Abstract
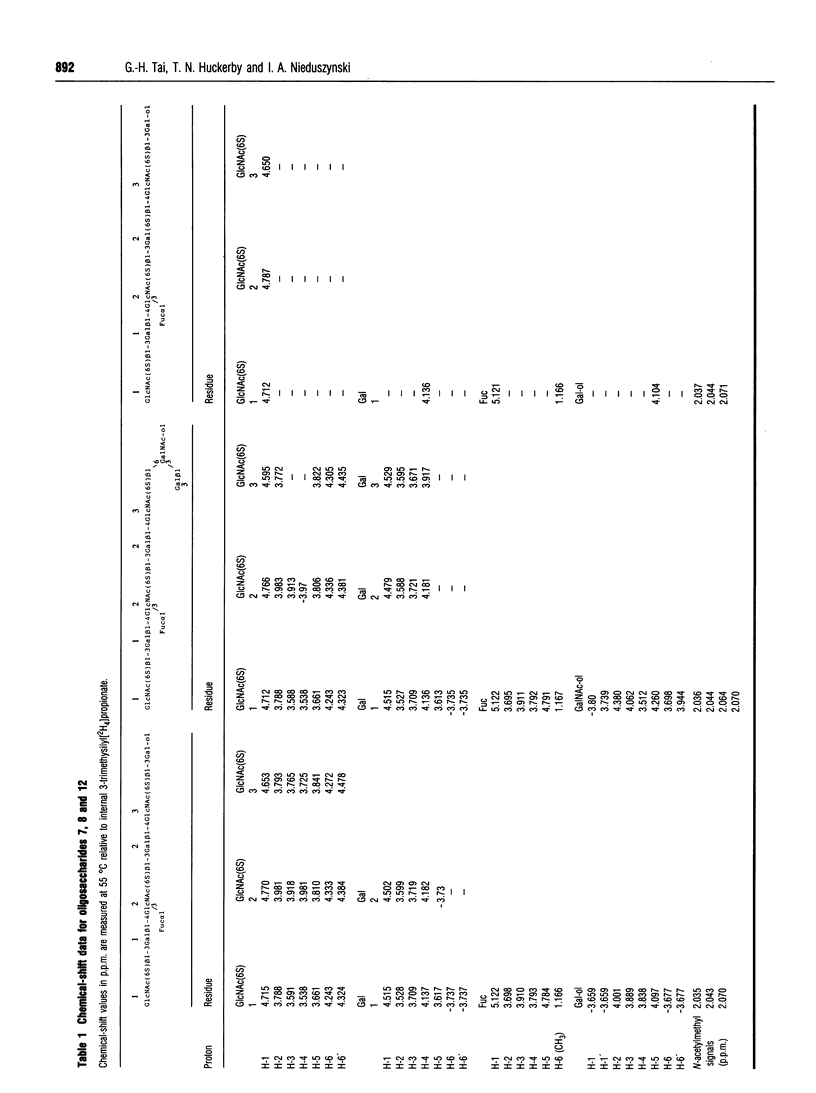
Keratan sulphate chains from bovine articular cartilage were fully digested with keratanase from Pseudomonas sp. and the products were reduced with alkaline borohydride. The resultant fragments were fractionated on a Nucleosil 5SB column and the earliest eluting fucose-containing oligosaccharides were isolated. Structural analysis using 1H n.m.r. spectroscopy (600 MHz) showed the two least-charged species to have the following structure: GlcNAc(6S) beta 1-3Gal beta 1-4(Fuc alpha 1-3)GlcNAc(6S) beta 1- 3Gal beta 1-4GlcNAc(6S) beta 1-3Gal-ol and GlcNAc(6S) beta 1-3Gal beta 1- 4(Fuc alpha 1-3)GlcNAc(6S) beta 1-3Gal beta 1-4GlcNAc(6S) beta 1-6(Gal beta 1- 3)GalNAc-ol. Both galactoses adjacent to the fucosylated N-acetylglucosamine residue are unsulphated. Therefore, it can be deduced from these structures that the presence of fucose on N-acetylglucosamine residues in keratan sulphates protects both of the adjacent unsulphated galactose residues from keratanase cleavage. This result has implications for the interpretation of keratanase fingerprints, because in articular cartilage keratan sulphates the keratanase-resistant blocks are not solely those with fully sulphated galactose residues, but also include the fucosylated sequences, which have unsulphated galactoses. It is, therefore, not possible to estimate their galactose sulphation or the size of the fully sulphated disaccharide-repeat sequences from keratan sulphates that contain fucose.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brown G. M., Huckerby T. N., Morris H. G., Nieduszynski I. A. Degradation of articular cartilage keratan sulphates using hydrazinolysis and nitrous acid. Environment of fucose residues. Biochem J. 1992 Aug 15;286(Pt 1):235–241. doi: 10.1042/bj2860235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caterson B., Christner J. E., Baker J. R. Identification of a monoclonal antibody that specifically recognizes corneal and skeletal keratan sulfate. Monoclonal antibodies to cartilage proteoglycan. J Biol Chem. 1983 Jul 25;258(14):8848–8854. [PubMed] [Google Scholar]
- Dickenson J. M., Huckerby T. N., Nieduszynski I. A. A non-reducing terminal fragment from tracheal cartilage keratan sulphate chains contains alpha (2-3)-linked N-acetylneuraminic acid. Biochem J. 1991 Sep 15;278(Pt 3):779–785. doi: 10.1042/bj2780779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson J. M., Huckerby T. N., Nieduszynski I. A. Skeletal keratan sulphate chains isolated from bovine intervertebral disc may terminate in alpha(2----6)-linked N-acetylneuraminic acid. Biochem J. 1992 Feb 15;282(Pt 1):267–271. doi: 10.1042/bj2820267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson J. M., Huckerby T. N., Nieduszynski I. A. Two linkage-region fragments isolated from skeletal keratan sulphate contain a sulphated N-acetylglucosamine residue. Biochem J. 1990 Jul 1;269(1):55–59. doi: 10.1042/bj2690055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickenson J. M., Morris H. G., Nieduszynski I. A., Huckerby T. N. Skeletal keratan sulfate chain molecular weight calibration by high-performance gel-permeation chromatography. Anal Biochem. 1990 Nov 1;190(2):271–275. doi: 10.1016/0003-2697(90)90192-c. [DOI] [PubMed] [Google Scholar]
- Hardingham T. E., Fosang A. J. Proteoglycans: many forms and many functions. FASEB J. 1992 Feb 1;6(3):861–870. [PubMed] [Google Scholar]
- Hopwood J. J., Elliott H. Selective depolymerisation of keratan sulfate: production of radiolabelled substrates for 6-O-sulfogalactose sulfatase and beta-D-galactosidase. Carbohydr Res. 1983 Jun 16;117:263–274. doi: 10.1016/0008-6215(83)88092-6. [DOI] [PubMed] [Google Scholar]
- Hounsell E. F., Feeney J., Scudder P., Tang P. W., Feizi T. 1H-NMR studies at 500 MHz of a neutral disaccharide and sulphated di-, tetra-, hexa- and larger oligosaccharides obtained by endo-beta-galactosidase treatment of keratan sulphate. Eur J Biochem. 1986 Jun 2;157(2):375–384. doi: 10.1111/j.1432-1033.1986.tb09679.x. [DOI] [PubMed] [Google Scholar]
- Huckerby T. N., Nieduszynski I. A., Brown G. M., Cockin G. H. A full assignment of proton resonances for an alpha(1-3)-linked fucose residue in keratan sulphate from bovine articular cartilage. Glycoconj J. 1991 Feb;8(1):39–44. doi: 10.1007/BF00731641. [DOI] [PubMed] [Google Scholar]
- Lohmander L. S. Markers of cartilage metabolism in arthrosis. A review. Acta Orthop Scand. 1991 Dec;62(6):623–632. doi: 10.3109/17453679108994513. [DOI] [PubMed] [Google Scholar]
- Mehmet H., Scudder P., Tang P. W., Hounsell E. F., Caterson B., Feizi T. The antigenic determinants recognized by three monoclonal antibodies to keratan sulphate involve sulphated hepta- or larger oligosaccharides of the poly(N-acetyllactosamine) series. Eur J Biochem. 1986 Jun 2;157(2):385–391. doi: 10.1111/j.1432-1033.1986.tb09680.x. [DOI] [PubMed] [Google Scholar]
- Nakazawa K., Suzuki S. Purification of Keratan Sulfate-endogalactosidase and its action on keratan sulfates of different origin. J Biol Chem. 1975 Feb 10;250(3):912–917. [PubMed] [Google Scholar]
- Nieduszynski I. A., Huckerby T. N., Dickenson J. M., Brown G. M., Tai G. H., Morris H. G., Eady S. There are two major types of skeletal keratan sulphates. Biochem J. 1990 Oct 1;271(1):243–245. doi: 10.1042/bj2710243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SENO N., MEYER K., ANDERSON B., HOFFMAN P. VARIATIONS IN KERATOSULFATES. J Biol Chem. 1965 Mar;240:1005–1010. [PubMed] [Google Scholar]
- Sanderson P. N., Huckerby T. N., Nieduszynski I. A. Conformational equilibria of alpha-L-iduronate residues in disaccharides derived from heparin. Biochem J. 1987 Apr 1;243(1):175–181. doi: 10.1042/bj2430175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai G. H., Brown G. M., Morris H. G., Huckerby T. N., Nieduszynski I. A. Fucose content of keratan sulphates from bovine articular cartilage. Biochem J. 1991 Jan 15;273(Pt 2):307–310. doi: 10.1042/bj2730307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thonar E. J., Lenz M. E., Klintworth G. K., Caterson B., Pachman L. M., Glickman P., Katz R., Huff J., Kuettner K. E. Quantification of keratan sulfate in blood as a marker of cartilage catabolism. Arthritis Rheum. 1985 Dec;28(12):1367–1376. doi: 10.1002/art.1780281209. [DOI] [PubMed] [Google Scholar]


