Abstract
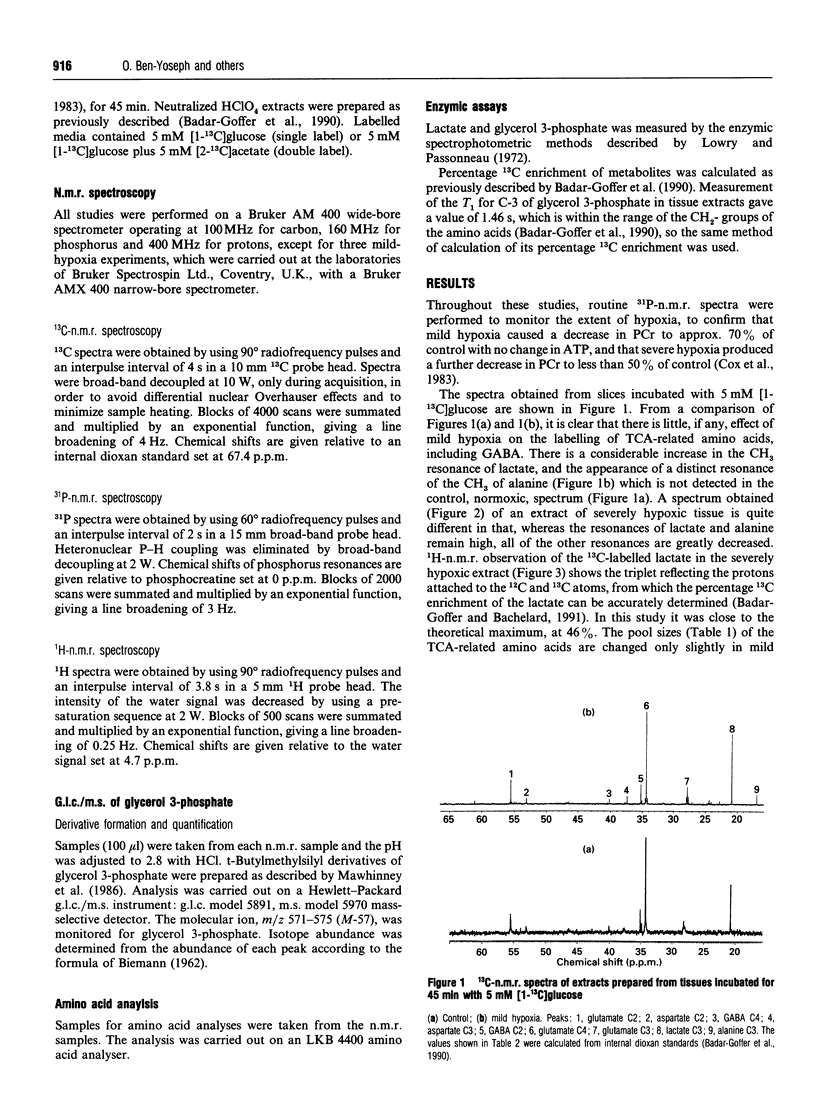
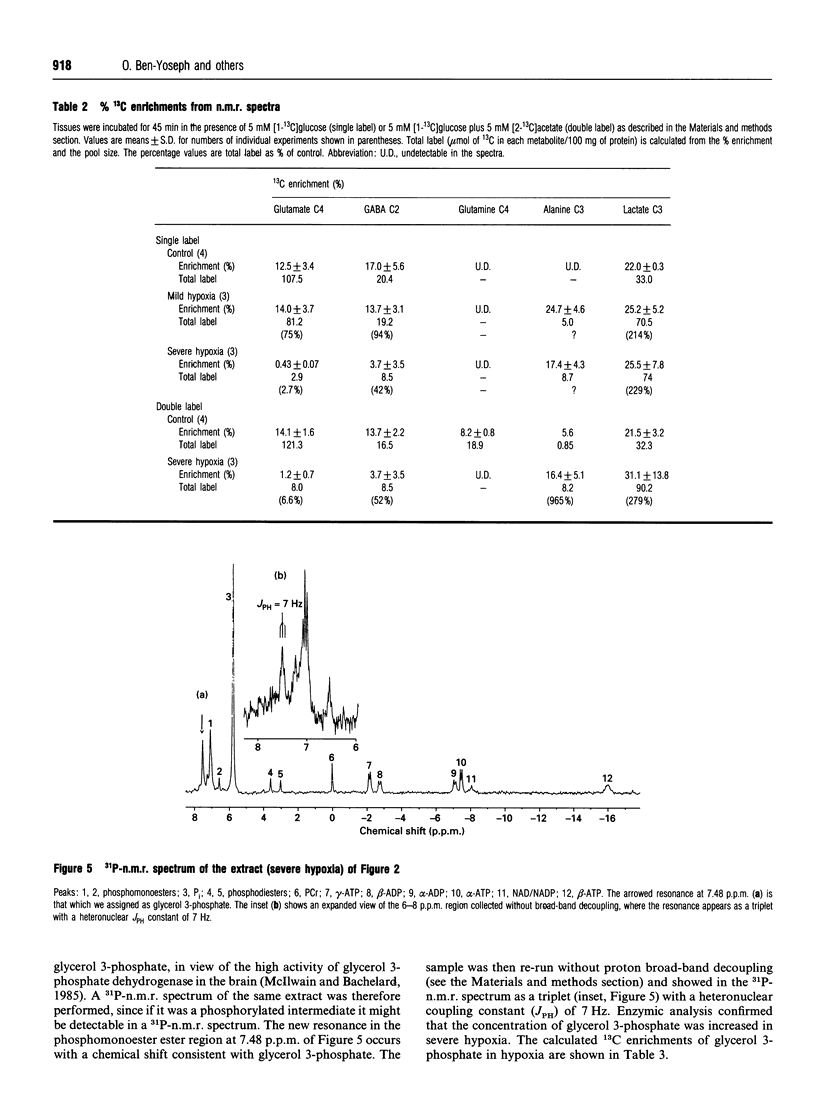
The incorporation of 13C from [1-13C]glucose and [2-13C]acetate into selected intermediary metabolites in extracts prepared from incubated cerebral-cortex slices was monitored by using 13C-n.m.r. spectroscopy under conditions of mild and severe hypoxia. Mild hypoxia had little effect on labelling of tricarboxylic-acid-cycle-related amino acids [glutamate, glutamine and gamma-aminobutyrate (GABA)], although the pool sizes of glutamate and glutamine decreased. There were large increases in the labelling of lactate and of alanine, and an increase in the pool size of lactate. In severe hypoxia, the resonances of lactate and alanine remained high, whereas those of the other intermediates decreased greatly. The pool size of GABA increased. Calculation of percentage 13C enrichments and total label incorporated showed that lactate was not further affected by severe hypoxia, but the total label in alanine and its pool size were further increased. A new resonance appeared in the phosphomonoester region of the 13C-n.m.r. spectrum only in severe hypoxia. This was unambiguously assigned to glycerol 3-phosphate from a combination of 31P- and 13C-n.m.r. spectroscopy. The percentage 13C-enrichment was calculated from the 13C-n.m.r. spectrum, and the total label incorporated was measured by g.l.c./m.s. The results are discussed in terms of the ability of lactate dehydrogenase to maintain normal levels of NADH in mild hypoxia, but not in severe hypoxia. The pyruvate which accumulates under the latter condition is channelled into alanine, and the increased NADH is reflected by the increase in glycerol 3-phosphate. We conclude that glycerol 3-phosphate and alanine may provide novel means of monitoring severe hypoxia, whereas lactate is a reliable indicator only of mild hypoxia.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badar-Goffer R. S., Bachelard H. S., Morris P. G. Cerebral metabolism of acetate and glucose studied by 13C-n.m.r. spectroscopy. A technique for investigating metabolic compartmentation in the brain. Biochem J. 1990 Feb 15;266(1):133–139. doi: 10.1042/bj2660133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badar-Goffer R. S., Ben-Yoseph O., Bachelard H. S., Morris P. G. Neuronal-glial metabolism under depolarizing conditions. A 13C-n.m.r. study. Biochem J. 1992 Feb 15;282(Pt 1):225–230. doi: 10.1042/bj2820225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badar-Goffer R., Bachelard H. Metabolic studies using 13C nuclear magnetic resonance spectroscopy. Essays Biochem. 1991;26:105–119. [PubMed] [Google Scholar]
- Cox D. W., Morris P. G., Bachelard H. S. Kinetic analysis of the cerebral creatine kinase reaction under hypoxic and hypoglycaemic conditions in vitro. A 31P-n.m.r. study. Biochem J. 1988 Oct 15;255(2):523–527. [PMC free article] [PubMed] [Google Scholar]
- Cox D. W., Morris P. G., Feeney J., Bachelard H. S. 31P-n.m.r. studies on cerebral energy metabolism under conditions of hypoglycaemia and hypoxia in vitro. Biochem J. 1983 May 15;212(2):365–370. doi: 10.1042/bj2120365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo O., Cox D. W., Bachelard H. S. Brain levels of NADH and NAD+ under hypoxic and hypoglycaemic conditions in vitro. J Neurochem. 1988 Jul;51(1):172–176. doi: 10.1111/j.1471-4159.1988.tb04851.x. [DOI] [PubMed] [Google Scholar]
- Glonek T., Kopp S. J., Kot E., Pettegrew J. W., Harrison W. H., Cohen M. M. P-31 nuclear magnetic resonance analysis of brain: the perchloric acid extract spectrum. J Neurochem. 1982 Nov;39(5):1210–1219. doi: 10.1111/j.1471-4159.1982.tb12557.x. [DOI] [PubMed] [Google Scholar]
- Leveille P. J., McGinnis J. F., Maxwell D. S., de Vellis J. Immunocytochemical localization of glycerol-3-phosphate dehydrogenase in rat oligodendrocytes. Brain Res. 1980 Sep 8;196(2):287–305. doi: 10.1016/0006-8993(80)90397-2. [DOI] [PubMed] [Google Scholar]
- Mawhinney T. P., Robinett R. S., Atalay A., Madson M. A. Analysis of amino acids as their tert.-butyldimethylsilyl derivatives by gas-liquid chromatography and mass spectrometry. J Chromatogr. 1986 May 16;358(1):231–242. doi: 10.1016/s0021-9673(01)90333-4. [DOI] [PubMed] [Google Scholar]
- Morris P. G., Feeney J., Cox D. W., Bachelard H. S. 31P-saturation-transfer nuclear-magnetic-resonance measurements of phosphocreatine turnover in guinea-pig brain slices. Biochem J. 1985 May 1;227(3):777–782. doi: 10.1042/bj2270777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TEWS J. K., CARTER S. H., ROA P. D., STONE W. E. FREE AMINO ACIDS AND RELATED COMPOUNDS IN DOG BRAIN: POST-MORTEM AND ANOXIC CHANGES, EFFECTS OF AMMONIUM CHLORIDE INFUSION, AND LEVELS DURING SEIZURES INDUCED BY PICROTOXIN AND BY PENTYLENETETRAZOL. J Neurochem. 1963 Sep;10:641–653. doi: 10.1111/j.1471-4159.1963.tb08936.x. [DOI] [PubMed] [Google Scholar]
- Walz W., Mukerji S. Simulation of aspects of ischemia in cell culture: changes in lactate compartmentation. Glia. 1990;3(6):522–528. doi: 10.1002/glia.440030611. [DOI] [PubMed] [Google Scholar]
- Wood J. D. A possible role for gamma-aminobutyric acid in the homeostatic control of brain metabolism under conditions of hypoxia. Exp Brain Res. 1967;4(1):81–84. doi: 10.1007/BF00235219. [DOI] [PubMed] [Google Scholar]


