MS Genetics Consortium. Meta-analysis of genome scans and replication identify CD6, IRF8 and TNFRSF1A as new multiple sclerosis susceptibility loci. Nat Genet 2009;41:776–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Mayberry JF, Judd D, Smart H, Rhodes J, Calcraft B, Morris JS.. Crohn’s disease in Jewish people–an epidemiological study in south-east Wales. Digestion 1986;35:237–40. [DOI] [PubMed] [Google Scholar]
- 126. Ibrahim ML, Klement JD, Lu C, et al. Myeloid-derived suppressor cells produce IL-10 to elicit DNMT3b-dependent IRF8 silencing to promote colitis-associated colon tumorigenesis. Cell Rep 2018;25:3036–3046.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Zhang K, Hocker JD, Miller M, et al. A single-cell atlas of chromatin accessibility in the human genome. Cell 2021;184:5985–6001.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Ouyang X, Zhang R, Yang J, et al. Transcription factor IRF8 directs a silencing programme for TH17 cell differentiation. Nat Commun 2011;2:314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Zhang R, Qi CF, Hu Y, et al. T follicular helper cells restricted by IRF8 contribute to T cell-mediated inflammation. J Autoimmun 2019;96:113–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Cytlak U, Resteu A, Pagan S, et al. Differential IRF8 transcription factor requirement defines two pathways of dendritic cell development in humans. Immunity 2020;53:353–370.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Langlais D, Barreiro LB, Gros P.. The macrophage IRF8/IRF1 regulome is required for protection against infections and is associated with chronic inflammation. J Exp Med 2016;213:585–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Sichien D, Scott CL, Martens L, et al. IRF8 transcription factor controls survival and function of terminally differentiated conventional and plasmacytoid dendritic cells, respectively. Immunity 2016;45:626–40. [DOI] [PubMed] [Google Scholar]
- 133. Tamura T, Kurotaki D, Koizumi S.. Regulation of myelopoiesis by the transcription factor IRF8. Int J Hematol 2015;101:342–51. [DOI] [PubMed] [Google Scholar]
- 134. Marquis JF, Kapoustina O, Langlais D, et al. Interferon regulatory factor 8 regulates pathways for antigen presentation in myeloid cells and during tuberculosis. PLoS Genet 2011;7:e1002097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Lanca T, Ungerback J, Da Silva C, et al. IRF8 deficiency induces the transcriptional, functional, and epigenetic reprogramming of cDC1 into the cDC2 lineage. Immunity 2022;55:1431–1447.e11. [DOI] [PubMed] [Google Scholar]
- 136. Waller K, Scott CL.. Who on IRF are you? IRF8 deficiency redirects cDC1 lineage commitment. Trends Immunol 2022;43:687–9. [DOI] [PubMed] [Google Scholar]
- 137. Salem S, Salem D, Gros P.. Role of IRF8 in immune cells functions, protection against infections, and susceptibility to inflammatory diseases. Hum Genet 2020;139:707–21. [DOI] [PubMed] [Google Scholar]
- 138. Honda K, Taniguchi T.. IRFs. master regulators of signalling by Toll-like receptors and cytosolic pattern-recognition receptors. Nat Rev Immunol 2006;6:644–58. [DOI] [PubMed] [Google Scholar]
- 139. Yan M, Wang H, Sun J, et al. Cutting Edge: expression of IRF8 in gastric epithelial cells confers protective innate immunity against Helicobacter pylori infection. Journal of Immunology 2016;196:1999–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Li JY, Xiao J, Gao M, et al. IRF/Type I IFN signaling serves as a valuable therapeutic target in the pathogenesis of inflammatory bowel disease. Int Immunopharmacol 2021;92:107350. [DOI] [PubMed] [Google Scholar]
- 141. Thumbigere-Math V, Foster BL, Bachu M, et al. Inactivating mutation in IRF8 promotes osteoclast transcriptional programs and increases susceptibility to tooth root resorption. J Bone Miner Res 2019;34:1155–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Chu EY, Deeb JG, Foster BL, Hajishengallis E, Somerman MJ, Thumbigere-Math V.. Multiple idiopathic cervical root resorption: a challenge for a transdisciplinary medical-dental team. Front Dent Med 2021;2:652605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Das A, Yesupatham SK, Allison D, et al. Murine IRF8 mutation offers new insight into osteoclast and root resorption. J Dent Res 2024;103:318–28. doi: 10.1177/00220345231222173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Santos MPC, Gomes C, Torres J.. Familial and ethnic risk in inflammatory bowel disease. Ann Gastroenterol 2018;31:14–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Monsen U, Bernell O, Johansson C, Hellers G.. Prevalence of inflammatory bowel disease among relatives of patients with Crohn’s disease. Scand J Gastroenterol 1991;26:302–6. [DOI] [PubMed] [Google Scholar]
- 146. Nimmons D, Limdi JK.. Elderly patients and inflammatory bowel disease. World J Gastrointest Pharmacol Ther 2016;7:51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Loftus EV, Jr, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR.. Ulcerative colitis in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gut 2000;46:336–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Loftus EV Jr, Silverstein MD, Sandborn WJ, Tremaine WJ, Harmsen WS, Zinsmeister AR.. Crohn’s disease in Olmsted County, Minnesota, 1940-1993: incidence, prevalence, and survival. Gastroenterology 1998;114:1161–8. [DOI] [PubMed] [Google Scholar]
- 149. Taleban S, Colombel JF, Mohler MJ, Fain MJ.. Inflammatory bowel disease and the elderly: a review. J Crohns Colitis 2015;9:507–15. [DOI] [PubMed] [Google Scholar]
- 150. Burisch J, Pedersen N, Cukovic-Cavka S, et al. ; EpiCom-group. East-West gradient in the incidence of inflammatory bowel disease in Europe: the ECCO-EpiCom inception cohort. Gut 2014;63:588–97. [DOI] [PubMed] [Google Scholar]
- 151. Charpentier C, Salleron J, Savoye G, et al. Natural history of elderly-onset inflammatory bowel disease: a population-based cohort study. Gut 2014;63:423–32. [DOI] [PubMed] [Google Scholar]
- 152. Zheng H, Zhang C, Wang Q, Feng S, Fang Y, Zhang S.. The impact of aging on intestinal mucosal immune function and clinical applications. Front Immunol 2022;13:1029948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Sovran B, Hugenholtz F, Elderman M, et al. Age-associated impairment of the mucus barrier function is associated with profound changes in microbiota and immunity. Sci Rep 2019;9:1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Buford TW. (Dis)Trust your gut: the gut microbiome in age-related inflammation, health, and disease. Microbiome 2017;5:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Roth MP, Petersen GM, McElree C, Feldman E, Rotter JI.. Geographic origins of Jewish patients with inflammatory bowel disease. Gastroenterology 1989;97:900–4. [DOI] [PubMed] [Google Scholar]
- 156. Aniwan S, Harmsen WS, Tremaine WJ, Loftus EV Jr, Incidence of inflammatory bowel disease by race and ethnicity in a population-based inception cohort from 1970 through 2010. Therap Adv Gastroenterol 2019;12:1756284819827692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Lakatos PL, Szamosi T, Lakatos L.. Smoking in inflammatory bowel diseases: good, bad or ugly? World J Gastroenterol 2007;13:6134–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Berkowitz L, Schultz BM, Salazar GA, et al. Impact of cigarette smoking on the gastrointestinal tract inflammation: opposing effects in Crohn’s disease and ulcerative colitis. Front Immunol 2018;9:74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Carter MJ, Lobo AJ, Travis SP; IBD Section, British Society of Gastroenterology. Guidelines for the management of inflammatory bowel disease in adults. Gut 2004;53:V1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Lamb CA, Kennedy NA, Raine T, et al. ; IBD guidelines eDelphi consensus group. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut 2019;68:s1–s106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Neurath MF. Current and emerging therapeutic targets for IBD. Nat Rev Gastroenterol Hepatol 2017;14:269–78. [DOI] [PubMed] [Google Scholar]
- 162. Brito F, de Barros FC, Zaltman C, et al. Prevalence of periodontitis and DMFT index in patients with Crohn’s disease and ulcerative colitis. J Clin Periodontol 2008;35:555–60. [DOI] [PubMed] [Google Scholar]
- 163. Vavricka SR, Manser CN, Hediger S, et al. Periodontitis and gingivitis in inflammatory bowel disease: a case-control study. Inflamm Bowel Dis 2013;19:2768–77. [DOI] [PubMed] [Google Scholar]
- 164. Katsanos KH, Torres J, Roda G, Brygo A, Delaporte E, Colombel JF.. Review article: non-malignant oral manifestations in inflammatory bowel diseases. Aliment Pharmacol Ther 2015;42:40–60. [DOI] [PubMed] [Google Scholar]
- 165. Natah SS, Konttinen YT, Enattah NS, Ashammakhi N, Sharkey KA, Hayrinen-Immonen R.. Recurrent aphthous ulcers today: a review of the growing knowledge. Int J Oral Maxillofac Surg 2004;33:221–34. [DOI] [PubMed] [Google Scholar]
- 166. Landova H, Danek Z, Gajdziok J, Vetchy D, Stembirek J.. Oral mucosa and therapy of recurrent aphthous stomatitis. Ceska Slov Farm 2013;62:12–8. [PubMed] [Google Scholar]
- 167. Stewart CM, Watson RE.. Experimental oral foreign body reactions. Commonly employed dental materials. Oral Surg Oral Med Oral Pathol 1990;69:713–9. [DOI] [PubMed] [Google Scholar]
- 168. Aleksandra Nielsen A, Nederby Nielsen J, Schmedes A, Brandslund I, Hey H.. Saliva interleukin-6 in patients with inflammatory bowel disease. Scand J Gastroenterol 2005;40:1444–8. [DOI] [PubMed] [Google Scholar]
- 169. Szczeklik K, Owczarek D, Pytko-Polonczyk J, Kesek B, Mach TH.. Proinflammatory cytokines in the saliva of patients with active and non-active Crohn’s disease. Pol Arch Med Wewn 2012;122:200–8. [DOI] [PubMed] [Google Scholar]
- 170. Figueredo CM, Martins AP, Lira-Junior R, et al. Activity of inflammatory bowel disease influences the expression of cytokines in gingival tissue. Cytokine 2017;95:1–6. [DOI] [PubMed] [Google Scholar]
- 171. Damen GM, Hol J, de Ruiter L, et al. Chemokine production by buccal epithelium as a distinctive feature of pediatric Crohn disease. J Pediatr Gastroenterol Nutr 2006;42:142–9. [DOI] [PubMed] [Google Scholar]
- 172. Glocker EO, Kotlarz D, Boztug K, et al. Inflammatory bowel disease and mutations affecting the interleukin-10 receptor. N Engl J Med 2009;361:2033–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Lin Z, Wang Z, Hegarty JP, et al. Genetic association and epistatic interaction of the interleukin-10 signaling pathway in pediatric inflammatory bowel disease. World J Gastroenterol 2017;23:4897–909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174. Engelhardt KR, Shah N, Faizura-Yeop I, et al. Clinical outcome in IL-10- and IL-10 receptor-deficient patients with or without hematopoietic stem cell transplantation. J Allergy Clin Immunol 2013;131:825–30. [DOI] [PubMed] [Google Scholar]
- 175. Bachtiar EW, Cornain S, Siregar B, Raharjo TW.. Decreased CD4+/CD8+ ratio in major type of recurrent aphthous ulcers: comparing major to minor types of ulcers. Asian Pac J Allergy Immunol 1998;16:75–9. [PubMed] [Google Scholar]
- 176. Schmidt J, Weigert M, Leuschner C, et al. Active matrix metalloproteinase-8 and periodontal bacteria-interlink between periodontitis and inflammatory bowel disease? J Periodontol 2018;89:699–707. [DOI] [PubMed] [Google Scholar]
- 177. Figueredo CM, Brito F, Barros FC, et al. Expression of cytokines in the gingival crevicular fluid and serum from patients with inflammatory bowel disease and untreated chronic periodontitis. J Periodontal Res 2011;46:141–6. [DOI] [PubMed] [Google Scholar]
- 178. Enver A, Ozmeric N, Isler SC, et al. Evaluation of periodontal status and cytokine levels in saliva and gingival crevicular fluid of patients with inflammatory bowel diseases. J Periodontol 2022;93:1649–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Das KM, Vecchi M, Sakamaki S.. A shared and unique epitope(s) on human colon, skin, and biliary epithelium detected by a monoclonal antibody. Gastroenterology 1990;98:464–9. [DOI] [PubMed] [Google Scholar]
- 180. Bhagat S, Das KM.. A shared and unique peptide in the human colon, eye, and joint detected by a monoclonal antibody. Gastroenterology 1994;107:103–8. [DOI] [PubMed] [Google Scholar]
- 181. Hedin CRH, Vavricka SR, Stagg AJ, et al. The pathogenesis of extraintestinal manifestations: implications for IBD research, diagnosis, and therapy. J Crohns Colitis 2019;13:541–54. [DOI] [PubMed] [Google Scholar]
- 182. Petrova G, Ferrante A, Gorski J.. Cross-reactivity of T cells and its role in the immune system. Crit Rev Immunol 2012;32:349–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Zhao Q, Elson CO.. Adaptive immune education by gut microbiota antigens. Immunology 2018;154:28–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol 2006;3:390–407. [DOI] [PubMed] [Google Scholar]
- 185. Belkaid Y, Hand TW.. Role of the microbiota in immunity and inflammation. Cell 2014;157:121–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Ficarra G, Baroni G, Massi D.. Pyostomatitis vegetans: cellular immune profile and expression of IL-6, IL-8 and TNF-alpha. Head Neck Pathol 2010;4:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187. Asquith P, Thompson RA, Cooke WT.. Oral manifestations of Crohn’s disease. Gut 1975;16:249–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Tan CX, Brand HS, de Boer NK, Forouzanfar T.. Gastrointestinal diseases and their oro-dental manifestations: part 1: Crohn’s disease. Br Dent J 2016;221:794–9. [DOI] [PubMed] [Google Scholar]
- 189. Lira-Junior R, Figueredo CM.. Periodontal and inflammatory bowel diseases: is there evidence of complex pathogenic interactions? World J Gastroenterol 2016;22:7963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190. Abrol N, Compton SM, Graf D, Parashar P, Heo G, Gibson MP.. Inflammatory bowel disease and periodontitis: a retrospective chart analysis. Clin Exp Dent Res 2022;8:1028–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Surna A, Kubilius R, Sakalauskiene J, et al. Lysozyme and microbiota in relation to gingivitis and periodontitis. Med Sci Monit 2009;15:CR66–73. [PubMed] [Google Scholar]
- 192. Forchielli ML, Walker WA.. The role of gut-associated lymphoid tissues and mucosal defence. Br J Nutr 2005;93:S41–8. [DOI] [PubMed] [Google Scholar]
- 193. Cesta MF. Normal structure, function, and histology of mucosa-associated lymphoid tissue. Toxicol Pathol 2006;34:599–608. [DOI] [PubMed] [Google Scholar]
- 194. Hevia A, Milani C, Lopez P, et al. Intestinal dysbiosis associated with systemic lupus erythematosus. mBio 2014;5:e01548–01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195. Cappello F, Rappa F, Canepa F, et al. Probiotics can cure oral aphthous-like ulcers in inflammatory bowel disease patients: a review of the literature and a working hypothesis. Int J Mol Sci 2019;20:5026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Dudeney TP. Crohn’s disease of the mouth. Proc R Soc Med 1969;62:1237. [PMC free article] [PubMed] [Google Scholar]
- 197. Bottomley WK, Giorgini GL, Julienne CH.. Oral extension of regional enteritis (Crohn’s disease). Report of a case. Oral Surg Oral Med Oral Pathol 1972;34:417–20. [DOI] [PubMed] [Google Scholar]
- 198. Croft CB, Wilkinson AR.. Ulceration of the mouth, pharynx, and larynx in Crohn’s disease of the intestine. Br J Surg 1972;59:249–52. [DOI] [PubMed] [Google Scholar]
- 199. Lamster IB, Rodrick ML, Sonis ST, Falchuk ZM.. An analysis of peripheral blood and salivary polymorphonuclear leukocyte function, circulating immune complex levels and oral status in patients with inflammatory bowel disease. J Periodontol 1982;53:231–8. [DOI] [PubMed] [Google Scholar]
- 200. Van Dyke TE, Dowell VR, Offenbacher VRS, Snyder W, Hersh T.. Potential role of microorganisms isolated from periodontal lesions in the pathogenesis of inflammatory bowel disease. Infect Immun 1986;53:671–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201. Flemmig TF, Shanahan F, Miyasaki KT.. Prevalence and severity of periodontal disease in patients with inflammatory bowel disease. J Clin Periodontol 1991;18:690–7. [DOI] [PubMed] [Google Scholar]
- 202. Grossner-Schreiber B, Fetter T, Hedderich J, Kocher T, Schreiber S, Jepsen S.. Prevalence of dental caries and periodontal disease in patients with inflammatory bowel disease: a case-control study. J Clin Periodontol 2006;33:478–84. [DOI] [PubMed] [Google Scholar]
- 203. Habashneh RA, Khader YS, Alhumouz MK, Jadallah K, Ajlouni Y.. The association between inflammatory bowel disease and periodontitis among Jordanians: a case-control study. J Periodontal Res 2012;47:293–8. [DOI] [PubMed] [Google Scholar]
- 204. Chi YC, Chen JL, Wang LH, et al. Increased risk of periodontitis among patients with Crohn’s disease: a population-based matched-cohort study. Int J Colorectal Dis 2018;33:1437–44. [DOI] [PubMed] [Google Scholar]
- 205. Yu HC, Chen TP, Chang YC.. Inflammatory bowel disease as a risk factor for periodontitis under Taiwanese National Health Insurance Research database. J Dent Sci 2018;13:242–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Zhang L, Gao X, Zhou J, et al. Increased risks of dental caries and periodontal disease in Chinese patients with inflammatory bowel disease. Int Dent J 2020;70:227–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Bertl K, Burisch J, Pandis N, Bruckmann C, Klinge B, Stavropoulos A.. Periodontitis prevalence in patients with ulcerative colitis and Crohn’s disease - PPCC: a case-control study. J Clin Periodontol 2022;49:1262–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208. Wang Z, Li S, Tan D, et al. Association between inflammatory bowel disease and periodontitis: a bidirectional two-sample Mendelian randomization study. J Clin Periodontol 2023;50:736–43. [DOI] [PubMed] [Google Scholar]
- 209. Cotti E, Mezzena S, Schirru E, et al. Healing of apical periodontitis in patients with inflammatory bowel diseases and under anti-tumor necrosis factor alpha therapy. J Endod 2018;44:1777–82. [DOI] [PubMed] [Google Scholar]
- 210. Nijakowski K, Rutkowski R, Eder P, Korybalska K, Witowski J, Surdacka A.. Changes in salivary parameters of oral immunity after biologic therapy for inflammatory bowel disease. Life (Basel) 2021;11:1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Peddis N, Musu D, Ideo F, Rossi-Fedele G, Cotti E.. Interaction of biologic therapy with apical periodontitis and periodontitis: a systematic review. Aust Dent J 2019;64:122–34. [DOI] [PubMed] [Google Scholar]
- 212. Zhang Y, Chen J, Fu H, et al. Exosomes derived from 3D-cultured MSCs improve therapeutic effects in periodontitis and experimental colitis and restore the Th17 cell/Treg balance in inflamed periodontium. Int J Oral Sci 2021;13:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213. Bajaj JS, Matin P, White MB, et al. Periodontal therapy favorably modulates the oral–gut–hepatic axis in cirrhosis. Am J Physiol Gastrointest Liver Physiol 2018;315:G824–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. de Oliveira AM, Lourenco TGB, Colombo APV.. Impact of systemic probiotics as adjuncts to subgingival instrumentation on the oral–gut microbiota associated with periodontitis: a randomized controlled clinical trial. J Periodontol 2022;93:31–44. [DOI] [PubMed] [Google Scholar]
- 215. Nasidze I, Li J, Quinque D, Tang K, Stoneking M.. Global diversity in the human salivary microbiome. Genome Res 2009;19:636–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216. Gao L, Xu T, Huang G, Jiang S, Gu Y, Chen F.. Oral microbiomes: more and more importance in oral cavity and whole body. Protein Cell 2018;9:488–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217. Humphrey SP, Williamson RT.. A review of saliva: normal composition, flow, and function. J Prosthet Dent 2001;85:162–9. [DOI] [PubMed] [Google Scholar]
- 218. Sender R, Fuchs S, Milo R.. Revised estimates for the number of human and bacteria cells in the body. PLoS Biol 2016;14:e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Giannella RA, Broitman SA, Zamcheck N.. Gastric acid barrier to ingested microorganisms in man: studies in vivo and in vitro. Gut 1972;13:251–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Okumura R, Takeda K.. Roles of intestinal epithelial cells in the maintenance of gut homeostasis. Exp Mol Med 2017;49:e338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Johansson ME, Sjovall H, Hansson GC.. The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol 2013;10:352–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Kamada N, Chen GY, Inohara N, Nunez G.. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol 2013;14:685–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Kitamoto S, Nagao-Kitamoto H, Hein R, Schmidt TM, Kamada N.. The bacterial connection between the oral cavity and the gut diseases. J Dent Res 2020;99:1021–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224. Zhu H, Hart CA, Sales D, Roberts NB.. Bacterial killing in gastric juice–effect of pH and pepsin on Escherichia coli and Helicobacter pylori. J Med Microbiol 2006;55:1265–70. [DOI] [PubMed] [Google Scholar]
- 225. Martinsen TC, Bergh K, Waldum HL.. Gastric juice: a barrier against infectious diseases. Basic Clin Pharmacol Toxicol 2005;96:94–102. [DOI] [PubMed] [Google Scholar]
- 226. Sohn J, Sun Y, Genco R, Kirkwood K.. The periodontal microenvironment: a potential reservoir for intestinal pathobionts in Crohn’s disease. Current Oral Health Reports 2020;7:37–44. [Google Scholar]
- 227. Byrd KM, Gulati AS.. The “Gum-Gut” axis in inflammatory bowel diseases: a hypothesis-driven review of associations and advances. Front Immunol 2021;12:620124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228. Read E, Curtis MA, Neves JF.. The role of oral bacteria in inflammatory bowel disease. Nat Rev Gastroenterol Hepatol 2021;18:731–42. [DOI] [PubMed] [Google Scholar]
- 229. Arimatsu K, Yamada H, Miyazawa H, et al. Oral pathobiont induces systemic inflammation and metabolic changes associated with alteration of gut microbiota. Sci Rep 2014;4:4828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230. Lorenzo-Pouso AI, Castelo-Baz P, Rodriguez-Zorrilla S, Perez-Sayans M, Vega P.. Association between periodontal disease and inflammatory bowel disease: a systematic review and meta-analysis. Acta Odontol Scand 2021;79:344–53. [DOI] [PubMed] [Google Scholar]
- 231. Sohn J, Li L, Zhang L, et al. Porphyromonas gingivalis indirectly elicits intestinal inflammation by altering the gut microbiota and disrupting epithelial barrier function through IL9-producing CD4+ T cells. Mol Oral Microbiol 2022;37:42–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232. Chen X, Sun B, Li L, et al. The oral microbiome analysis reveals the similarities and differences between periodontitis and Crohn’s disease-associated periodontitis. FEMS Microbiol Lett 2022;369:fnac054. [DOI] [PubMed] [Google Scholar]
- 233. Pelaseyed T, Bergstrom JH, Gustafsson JK, et al. The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev 2014;260:8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234. Johansson ME, Hansson GC.. Immunological aspects of intestinal mucus and mucins. Nat Rev Immunol 2016;16:639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. De Lisle RC, Borowitz D.. The cystic fibrosis intestine. Cold Spring Harb Perspect Med 2013;3:a009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236. Allen JM, Mackos AR, Jaggers RM, et al. Psychological stress disrupts intestinal epithelial cell function and mucosal integrity through microbe and host-directed processes. Gut Microbes 2022;14:2035661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237. Bergstrom KS, Kissoon-Singh V, Gibson DL, et al. Muc2 protects against lethal infectious colitis by disassociating pathogenic and commensal bacteria from the colonic mucosa. PLoS Pathog 2010;6:e1000902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238. Tsukita S, Furuse M, Itoh M.. Multifunctional strands in tight junctions. Nat Rev Mol Cell Biol 2001;2:285–93. [DOI] [PubMed] [Google Scholar]
- 239. Michielan A, D’Inca R.. Intestinal permeability in inflammatory bowel disease: pathogenesis, clinical evaluation, and therapy of leaky gut. Mediators Inflamm 2015;2015:628157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240. Rolhion N, Chassaing B.. When pathogenic bacteria meet the intestinal microbiota. Philos Trans R Soc Lond B Biol Sci 2016;371:20150504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241. Sorbara MT, Pamer EG.. Correction: interbacterial mechanisms of colonization resistance and the strategies pathogens use to overcome them. Mucosal Immunol 2019;12:840. [DOI] [PubMed] [Google Scholar]
- 242. Rios-Covian D, Ruas-Madiedo P, Margolles A, Gueimonde M, de Los Reyes-Gavilan CG, Salazar N.. Intestinal short chain fatty acids and their link with diet and human health. Front Microbiol 2016;7:185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243. Petersen C, Round JL.. Defining dysbiosis and its influence on host immunity and disease. Cell Microbiol 2014;16:1024–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Freedberg DE, Toussaint NC, Chen SP, et al. Proton pump inhibitors alter specific taxa in the human gastrointestinal microbiome: a crossover trial. Gastroenterology 2015;149:883–5.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245. Belstrom D, Fiehn NE, Nielsen CH, et al. Differences in bacterial saliva profile between periodontitis patients and a control cohort. J Clin Periodontol 2014;41:104–12. [DOI] [PubMed] [Google Scholar]
- 246. Marcotte H, Lavoie MC.. Oral microbial ecology and the role of salivary immunoglobulin A. Microbiol Mol Biol Rev 1998;62:71–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247. Sedghi L, DiMassa V, Harrington A, Lynch SV, Kapila YL.. The oral microbiome: Role of key organisms and complex networks in oral health and disease. Periodontol 2000 2021;87:107–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248. Sato K, Takahashi N, Kato T, et al. Aggravation of collagen-induced arthritis by orally administered Porphyromonas gingivalis through modulation of the gut microbiota and gut immune system. Sci Rep 2017;7:6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Ismail Y, Mahendran V, Octavia S, et al. Investigation of the enteric pathogenic potential of oral Campylobacter concisus strains isolated from patients with inflammatory bowel disease. PLoS One 2012;7:e38217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250. Bhattacharyya A, Chattopadhyay R, Mitra S, Crowe SE.. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol Rev 2014;94:329–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Sigthorsson G, Tibble J, Hayllar J, et al. Intestinal permeability and inflammation in patients on NSAIDs. Gut 1998;43:506–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252. Rogers MAM, Aronoff DM.. The influence of non-steroidal anti-inflammatory drugs on the gut microbiome. Clin Microbiol Infect 2016;22:178.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Zhernakova A, Kurilshikov A, Bonder MJ, et al. ; LifeLines cohort study. Population-based metagenomics analysis reveals markers for gut microbiome composition and diversity. Science 2016;352:565–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Imhann F, Bonder MJ, Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut 2016;65:740–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Abed J, Maalouf N, Manson AL, et al. Colon cancer-associated Fusobacterium nucleatum may originate from the oral cavity and reach colon tumors via the circulatory system. Front Cell Infect Microbiol 2020;10:400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Round JL, Mazmanian SK.. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol 2009;9:313–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Maynard CL, Elson CO, Hatton RD, Weaver CT.. Reciprocal interactions of the intestinal microbiota and immune system. Nature 2012;489:231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Caselli E, Fabbri C, D’Accolti M, et al. Defining the oral microbiome by whole-genome sequencing and resistome analysis: the complexity of the healthy picture. BMC Microbiol 2020;20:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259. Wade WG. The oral microbiome in health and disease. Pharmacol Res 2013;69:137–43. [DOI] [PubMed] [Google Scholar]
- 260. Curtis MA, Diaz PI, Van Dyke TE.. The role of the microbiota in periodontal disease. Periodontol 2000 2020;83:14–25. [DOI] [PubMed] [Google Scholar]
- 261. Escapa IF, Chen T, Huang Y, Gajare P, Dewhirst FE, Lemon KP.. New insights into human nostril microbiome from the expanded human oral microbiome database (eHOMD): a resource for the microbiome of the human aerodigestive Tract. mSystems 2018;3:e00187-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262. Aleti G, Baker JL, Tang X, et al. Identification of the bacterial biosynthetic gene clusters of the oral microbiome illuminates the unexplored social language of bacteria during health and disease. mBio 2019;10:e00321-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. McLean JS, Bor B, Kerns KA, et al. Acquisition and adaptation of ultra-small parasitic reduced genome bacteria to mammalian hosts. Cell Rep 2020;32:107939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264. He X, McLean JS, Edlund A, et al. Cultivation of a human-associated TM7 phylotype reveals a reduced genome and epibiotic parasitic lifestyle. Proc Natl Acad Sci U S A 2015;112:244–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Bor B, Bedree JK, Shi W, McLean JS, He X.. Saccharibacteria (TM7) in the human oral microbiome. J Dent Res 2019;98:500–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Hajishengallis G, Liang S, Payne MA, et al. Low-abundance biofilm species orchestrates inflammatory periodontal disease through the commensal microbiota and complement. Cell Host Microbe 2011;10:497–506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Hajishengallis G, Darveau RP, Curtis MA.. The keystone-pathogen hypothesis. Nat Rev Microbiol 2012;10:717–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Lamont RJ, Jenkinson HF.. Subgingival colonization by Porphyromonas gingivalis. Oral Microbiol Immunol 2000;15:341–9. [DOI] [PubMed] [Google Scholar]
- 269. Lamont RJ, Jenkinson HF.. Life below the gum line: pathogenic mechanisms of Porphyromonas gingivalis. Microbiol Mol Biol Rev 1998;62:1244–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270. Holt SC, Kesavalu L, Walker S, Genco CA.. Virulence factors of Porphyromonas gingivalis. Periodontology 2000 1999;20:168–238. [DOI] [PubMed] [Google Scholar]
- 271. Travis J, Pike R, Imamura T, Potempa J.. Porphyromonas gingivalis proteinases as virulence factors in the development of periodontitis. J Periodontal Res 1997;32:120–5. [DOI] [PubMed] [Google Scholar]
- 272. Signat B, Roques C, Poulet P, Duffaut D.. Role of Fusobacterium nucleatum in periodontal health and disease. Curr Issues Mol Biol 2011;13:25–36. [PubMed] [Google Scholar]
- 273. Settem RP, El-Hassan AT, Honma K, Stafford GP, Sharma A.. Fusobacterium nucleatum and Tannerella forsythia induce synergistic alveolar bone loss in a mouse periodontitis model. Infect Immun 2012;80:2436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274. Zambon JJ. Actinobacillus actinomycetemcomitans in human periodontal disease. J Clin Periodontol 1985;12:1–20. [DOI] [PubMed] [Google Scholar]
- 275. Slots J, Reynolds HS, Genco RJ.. Actinobacillus actinomycetemcomitans in human periodontal disease: a cross-sectional microbiological investigation. Infect Immun 1980;29:1013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276. Zambon JJ, Christersson LA, Slots J.. Actinobacillus actinomycetemcomitans in human periodontal disease: prevalence in patient groups and distribution of biotypes and serotypes within families. J Periodontol 1983;54:707–11. [DOI] [PubMed] [Google Scholar]
- 277. Fives-Taylor PM, Meyer DH, Mintz KP, Brissette C.. Virulence factors of Actinobacillus actinomycetemcomitans. Periodontology 2000 1999;20:136–67. [DOI] [PubMed] [Google Scholar]
- 278. Wilson M, Henderson B.. Virulence factors of Actinobacillus actinomycetemcomitans relevant to the pathogenesis of inflammatory periodontal diseases. FEMS Microbiol Rev 1995;17:365–79. [DOI] [PubMed] [Google Scholar]
- 279. Kolenbrander PE, Andersen RN, Blehert DS, Egland PG, Foster JS, Palmer RJ. Jr.. Communication among oral bacteria. Microbiol Mol Biol Rev 2002;66:486–505, table of contents. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Kaplan CW, Ma X, Paranjpe A, et al. Fusobacterium nucleatum outer membrane proteins Fap2 and RadD induce cell death in human lymphocytes. Infect Immun 2010;78:4773–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Han YW, Shi W, Huang GT, et al. Interactions between periodontal bacteria and human oral epithelial cells: Fusobacterium nucleatum adheres to and invades epithelial cells. Infect Immun 2000;68:3140–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282. Sharma A. Virulence mechanisms of Tannerella forsythia. Periodontol 2000 2010;54:106–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Sharma A, Inagaki S, Honma K, Sfintescu C, Baker PJ, Evans RT.. Tannerella forsythia-induced alveolar bone loss in mice involves leucine-rich-repeat BspA protein. J Dent Res 2005;84:462–7. [DOI] [PubMed] [Google Scholar]
- 284. Sekirov I, Russell SL, Antunes LCM, Finlay BB.. Gut microbiota in health and disease. Physiol Rev 2010;90:859–904. [DOI] [PubMed] [Google Scholar]
- 285. Kamada N, Chen GY, Inohara N, Núñez G.. Control of pathogens and pathobionts by the gut microbiota. Nat Immunol 2013;14:685–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286. Eckburg PB, Bik EM, Bernstein CN, et al. Diversity of the human intestinal microbial flora. Science 2005;308:1635–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287. Thursby E, Juge N.. Introduction to the human gut microbiota. Biochem J 2017;474:1823–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288. Wu HJ, Wu E.. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012;3:4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289. Rowland I, Gibson G, Heinken A, et al. Gut microbiota functions: metabolism of nutrients and other food components. Eur J Nutr 2018;57:1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Drewes JL, White JR, Dejea CM, et al. High-resolution bacterial 16S rRNA gene profile meta-analysis and biofilm status reveal common colorectal cancer consortia. NPJ Biofilms Microbiomes 2017;3:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Vieira-Silva S, Sabino J, Valles-Colomer M, et al. Quantitative microbiome profiling disentangles inflammation- and bile duct obstruction-associated microbiota alterations across PSC/IBD diagnoses. Nat Microbiol 2019;4:1826–31. [DOI] [PubMed] [Google Scholar]
- 292. Liu H, Hong XL, Sun TT, Huang XW, Wang JL, Xiong H.. Fusobacterium nucleatum exacerbates colitis by damaging epithelial barriers and inducing aberrant inflammation. J Dig Dis 2020;21:385–98. [DOI] [PubMed] [Google Scholar]
- 293. Flemer B, Lynch DB, Brown JM, et al. Tumour-associated and non-tumour-associated microbiota in colorectal cancer. Gut 2017;66:633–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294. Ahn J, Sinha R, Pei Z, et al. Human gut microbiome and risk for colorectal cancer. J Natl Cancer Inst 2013;105:1907–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Yang Y, Misra BB, Liang L, et al. Integrated microbiome and metabolome analysis reveals a novel interplay between commensal bacteria and metabolites in colorectal cancer. Theranostics 2019;9:4101–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Wang X, Jia Y, Wen L, et al. Porphyromonas gingivalis promotes colorectal carcinoma by activating the hematopoietic NLRP3 inflammasome. Cancer Res 2021;81:2745–59. [DOI] [PubMed] [Google Scholar]
- 297. Lee YC, Liu CY, Lee CL, Zhang RH, Huang CJ, Yen TL.. The periodontopathic pathogen, Porphyromonas gingivalis, involves a gut inflammatory response and exacerbates inflammatory bowel disease. Pathogens 2022;11:84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298. Lloyd-Price J, Arze C, Ananthakrishnan AN, et al. ; IBDMDB Investigators. Multi-omics of the gut microbial ecosystem in inflammatory bowel diseases. Nature 2019;569:655–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299. Imai J, Ichikawa H, Kitamoto S, et al. A potential pathogenic association between periodontal disease and Crohn’s disease. JCI Insight 2021;6:e148543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300. Man SM, Zhang L, Day AS, Leach ST, Lemberg DA, Mitchell H.. Campylobacter concisus and other Campylobacter species in children with newly diagnosed Crohn’s disease. Inflamm Bowel Dis 2010;16:1008–16. [DOI] [PubMed] [Google Scholar]
- 301. Kirk KF, Nielsen HL, Thorlacius-Ussing O, Nielsen H.. Optimized cultivation of Campylobacter concisus from gut mucosal biopsies in inflammatory bowel disease. Gut Pathog 2016;8:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302. Meurman JH, Halme L, Laine P, von Smitten K, Lindqvist C.. Gingival and dental status, salivary acidogenic bacteria, and yeast counts of patients with active or inactive Crohn’s disease. Oral Surg Oral Med Oral Pathol 1994;77:465–8. [DOI] [PubMed] [Google Scholar]
- 303. Stein JM, Lammert F, Zimmer V, et al. Clinical periodontal and microbiologic parameters in patients with Crohn’s disease with consideration of the CARD15 genotype. J Periodontol 2010;81:535–45. [DOI] [PubMed] [Google Scholar]
- 304. Docktor MJ, Paster BJ, Abramowicz S, et al. Alterations in diversity of the oral microbiome in pediatric inflammatory bowel disease. Inflamm Bowel Dis 2012;18:935–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305. Kelsen J, Bittinger K, Pauly-Hubbard H, et al. Alterations of the subgingival microbiota in pediatric Crohn’s disease studied longitudinally in discovery and validation cohorts. Inflamm Bowel Dis 2015;21:2797–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306. Baima G, Massano A, Squillace E, et al. Shared microbiological and immunological patterns in periodontitis and IBD: a scoping review. Oral Dis 2022;28:1029–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307. Brewer RC, Lanz TV, Hale CR, et al. Oral mucosal breaks trigger anti-citrullinated bacterial and human protein antibody responses in rheumatoid arthritis. Sci Transl Med 2023;15:eabq8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308. de Molon RS, Rossa C Jr, Thurlings RM, Cirelli JA, Koenders MI.. Linkage of periodontitis and rheumatoid arthritis: current evidence and potential biological interactions. Int J Mol Sci 2019;20:4541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309. Brakenhoff LK, van der Heijde DM, Hommes DW, Huizinga TW, Fidder HH.. The joint-gut axis in inflammatory bowel diseases. J Crohns Colitis 2010;4:257–68. [DOI] [PubMed] [Google Scholar]
- 310. Rodriguez-Reyna TS, Martinez-Reyes C, Yamamoto-Furusho JK.. Rheumatic manifestations of inflammatory bowel disease. World J Gastroenterol 2009;15:5517–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 311. Voulgari PV. Rheumatological manifestations in inflammatory bowel disease. Ann Gastroenterol 2011;24:173–80. [PMC free article] [PubMed] [Google Scholar]
- 312. Vitkov L, Hannig M, Minnich B, Herrmann M.. Periodontal sources of citrullinated antigens and TLR agonists related to RA. Autoimmunity 2018;51:304–9. [DOI] [PubMed] [Google Scholar]
- 313. Gomez-Banuelos E, Mukherjee A, Darrah E, Andrade F.. Rheumatoid Arthritis-associated mechanisms of Porphyromonas gingivalis and Aggregatibacter actinomycetemcomitans. J Clin Med 2019;8:1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 314. Moller B, Kollert F, Sculean A, Villiger PM.. Infectious triggers in periodontitis and the gut in rheumatoid arthritis (RA): a complex story about association and causality. Front Immunol 2020;11:1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315. Ciesielski O, Biesiekierska M, Panthu B, Soszynski M, Pirola L, Balcerczyk A.. Citrullination in the pathology of inflammatory and autoimmune disorders: recent advances and future perspectives. Cell Mol Life Sci 2022;79:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316. Gyorgy B, Toth E, Tarcsa E, Falus A, Buzas EI.. Citrullination: a posttranslational modification in health and disease. Int J Biochem Cell Biol 2006;38:1662–77. [DOI] [PubMed] [Google Scholar]
- 317. Falcao AM, Meijer M, Scaglione A, et al. PAD2-mediated citrullination contributes to efficient oligodendrocyte differentiation and myelination. Cell Rep 2019;27:1090–1102.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318. Hensen SM, Pruijn GJ.. Methods for the detection of peptidylarginine deiminase (PAD) activity and protein citrullination. Mol Cell Proteomics 2014;13:388–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319. Curran AM, Girgis AA, Jang Y, et al. Citrullination modulates antigen processing and presentation by revealing cryptic epitopes in rheumatoid arthritis. Nat Commun 2023;14:1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320. Darrah E, Andrade F.. Rheumatoid arthritis and citrullination. Curr Opin Rheumatol 2018;30:72–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 321. Roudier J, Auger I.. How does citrullination contribute to RA autoantibody development? Nat Rev Rheumatol 2023;19:329–30. [DOI] [PubMed] [Google Scholar]
- 322. Dragoni G, De Hertogh G, Vermeire S.. The role of citrullination in inflammatory bowel disease: a neglected player in triggering inflammation and fibrosis? Inflamm Bowel Dis 2021;27:134–44. [DOI] [PubMed] [Google Scholar]
- 323. Sherina N, de Vries C, Kharlamova N, et al. Antibodies to a citrullinated Porphyromonas gingivalis epitope are increased in early rheumatoid arthritis, and can be produced by gingival tissue B cells: implications for a bacterial origin in RA etiology. Front Immunol 2022;13:804822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324. Gomez-Banuelos E, Konig MF, Andrade F.. Microbial pathways to subvert host immunity generate citrullinated neoantigens targeted in rheumatoid arthritis. Curr Opin Struct Biol 2022;75:102423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 325. Garabatos N, Santamaria P.. Gut microbial antigenic mimicry in autoimmunity. Front Immunol 2022;13:873607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 326. Yusung S, Braun J.. Molecular mimicry, inflammatory bowel disease, and the vaccine safety debate. BMC Med 2014;12:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327. Verma A, Sharda S, Rathi B, Somvanshi P, Pandey BD.. Elucidating potential molecular signatures through host-microbe interactions for reactive arthritis and inflammatory bowel disease using combinatorial approach. Sci Rep 2020;10:15131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328. Janssen KMJ, Hop H, Vissink A, et al. Levels of anti-citrullinated protein antibodies and rheumatoid factor, including iga isotypes, and articular manifestations in ulcerative colitis and Crohn’s disease. Int J Environ Res Public Health 2020;17:8054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329. Yamazaki H, Kuroiwa T, Shinmura K, Yukioka M, Murata N.. Prevalence of anti-cyclic citrullinated peptide antibodies in patients with spondyloarthritis: a retrospective study. Mod Rheumatol 2021;31:458–61. [DOI] [PubMed] [Google Scholar]
- 330. Koutroubakis IE, Karmiris K, Bourikas L, Kouroumalis EA, Drygiannakis I, Drygiannakis D.. Antibodies against cyclic citrullinated peptide (CCP) in inflammatory bowel disease patients with or without arthritic manifestations. Inflamm Bowel Dis 2007;13:504–5. [DOI] [PubMed] [Google Scholar]
- 331. Afrasiabi S, Chiniforush N, Partoazar A, Goudarzi R.. The role of bacterial infections in rheumatoid arthritis development and novel therapeutic interventions: focus on oral infections. J Clin Lab Anal 2023;37:e24897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332. Kurowska W, Kuca-Warnawin EH, Radzikowska A, Maslinski W.. The role of anti-citrullinated protein antibodies (ACPA) in the pathogenesis of rheumatoid arthritis. Cent Eur J Immunol 2017;42:390–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333. Drury B, Hardisty G, Gray RD, Ho GT.. Neutrophil extracellular traps in inflammatory bowel disease: pathogenic mechanisms and clinical translation. Cell Mol Gastroenterol Hepatol 2021;12:321–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334. Dos Santos Ramos A, Viana GCS, de Macedo Brigido M, Almeida JF.. Neutrophil extracellular traps in inflammatory bowel diseases: implications in pathogenesis and therapeutic targets. Pharmacol Res 2021;171:105779. [DOI] [PubMed] [Google Scholar]
- 335. Maronek M, Gardlik R.. The citrullination-neutrophil extracellular trap axis in chronic diseases. J Innate Immun 2022;14:393–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 336. Hajishengallis G, Chavakis T, Hajishengallis E, Lambris JD.. Neutrophil homeostasis and inflammation: novel paradigms from studying periodontitis. J Leukoc Biol 2015;98:539–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337. Moutsopoulos NM, Konkel J, Sarmadi M, et al. Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss. Sci Transl Med 2014;6:229ra240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338. Fournier BM, Parkos CA.. The role of neutrophils during intestinal inflammation. Mucosal Immunol 2012;5:354–66. [DOI] [PubMed] [Google Scholar]
- 339. Wera O, Lancellotti P, Oury C.. The dual role of neutrophils in inflammatory bowel diseases. J Clin Med 2016;5:118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340. Yucel-Lindberg T, Bage T.. Inflammatory mediators in the pathogenesis of periodontitis. Expert Rev Mol Med 2013;15:e7. [DOI] [PubMed] [Google Scholar]
- 341. Sochalska M, Potempa J.. Manipulation of neutrophils by porphyromonas gingivalis in the development of periodontitis. Front Cell Infect Microbiol 2017;7:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342. Zundler S, Becker E, Schulze LL, Neurath MF.. Immune cell trafficking and retention in inflammatory bowel disease: mechanistic insights and therapeutic advances. Gut 2019;68:1688–700. [DOI] [PubMed] [Google Scholar]
- 343. Zhan C, Zhou Z, Huang Y, et al. Exploration of the shared gene signatures and molecular mechanisms between periodontitis and inflammatory bowel disease: evidence from transcriptome data. Gastroenterol Rep (Oxf) 2023;11:goad041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344. Foell D, Wittkowski H, Vogl T, Roth J.. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J Leukoc Biol 2007;81:28–37. [DOI] [PubMed] [Google Scholar]
- 345. Johnstone KF, Wei Y, Bittner-Eddy PD, et al. Calprotectin (S100A8/A9) is an innate immune effector in experimental periodontitis. Infect Immun 2021;89:e0012221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346. Jukic A, Bakiri L, Wagner EF, Tilg H, Adolph TE.. Calprotectin: from biomarker to biological function. Gut 2021;70:1978–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347. Figueredo CM, Nunes JGR, Mello-Neto JM, Carvalho AT, Ipe DS.. Higher salivary expression of S100A12 in patients with ulcerative colitis and chronic periodontitis. Eur J Gastroenterol Hepatol 2021;33:116–7. [DOI] [PubMed] [Google Scholar]
- 348. Yu S, Ding L, Liang D, Luo L.. Porphyromonas gingivalis inhibits M2 activation of macrophages by suppressing alpha-ketoglutarate production in mice. Mol Oral Microbiol 2018;33:388–95. [DOI] [PubMed] [Google Scholar]
- 349. Liu L, Liang L, Liang H, et al. Fusobacterium nucleatum aggravates the progression of colitis by regulating M1 macrophage polarization via AKT2 pathway. Front Immunol 2019;10:1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 350. Lin Y, Yang X, Yue W, et al. Chemerin aggravates DSS-induced colitis by suppressing M2 macrophage polarization. Cell Mol Immunol 2014;11:355–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351. Kuhl AA, Erben U, Kredel LI, Siegmund B.. Diversity of intestinal macrophages in inflammatory bowel diseases. Front Immunol 2015;6:613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352. Banchereau J, Steinman RM.. Dendritic cells and the control of immunity. Nature 1998;392:245–52. [DOI] [PubMed] [Google Scholar]
- 353. Niess JH, Brand S, Gu X, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science 2005;307:254–8. [DOI] [PubMed] [Google Scholar]
- 354. Cutler CW, Jotwani R.. Dendritic cells at the oral mucosal interface. J Dent Res 2006;85:678–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355. Herbert BA, Novince CM, Kirkwood KL.. Aggregatibacter actinomycetemcomitans, a potent immunoregulator of the periodontal host defense system and alveolar bone homeostasis. Mol Oral Microbiol 2016;31:207–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356. Jotwani R, Pulendran B, Agrawal S, Cutler CW.. Human dendritic cells respond to Porphyromonas gingivalis LPS by promoting a Th2 effector response in vitro. Eur J Immunol 2003;33:2980–6. [DOI] [PubMed] [Google Scholar]
- 357. Becker C, Wirtz S, Blessing M, et al. Constitutive p40 promoter activation and IL-23 production in the terminal ileum mediated by dendritic cells. J Clin Invest 2003;112:693–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358. Stagg AJ, Hart AL, Knight SC, Kamm MA.. The dendritic cell: its role in intestinal inflammation and relationship with gut bacteria. Gut 2003;52:1522–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359. Moutsopoulos NM, Kling HM, Angelov N, et al. Porphyromonas gingivalis promotes Th17 inducing pathways in chronic periodontitis. J Autoimmun 2012;39:294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 360. Jia L, Wu R, Han N, et al. Porphyromonas gingivalis and Lactobacillus rhamnosus GG regulate the Th17/Treg balance in colitis via TLR4 and TLR2. Clin Transl Immunology 2020;9:e1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361. Menegat JS, Lira-Junior R, Siqueira MA, et al. Cytokine expression in gingival and intestinal tissues of patients with periodontitis and inflammatory bowel disease: An exploratory study. Arch Oral Biol 2016;66:141–6. [DOI] [PubMed] [Google Scholar]
- 362. Champaiboon C, Yongvanitchit K, Pichyangkul S, Mahanonda R.. The immune modulation of B-cell responses by Porphyromonas ginginvalis and interleukin-10. J Periodontol 2000;71:468–75. [DOI] [PubMed] [Google Scholar]
- 363. Danielsen AK, Damgaard C, Massarenti L, et al. B-cell cytokine responses to Porphyromonas gingivalis in patients with periodontitis and healthy controls. J Periodontol 2023;94:997–1007. [DOI] [PubMed] [Google Scholar]
- 364. Noronha AM, Liang Y, Hetzel JT, et al. Hyperactivated B cells in human inflammatory bowel disease. J Leukoc Biol 2009;86:1007–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365. Uzzan M, Martin JC, Mesin L, et al. Ulcerative colitis is characterized by a plasmablast-skewed humoral response associated with disease activity. Nat Med 2022;28:766–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366. Nakajima M, Arimatsu K, Kato T, et al. Oral administration of P. gingivalis induces dysbiosis of gut microbiota and impaired barrier function leading to dissemination of enterobacteria to the liver. PLoS One 2015;10:e0134234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 367. Liu Y, Huang W, Dai K, et al. Inflammatory response of gut, spleen, and liver in mice induced by orally administered Porphyromonas gingivalis. J Oral Microbiol 2022;14:2088936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368. Qian J, Lu J, Huang Y, et al. Periodontitis salivary microbiota worsens colitis. J Dent Res 2022;101:559–68. [DOI] [PubMed] [Google Scholar]
- 369. Baumgart DC, Carding SR.. Inflammatory bowel disease: cause and immunobiology. Lancet 2007;369:1627–40. [DOI] [PubMed] [Google Scholar]
- 370. Sohn J, Li L, Zhang L, et al. Periodontal disease is associated with increased gut colonization of pathogenic Haemophilus parainfluenzae in patients with Crohn’s disease. Cell Rep 2023;42:112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371. Chassaing B, Aitken JD, Malleshappa M, Vijay-Kumar M.. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr Protoc Immunol. 2014;104:15 25 11–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372. Kiesler P, Fuss IJ, Strober W.. Experimental models of inflammatory bowel diseases. Cell Mol Gastroenterol Hepatol 2015;1:154–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373. Wirtz S, Popp V, Kindermann M, et al. Chemically induced mouse models of acute and chronic intestinal inflammation. Nat Protoc 2017;12:1295–309. [DOI] [PubMed] [Google Scholar]
- 374. Mizoguchi A. Animal models of inflammatory bowel disease. Prog Mol Biol Transl Sci 2012;105:263–320. [DOI] [PubMed] [Google Scholar]
- 375. Tsuzuno T, Takahashi N, Yamada-Hara M, et al. Ingestion of Porphyromonas gingivalis exacerbates colitis via intestinal epithelial barrier disruption in mice. J Periodontal Res 2021;56:275–88. [DOI] [PubMed] [Google Scholar]
- 376. Wang X, Luo Y, Huang Y, et al. Periodontitis-induced oral microbiome alterations provide clues on how periodontitis exacerbates colitis. J Clin Periodontol 2023;50:627–41. [DOI] [PubMed] [Google Scholar]
- 377. Lin S, Zhang X, Zhu X, et al. Fusobacterium nucleatum aggravates ulcerative colitis through promoting gut microbiota dysbiosis and dysmetabolism. J Periodontol 2023;94:405–18. [DOI] [PubMed] [Google Scholar]
- 378. Oz HS, Ebersole JL.. A novel murine model for chronic inflammatory alveolar bone loss. J Periodontal Res 2010;45:94–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379. Oz HS, Chen T, Ebersole JL.. A model for chronic mucosal inflammation in IBD and periodontitis. Dig Dis Sci 2010;55:2194–202. [DOI] [PubMed] [Google Scholar]
- 380. de Mello-Neto JM, Elangovan G, Ervolino E, Johnson NW, Gustafsson A, da Figueredo CM.. Colitis induced by dextran sulphate sodium causes histopathological and immunological changes in the periodontal tissues of Wistar rats. J Periodontal Res 2022;57:1267–76. [DOI] [PubMed] [Google Scholar]
- 381. Rautava J, Pinnell LJ, Vong L, Akseer N, Assa A, Sherman PM.. Oral microbiome composition changes in mouse models of colitis. J Gastroenterol Hepatol 2015;30:521–7. [DOI] [PubMed] [Google Scholar]
- 382. Metzger CE, Narayanan SA, Elizondo JP, et al. DSS-induced colitis produces inflammation-induced bone loss while irisin treatment mitigates the inflammatory state in both gut and bone. Sci Rep 2019;9:15144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 383. Hamdani G, Gabet Y, Rachmilewitz D, Karmeli F, Bab I, Dresner-Pollak R.. Dextran sodium sulfate-induced colitis causes rapid bone loss in mice. Bone 2008;43:945–50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.