Abstract
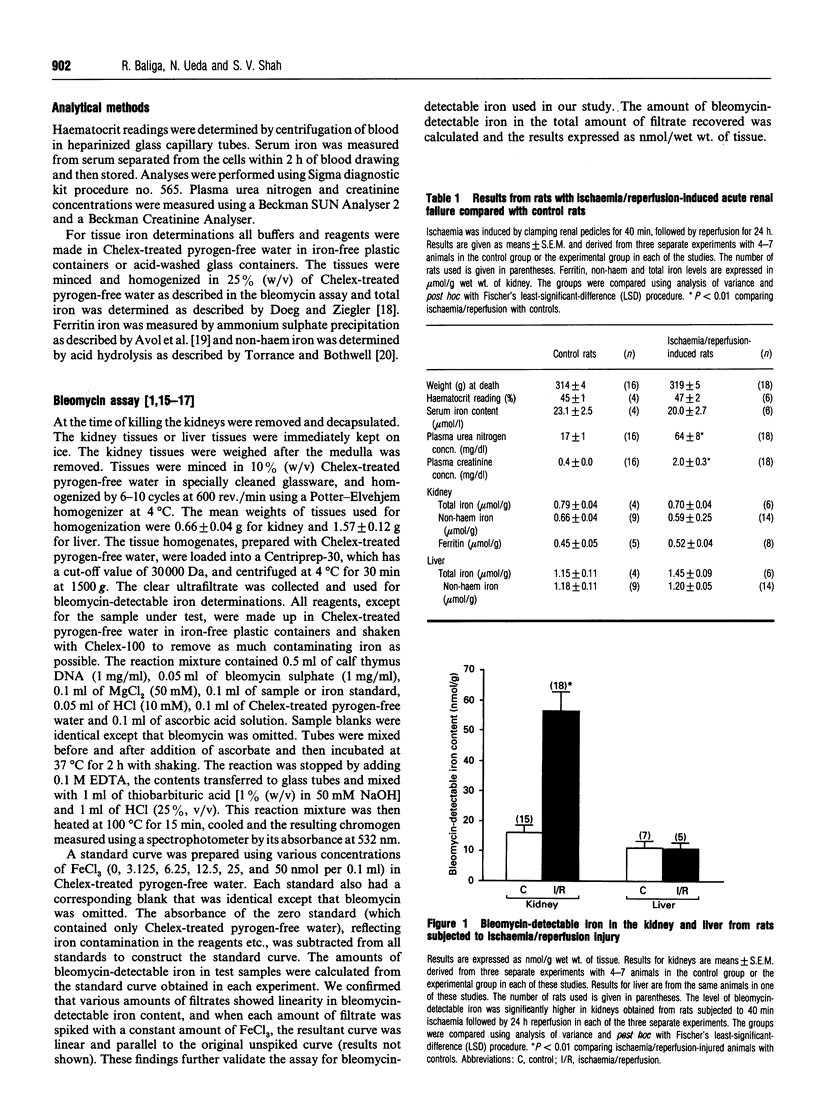
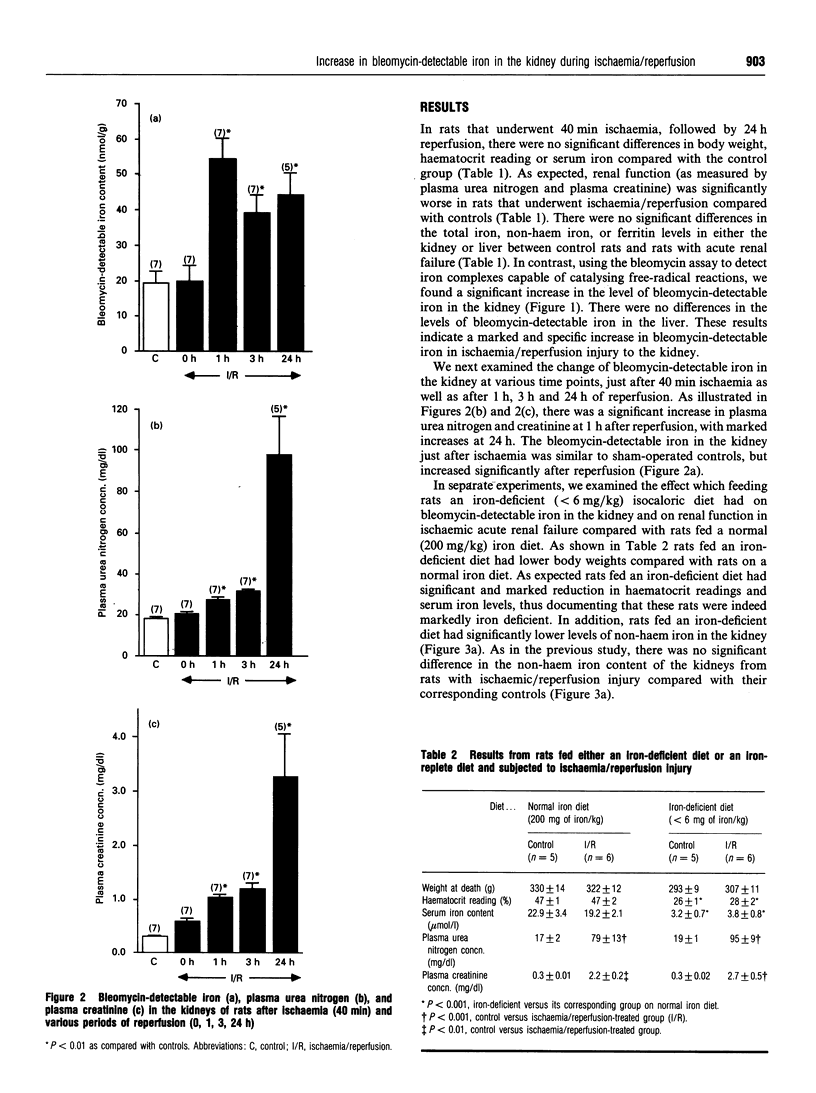
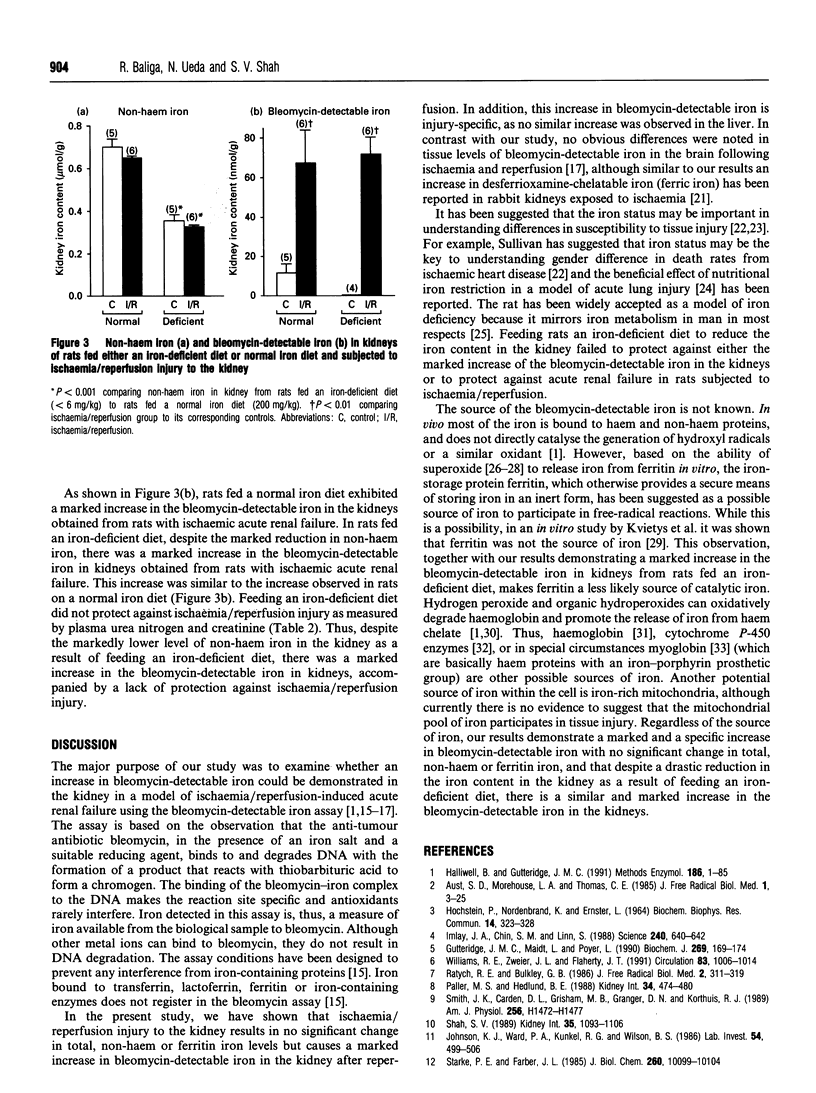
Iron has been shown to be important in ischaemic, immune and toxic forms of tissue injury in various organs. Although it is generally accepted that iron participates in the generation of powerful oxidant species (e.g. hydroxyl radicals) there has not been any direct evidence that iron capable of catalysing free-radical reactions is increased in tissues in these models of injury. In the present study we demonstrate that ischaemia/reperfusion injury to the kidney results in no significant change in total, nonhaem or ferritin iron levels, but there is a marked and specific increase in bleomycin-detectable iron (capable of catalysing free-radical reactions) in the kidney. The increase in bleomycin-detectable iron is observed only after reperfusion but not during the ischaemic period. In a separate study we demonstrate that despite a drastic reduction in the iron content in the kidney, as a result of feeding an iron-deficient diet, there is a similar and a marked increase in the bleomycin-detectable iron in kidneys accompanied by a lack of protection against ischaemia/reperfusion injury.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aust S. D., Morehouse L. A., Thomas C. E. Role of metals in oxygen radical reactions. J Free Radic Biol Med. 1985;1(1):3–25. doi: 10.1016/0748-5514(85)90025-x. [DOI] [PubMed] [Google Scholar]
- Avol E., Carmichael D., Hegenauer J., Saltman P. Rapid induction of ferritin in laboratory animals prior to its isolation. Prep Biochem. 1973;3(3):279–290. doi: 10.1080/00327487308061512. [DOI] [PubMed] [Google Scholar]
- Biemond P., Swaak A. J., Beindorff C. M., Koster J. F. Superoxide-dependent and -independent mechanisms of iron mobilization from ferritin by xanthine oxidase. Implications for oxygen-free-radical-induced tissue destruction during ischaemia and inflammation. Biochem J. 1986 Oct 1;239(1):169–173. doi: 10.1042/bj2390169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biemond P., van Eijk H. G., Swaak A. J., Koster J. F. Iron mobilization from ferritin by superoxide derived from stimulated polymorphonuclear leukocytes. Possible mechanism in inflammation diseases. J Clin Invest. 1984 Jun;73(6):1576–1579. doi: 10.1172/JCI111364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolann B. J., Ulvik R. J. Release of iron from ferritin by xanthine oxidase. Role of the superoxide radical. Biochem J. 1987 Apr 1;243(1):55–59. doi: 10.1042/bj2430055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bysani G. K., Kennedy T. P., Ky N., Rao N. V., Blaze C. A., Hoidal J. R. Role of cytochrome P-450 in reperfusion injury of the rabbit lung. J Clin Invest. 1990 Nov;86(5):1434–1441. doi: 10.1172/JCI114859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOEG K. A., ZIEGLER D. M. Simplified methods for the estimation of iron in mitochondria and submitochondrial fractions. Arch Biochem Biophys. 1962 Apr;97:37–40. doi: 10.1016/0003-9861(62)90041-3. [DOI] [PubMed] [Google Scholar]
- Dallman P. R. Biochemical basis for the manifestations of iron deficiency. Annu Rev Nutr. 1986;6:13–40. doi: 10.1146/annurev.nu.06.070186.000305. [DOI] [PubMed] [Google Scholar]
- Gower J., Healing G., Green C. Measurement by HPLC of desferrioxamine-available iron in rabbit kidneys to assess the effect of ischaemia on the distribution of iron within the total pool. Free Radic Res Commun. 1989;5(4-5):291–299. doi: 10.3109/10715768909074713. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Cao W., Chevion M. Bleomycin-detectable iron in brain tissue. Free Radic Res Commun. 1991;11(6):317–320. doi: 10.3109/10715769109088929. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett. 1986 Jun 9;201(2):291–295. doi: 10.1016/0014-5793(86)80626-3. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Maidt L., Poyer L. Superoxide dismutase and Fenton chemistry. Reaction of ferric-EDTA complex and ferric-bipyridyl complex with hydrogen peroxide without the apparent formation of iron(II). Biochem J. 1990 Jul 1;269(1):169–174. doi: 10.1042/bj2690169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Rowley D. A., Halliwell B. Superoxide-dependent formation of hydroxyl radicals and lipid peroxidation in the presence of iron salts. Detection of 'catalytic' iron and anti-oxidant activity in extracellular fluids. Biochem J. 1982 Sep 15;206(3):605–609. doi: 10.1042/bj2060605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M., Rowley D. A., Halliwell B. Superoxide-dependent formation of hydroxyl radicals in the presence of iron salts. Detection of 'free' iron in biological systems by using bleomycin-dependent degradation of DNA. Biochem J. 1981 Oct 1;199(1):263–265. doi: 10.1042/bj1990263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Role of free radicals and catalytic metal ions in human disease: an overview. Methods Enzymol. 1990;186:1–85. doi: 10.1016/0076-6879(90)86093-b. [DOI] [PubMed] [Google Scholar]
- Hiraishi H., Terano A., Ota S., Mutoh H., Razandi M., Sugimoto T., Ivey K. J. Role for iron in reactive oxygen species-mediated cytotoxicity to cultured rat gastric mucosal cells. Am J Physiol. 1991 Apr;260(4 Pt 1):G556–G563. doi: 10.1152/ajpgi.1991.260.4.G556. [DOI] [PubMed] [Google Scholar]
- Hochstein P., Nordenbrand K., Ernster L. Evidence for the involvement of iron in the ADP-activated peroxidation of lipids in microsomes and mitochondria. Biochem Biophys Res Commun. 1964;14:323–328. doi: 10.1016/s0006-291x(64)80004-8. [DOI] [PubMed] [Google Scholar]
- Imlay J. A., Chin S. M., Linn S. Toxic DNA damage by hydrogen peroxide through the Fenton reaction in vivo and in vitro. Science. 1988 Apr 29;240(4852):640–642. doi: 10.1126/science.2834821. [DOI] [PubMed] [Google Scholar]
- Johnson K. J., Ward P. A., Kunkel R. G., Wilson B. S. Mediation of IgA induced lung injury in the rat. Role of macrophages and reactive oxygen products. Lab Invest. 1986 May;54(5):499–506. [PubMed] [Google Scholar]
- McCord J. M. Is iron sufficiency a risk factor in ischemic heart disease? Circulation. 1991 Mar;83(3):1112–1114. doi: 10.1161/01.cir.83.3.1112. [DOI] [PubMed] [Google Scholar]
- Paller M. S., Hedlund B. E. Role of iron in postischemic renal injury in the rat. Kidney Int. 1988 Oct;34(4):474–480. doi: 10.1038/ki.1988.205. [DOI] [PubMed] [Google Scholar]
- Puppo A., Halliwell B. Formation of hydroxyl radicals in biological systems. Does myoglobin stimulate hydroxyl radical formation from hydrogen peroxide? Free Radic Res Commun. 1988;4(6):415–422. doi: 10.3109/10715768809066910. [DOI] [PubMed] [Google Scholar]
- Ratych R. E., Bulkley G. B. Free-radical-mediated postischemic reperfusion injury in the kidney. J Free Radic Biol Med. 1986;2(5-6):311–319. doi: 10.1016/s0748-5514(86)80030-7. [DOI] [PubMed] [Google Scholar]
- Sadrzadeh S. M., Anderson D. K., Panter S. S., Hallaway P. E., Eaton J. W. Hemoglobin potentiates central nervous system damage. J Clin Invest. 1987 Feb;79(2):662–664. doi: 10.1172/JCI112865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. V. Role of reactive oxygen metabolites in experimental glomerular disease. Kidney Int. 1989 May;35(5):1093–1106. doi: 10.1038/ki.1989.96. [DOI] [PubMed] [Google Scholar]
- Smith J. K., Carden D. L., Grisham M. B., Granger D. N., Korthuis R. J. Role of iron in postischemic microvascular injury. Am J Physiol. 1989 May;256(5 Pt 2):H1472–H1477. doi: 10.1152/ajpheart.1989.256.5.H1472. [DOI] [PubMed] [Google Scholar]
- Starke P. E., Farber J. L. Ferric iron and superoxide ions are required for the killing of cultured hepatocytes by hydrogen peroxide. Evidence for the participation of hydroxyl radicals formed by an iron-catalyzed Haber-Weiss reaction. J Biol Chem. 1985 Aug 25;260(18):10099–10104. [PubMed] [Google Scholar]
- Sullivan J. L. The iron paradigm of ischemic heart disease. Am Heart J. 1989 May;117(5):1177–1188. doi: 10.1016/0002-8703(89)90887-9. [DOI] [PubMed] [Google Scholar]
- Torrance J. D., Bothwell T. H. A simple technique for measuring storage iron concentrations in formalinised liver samples. S Afr J Med Sci. 1968 Apr;33(1):9–11. [PubMed] [Google Scholar]
- Walker P. D., Shah S. V. Hydrogen peroxide cytotoxicity in LLC-PK1 cells: a role for iron. Kidney Int. 1991 Nov;40(5):891–898. doi: 10.1038/ki.1991.290. [DOI] [PubMed] [Google Scholar]
- Williams R. E., Zweier J. L., Flaherty J. T. Treatment with deferoxamine during ischemia improves functional and metabolic recovery and reduces reperfusion-induced oxygen radical generation in rabbit hearts. Circulation. 1991 Mar;83(3):1006–1014. doi: 10.1161/01.cir.83.3.1006. [DOI] [PubMed] [Google Scholar]


