Abstract
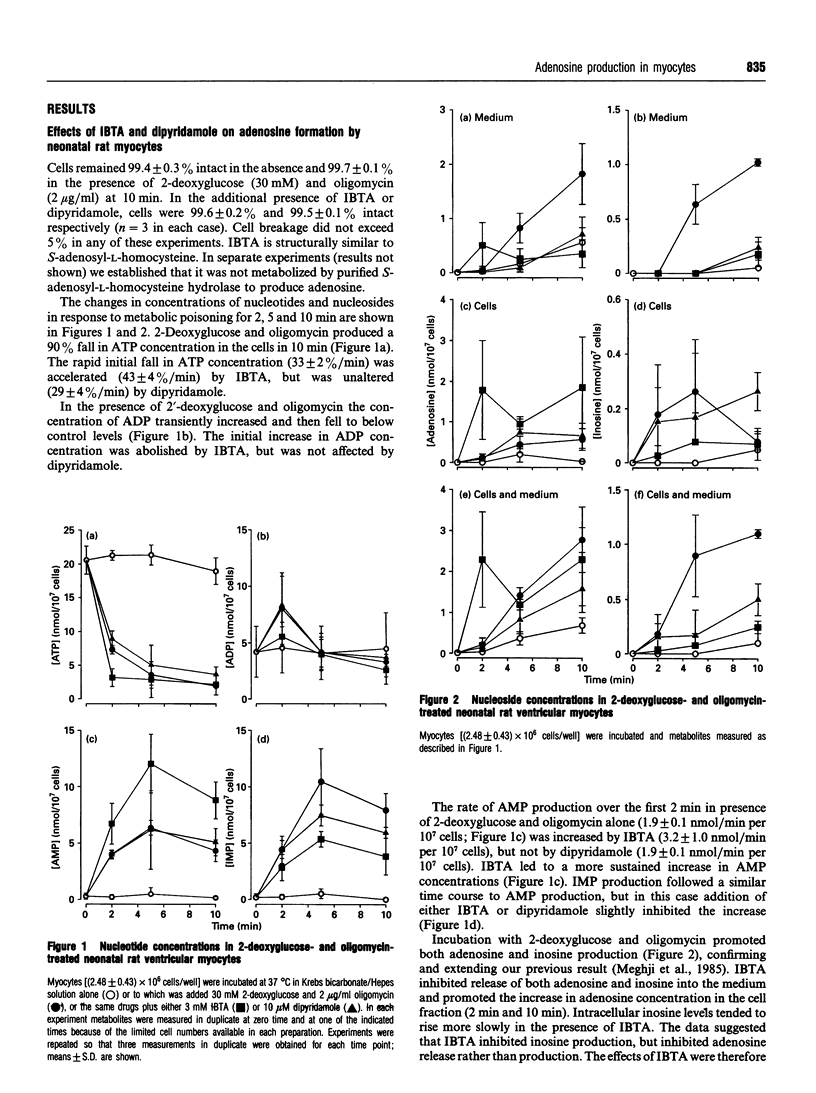
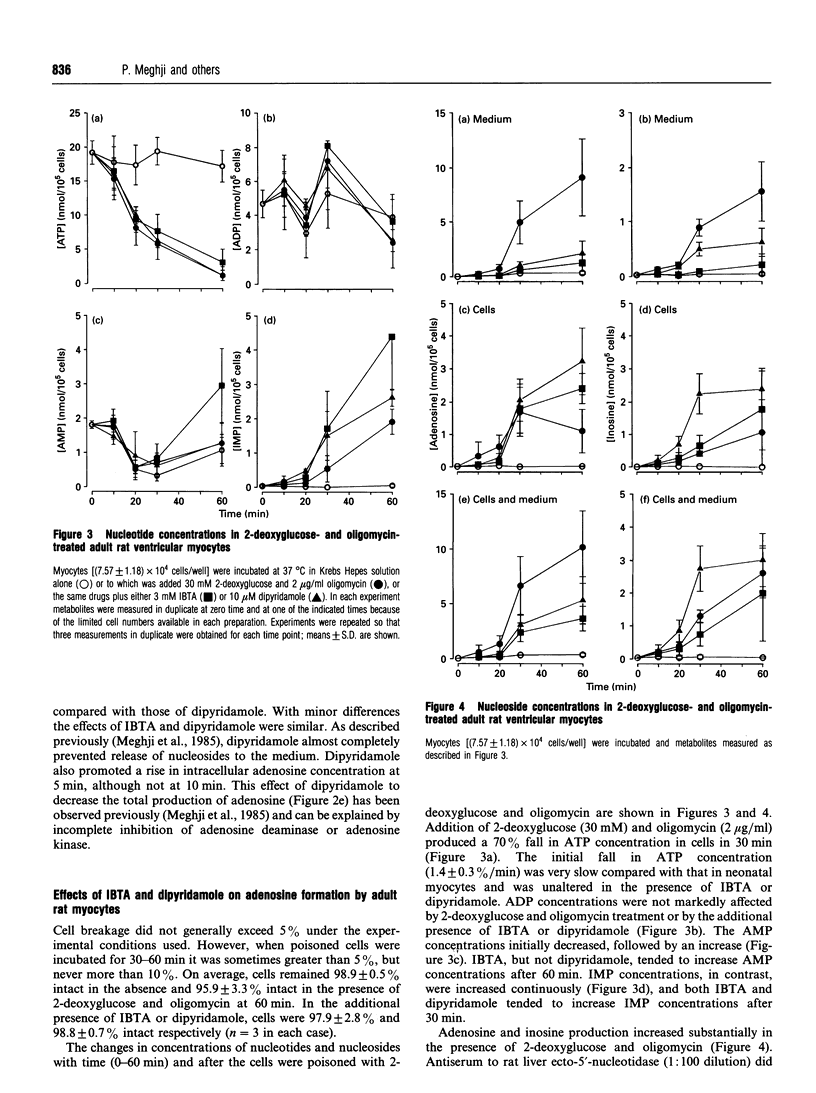
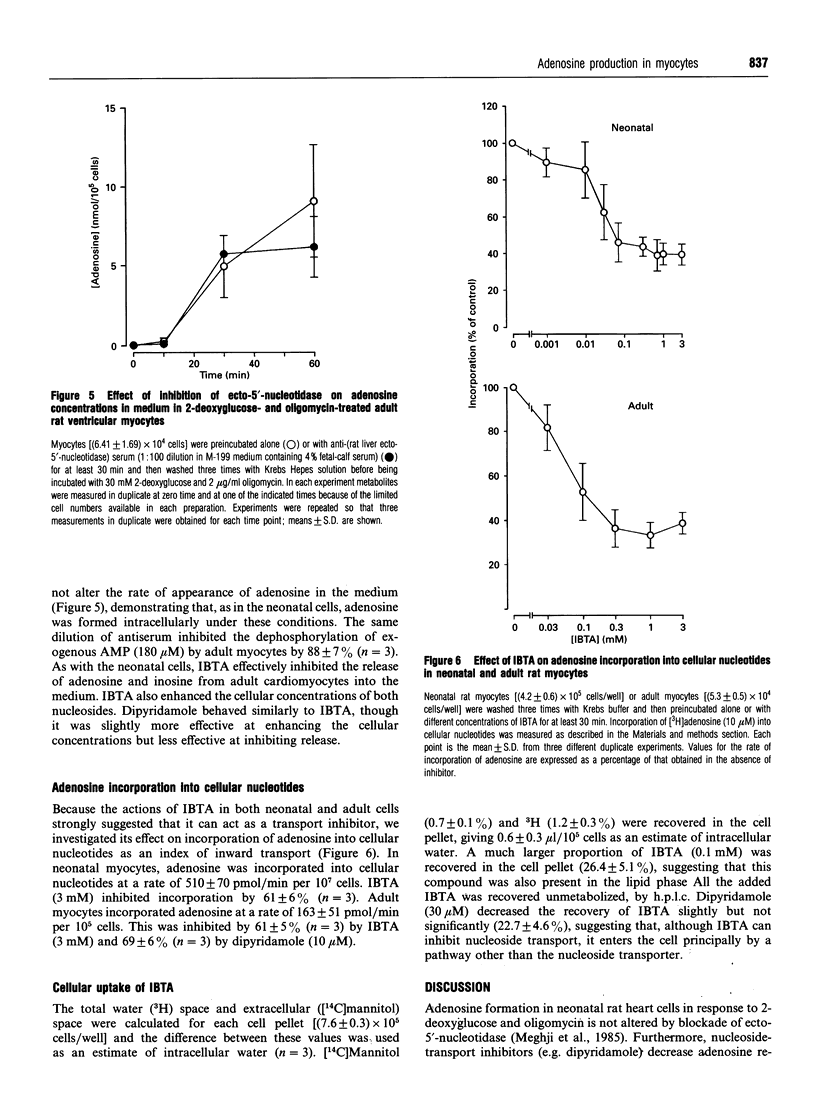
1. Studies in rat polymorphonuclear leucocytes have suggested that 5'-deoxy-5'-isobutylthioadenosine (IBTA), an inhibitor of the IMP-selective cytosolic 5'-nucleotidase, may be used to test its role in adenosine formation in intact cells. We investigated adenosine formation in neonatal and adult rat cardiomyocytes. 2. 2-Deoxyglucose (30 mM) with oligomycin (2 micrograms/ml) induced a 90-100% fall in ATP concentration in 10 min in neonatal and 60 min in adult heart cells. Adenosine accumulation was substantially increased, accounting for 13% of the fall in ATP concentration in neonatal cells and 56% in adult cells. 3. Anti-(rat liver ecto-5'-nucleotidase) serum did not inhibit adenosine accumulation. Furthermore, dipyridamole (10 microM), a nucleoside-transport blocker, inhibited by 80% the appearance of the newly formed adenosine in the medium, showing that adenosine is produced intracellularly by both adult and neonatal-rat myocytes in response to inhibition of oxidative metabolism. 4. IBTA (3 mM) inhibited by 80% the appearance of adenosine in the medium, but did not inhibit total adenosine accumulation by neonatal-rat myocytes and only modestly inhibited total adenosine accumulation by adult myocytes. 5. IBTA, like dipyridamole, inhibited incorporation of extracellular adenosine (10 microM) into neonatal and adult ventricular myocyte nucleotides by 60-70%. Transport of IBTA (100 microM) into the cells did not appear to be inhibited by dipyridamole (30 microM). 6. We conclude that IBTA acted primarily to inhibit adenosine release from myocytes. The small effect on adenosine formation rates implies that the IMP-selective cytosolic 5'-nucleotidase plays a minor role in this tissue.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bailyes E. M., Ferguson M. A., Colaco C. A., Luzio J. P. Inositol is a constituent of detergent-solubilized immunoaffinity-purified rat liver 5'-nucleotidase. Biochem J. 1990 Feb 1;265(3):907–909. doi: 10.1042/bj2650907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst M. M., Schrader J. Adenine nucleotide release from isolated perfused guinea pig hearts and extracellular formation of adenosine. Circ Res. 1991 Mar;68(3):797–806. doi: 10.1161/01.res.68.3.797. [DOI] [PubMed] [Google Scholar]
- Bukoski R. D., Sparks H. V., Jr Adenosine production and release by adult rat cardiocytes. J Mol Cell Cardiol. 1986 Jun;18(6):595–605. doi: 10.1016/s0022-2828(86)80967-1. [DOI] [PubMed] [Google Scholar]
- Clemens M. G., Forrester T. Appearance of adenosine triphosphate in the coronary sinus effluent from isolated working rat heart in response to hypoxia. J Physiol. 1981 Mar;312:143–158. doi: 10.1113/jphysiol.1981.sp013621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deussen A., Borst M., Schrader J. Formation of S-adenosylhomocysteine in the heart. I: An index of free intracellular adenosine. Circ Res. 1988 Jul;63(1):240–249. doi: 10.1161/01.res.63.1.240. [DOI] [PubMed] [Google Scholar]
- Forrester T., Williams C. A. Release of adenosine triphosphate from isolated adult heart cells in response to hypoxia. J Physiol. 1977 Jun;268(2):371–390. doi: 10.1113/jphysiol.1977.sp011862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frick G. P., Lowenstein J. M. Studies of 5'-nucleotidase in the perfused rat heart. Including measurements of the enzyme in perfused skeletal muscle and liver. J Biol Chem. 1976 Oct 25;251(20):6372–6378. [PubMed] [Google Scholar]
- Gordon E. L., Pearson J. D., Slakey L. L. The hydrolysis of extracellular adenine nucleotides by cultured endothelial cells from pig aorta. Feed-forward inhibition of adenosine production at the cell surface. J Biol Chem. 1986 Nov 25;261(33):15496–15507. [PubMed] [Google Scholar]
- Imai S., Chin W. P., Jin H., Nakazawa M. Production of AMP and adenosine in the interstitial fluid compartment of the isolated perfused normoxic guinea pig heart. Pflugers Arch. 1989 Aug;414(4):443–449. doi: 10.1007/BF00585055. [DOI] [PubMed] [Google Scholar]
- Itoh R., Oka J. Evidence for existence of a cytosol 5'-nucleotidase in chicken heart: comparison of some properties of heart and liver enzymes. Comp Biochem Physiol B. 1985;81(1):159–163. doi: 10.1016/0305-0491(85)90177-4. [DOI] [PubMed] [Google Scholar]
- Itoh R., Oka J., Ozasa H. Regulation of rat heart cytosol 5'-nucleotidase by adenylate energy charge. Biochem J. 1986 May 1;235(3):847–851. doi: 10.1042/bj2350847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh R. Purification and some properties of cytosol 5'-nucleotidase from rat liver. Biochim Biophys Acta. 1981 Feb 13;657(2):402–410. doi: 10.1016/0005-2744(81)90326-0. [DOI] [PubMed] [Google Scholar]
- Itoh R. Regulation of cytosol 5'-nucleotidase by adenylate energy charge. Biochim Biophys Acta. 1981 May 14;659(1):31–37. doi: 10.1016/0005-2744(81)90268-0. [DOI] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Katzberg A. A., Farmer B. B., Harris R. A. The predominance of binucleation in isolated rat heart myocytes. Am J Anat. 1977 Aug;149(4):489–499. doi: 10.1002/aja.1001490406. [DOI] [PubMed] [Google Scholar]
- Lawrence F., Richou M., Robert-Gero M. Simultaneous decrease in deamination of 5'-adenylic acid and 5'-deoxy-5'-S-isobutylthioadenosine in chick-embryo fibroblasts infected by Rous sarcoma virus. Eur J Biochem. 1980 Jun;107(2):467–473. doi: 10.1111/j.1432-1033.1980.tb06052.x. [DOI] [PubMed] [Google Scholar]
- Meghji P., Holmquist C. A., Newby A. C. Adenosine formation and release from neonatal-rat heart cells in culture. Biochem J. 1985 Aug 1;229(3):799–805. doi: 10.1042/bj2290799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meghji P., Pearson J. D., Slakey L. L. Regulation of extracellular adenosine production by ectonucleotidases of adult rat ventricular myocytes. Am J Physiol. 1992 Jul;263(1 Pt 2):H40–H47. doi: 10.1152/ajpheart.1992.263.1.H40. [DOI] [PubMed] [Google Scholar]
- Meghji P., Rubio R., Berne R. M. Intracellular adenosine formation and its carrier-mediated release in cultured embryonic chick heart cells. Life Sci. 1988;43(23):1851–1859. doi: 10.1016/s0024-3205(88)80002-x. [DOI] [PubMed] [Google Scholar]
- Meghji P., Tuttle J. B., Rubio R. Adenosine formation and release by embryonic chick neurons and glia in cell culture. J Neurochem. 1989 Dec;53(6):1852–1860. doi: 10.1111/j.1471-4159.1989.tb09252.x. [DOI] [PubMed] [Google Scholar]
- Misumi Y., Ogata S., Hirose S., Ikehara Y. Primary structure of rat liver 5'-nucleotidase deduced from the cDNA. Presence of the COOH-terminal hydrophobic domain for possible post-translational modification by glycophospholipid. J Biol Chem. 1990 Feb 5;265(4):2178–2183. [PubMed] [Google Scholar]
- Newby A. C. Role of adenosine deaminase, ecto-(5'-nucleotidase) and ecto-(non-specific phosphatase) in cyanide-induced adenosine monophosphate catabolism in rat polymorphonuclear leucocytes. Biochem J. 1980 Mar 15;186(3):907–918. doi: 10.1042/bj1860907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newby A. C. The pigeon heart 5'-nucleotidase responsible for ischaemia-induced adenosine formation. Biochem J. 1988 Jul 1;253(1):123–130. doi: 10.1042/bj2530123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paddle B. M., Burnstock G. Release of ATP from perfused heart during coronary vasodilatation. Blood Vessels. 1974;11(3):110–119. doi: 10.1159/000158005. [DOI] [PubMed] [Google Scholar]
- Pearson J. D., Gordon J. L. Vascular endothelial and smooth muscle cells in culture selectively release adenine nucleotides. Nature. 1979 Oct 4;281(5730):384–386. doi: 10.1038/281384a0. [DOI] [PubMed] [Google Scholar]
- Pierré A., Robert-Géro M. Inhibition of nucleoside and sugar transport into cells by an oncostatic methylase inhibitor, 5'-deoxy-5'-S-isobutylthioadenosine (SIBA). FEBS Lett. 1979 May 15;101(2):233–238. doi: 10.1016/0014-5793(79)81015-7. [DOI] [PubMed] [Google Scholar]
- Piper H. M., Probst I., Schwartz P., Hütter F. J., Spieckermann P. G. Culturing of calcium stable adult cardiac myocytes. J Mol Cell Cardiol. 1982 Jul;14(7):397–412. doi: 10.1016/0022-2828(82)90171-7. [DOI] [PubMed] [Google Scholar]
- Recommended methods for the determination of four enzymes in blood. Scand J Clin Lab Invest. 1974 Jun;33(4):291–306. doi: 10.1080/00365517409082499. [DOI] [PubMed] [Google Scholar]
- Schrader J., Schütz W., Bardenheuer H. Role of S-adenosylhomocysteine hydrolase in adenosine metabolism in mammalian heart. Biochem J. 1981 Apr 15;196(1):65–70. doi: 10.1042/bj1960065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader J., Thompson C. I., Hiendlmayer G., Gerlach E. Role of purines in acetylcholine-induced coronary vasodilation. J Mol Cell Cardiol. 1982 Jul;14(7):427–430. doi: 10.1016/0022-2828(82)90174-2. [DOI] [PubMed] [Google Scholar]
- Schütz W., Schrader J., Gerlach E. Different sites of adenosine formation in the heart. Am J Physiol. 1981 Jun;240(6):H963–H970. doi: 10.1152/ajpheart.1981.240.6.H963. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Creveling C. R., Daly J. Stimulated formation of adenosine 3',5'-cyclic phosphate in cerebral cortex: synergism between electrical activity and biogenic amines. Proc Natl Acad Sci U S A. 1970 Apr;65(4):1033–1040. doi: 10.1073/pnas.65.4.1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skladanowski A. C., Newby A. C. Partial purification and properties of an AMP-specific soluble 5'-nucleotidase from pigeon heart. Biochem J. 1990 May 15;268(1):117–122. doi: 10.1042/bj2680117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skladanowski A. C., Sala G. B., Newby A. C. Inhibition of IMP-specific cytosolic 5'-nucleotidase and adenosine formation in rat polymorphonuclear leucocytes by 5'-deoxy-5'-isobutylthio derivatives of adenosine and inosine. Biochem J. 1989 Aug 15;262(1):203–208. doi: 10.1042/bj2620203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolenski R. T., Suitters A., Yacoub M. H. Adenine nucleotide catabolism and adenosine formation in isolated human cardiomyocytes. J Mol Cell Cardiol. 1992 Jan;24(1):91–96. doi: 10.1016/0022-2828(92)91162-x. [DOI] [PubMed] [Google Scholar]
- Truong V. L., Collinson A. R., Lowenstein J. M. 5'-Nucleotidases in rat heart. Evidence for the occurrence of two soluble enzymes with different substrate specificities. Biochem J. 1988 Jul 1;253(1):117–121. doi: 10.1042/bj2530117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Belle H., Goossens F., Wynants J. Formation and release of purine catabolites during hypoperfusion, anoxia, and ischemia. Am J Physiol. 1987 May;252(5 Pt 2):H886–H893. doi: 10.1152/ajpheart.1987.252.5.H886. [DOI] [PubMed] [Google Scholar]
- Williams C. A., Forrester T. Possible source of adenosine triphosphate released from rat myocytes in response to hypoxia and acidosis. Cardiovasc Res. 1983 May;17(5):301–312. doi: 10.1093/cvr/17.5.301. [DOI] [PubMed] [Google Scholar]
- Yokota H., Nakazawa S., Kobayashi S., Taniguchi Y., Yukihide T. [Clinical study of two cases of traumatic cerebellar injury]. No Shinkei Geka. 1990 Jan;18(1):67–70. [PubMed] [Google Scholar]
- van den Berghe G., van Pottelsberghe C., Hers H. G. A kinetic study of the soluble 5'-nucleotidase of rat liver. Biochem J. 1977 Mar 15;162(3):611–616. doi: 10.1042/bj1620611. [DOI] [PMC free article] [PubMed] [Google Scholar]


