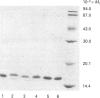
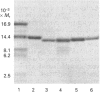
Abstract
Human cystatin C variants in which the evolutionarily conserved Gly-11 residue has been replaced by residues with positively charged (Arg), negatively charged (Glu), bulky hydrophobic (Trp), or small (Ser or Ala) side-chains have been produced by site-directed mutagenesis and expression in Escherichia coli. The five variants were isolated and structurally verified. Their inhibitory properties were compared with those of wild-type recombinant cystatin C by determination of the equilibrium constants for dissociation (Ki) of their complexes with the cysteine endopeptidases papain and human cathepsin B and with the cysteine exopeptidase dipeptidyl peptidase I. The Ser-11 and Ala-11 cystatin C variants displayed Ki values for the two endopeptidases that were approx. 20-fold higher than those of wild-type cystatin C, while the corresponding values for the Trp-11. Arg-11 and Glu-11 variants were increased by a factor of about 2000. In contrast, the Ki values for the interactions of all five variants with the exopeptidase differed from that of wild-type cystatin C by a factor of less than 10. Wild-type cystatin C and the Ser-11, Ala-11 and Glu-11 variants were incubated with neutrophil elastase, which in all cases resulted in the rapid hydrolysis of a single peptide bond, between amino acid residues 10 and 11. The Ki values for the interactions with papain of these three N-terminal-decapeptide-lacking cystatin C variants were 20-50 nM, just one order of magnitude higher than the value for N-terminally truncated wild-type cystatin C, which in turn was similar to the corresponding values for the full-length Glu-11, Arg-11 and Trp-11 variants. These data indicate that the crucial feature of the conserved Gly residue in position 11 of wild-type cystatin C is that this residue, devoid of a side-chain, will allow the N-terminal segment of cystatin C to adopt a conformation suitable for interaction with the substrate-binding pockets of cysteine endopeptidases, resulting in high-affinity binding and efficient inhibition. The functional properties of the remaining part of the proteinase contact area, which is built from more C-terminal inhibitor segments, are not significantly affected even when amino acids with bulky or charged side-chains replace the Gly-11 residue of the N-terminal segment.
Full text
PDF


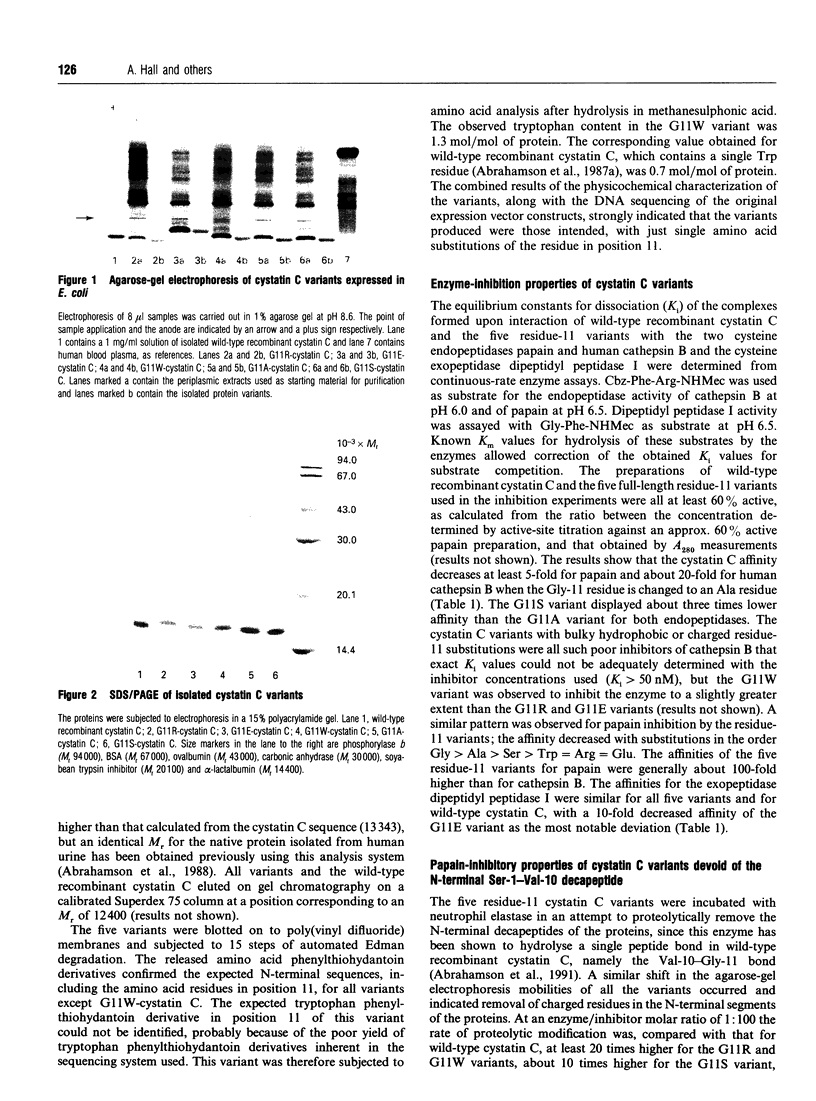
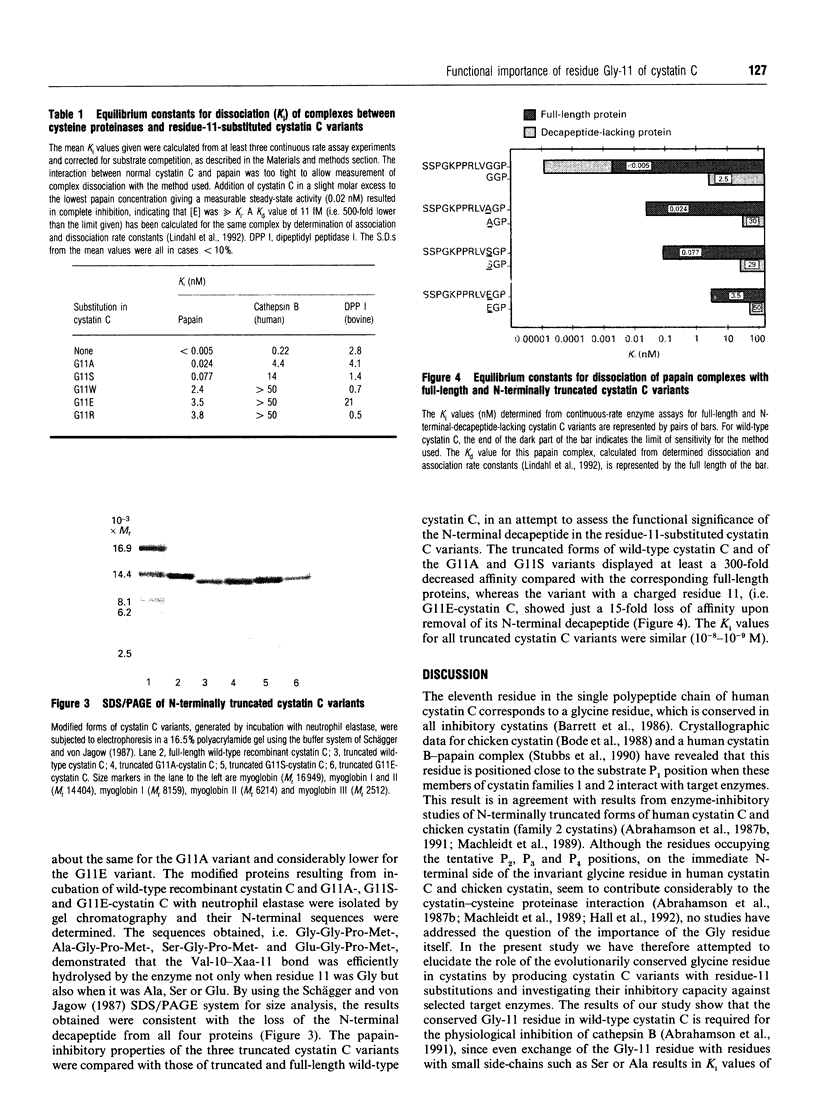


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamson M., Barrett A. J., Salvesen G., Grubb A. Isolation of six cysteine proteinase inhibitors from human urine. Their physicochemical and enzyme kinetic properties and concentrations in biological fluids. J Biol Chem. 1986 Aug 25;261(24):11282–11289. [PubMed] [Google Scholar]
- Abrahamson M., Dalbøge H., Olafsson I., Carlsen S., Grubb A. Efficient production of native, biologically active human cystatin C by Escherichia coli. FEBS Lett. 1988 Aug 15;236(1):14–18. doi: 10.1016/0014-5793(88)80276-x. [DOI] [PubMed] [Google Scholar]
- Abrahamson M., Grubb A., Olafsson I., Lundwall A. Molecular cloning and sequence analysis of cDNA coding for the precursor of the human cysteine proteinase inhibitor cystatin C. FEBS Lett. 1987 Jun 1;216(2):229–233. doi: 10.1016/0014-5793(87)80695-6. [DOI] [PubMed] [Google Scholar]
- Abrahamson M., Mason R. W., Hansson H., Buttle D. J., Grubb A., Ohlsson K. Human cystatin C. role of the N-terminal segment in the inhibition of human cysteine proteinases and in its inactivation by leucocyte elastase. Biochem J. 1991 Feb 1;273(Pt 3):621–626. doi: 10.1042/bj2730621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamson M., Olafsson I., Palsdottir A., Ulvsbäck M., Lundwall A., Jensson O., Grubb A. Structure and expression of the human cystatin C gene. Biochem J. 1990 Jun 1;268(2):287–294. doi: 10.1042/bj2680287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamson M., Ritonja A., Brown M. A., Grubb A., Machleidt W., Barrett A. J. Identification of the probable inhibitory reactive sites of the cysteine proteinase inhibitors human cystatin C and chicken cystatin. J Biol Chem. 1987 Jul 15;262(20):9688–9694. [PubMed] [Google Scholar]
- Barrett A. J., Davies M. E., Grubb A. The place of human gamma-trace (cystatin C) amongst the cysteine proteinase inhibitors. Biochem Biophys Res Commun. 1984 Apr 30;120(2):631–636. doi: 10.1016/0006-291x(84)91302-0. [DOI] [PubMed] [Google Scholar]
- Barrett A. J., Fritz H., Grubb A., Isemura S., Järvinen M., Katunuma N., Machleidt W., Müller-Esterl W., Sasaki M., Turk V. Nomenclature and classification of the proteins homologous with the cysteine-proteinase inhibitor chicken cystatin. Biochem J. 1986 May 15;236(1):312–312. doi: 10.1042/bj2360312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett A. J., Kembhavi A. A., Brown M. A., Kirschke H., Knight C. G., Tamai M., Hanada K. L-trans-Epoxysuccinyl-leucylamido(4-guanidino)butane (E-64) and its analogues as inhibitors of cysteine proteinases including cathepsins B, H and L. Biochem J. 1982 Jan 1;201(1):189–198. doi: 10.1042/bj2010189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg S., Schechter I., Berger A. The purification of papain by affinity chromatography. Eur J Biochem. 1970 Jul;15(1):97–102. doi: 10.1111/j.1432-1033.1970.tb00981.x. [DOI] [PubMed] [Google Scholar]
- Bode W., Engh R., Musil D., Thiele U., Huber R., Karshikov A., Brzin J., Kos J., Turk V. The 2.0 A X-ray crystal structure of chicken egg white cystatin and its possible mode of interaction with cysteine proteinases. EMBO J. 1988 Aug;7(8):2593–2599. doi: 10.1002/j.1460-2075.1988.tb03109.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttle D. J., Ritonja A., Dando P. M., Abrahamson M., Shaw E. N., Wikstrom P., Turk V., Barrett A. J. Interactions of papaya proteinase IV with inhibitors. FEBS Lett. 1990 Mar 12;262(1):58–60. doi: 10.1016/0014-5793(90)80153-a. [DOI] [PubMed] [Google Scholar]
- Dalbøge H., Jensen E. B., Tøttrup H., Grubb A., Abrahamson M., Olafsson I., Carlsen S. High-level expression of active human cystatin C in Escherichia coli. Gene. 1989 Jul 15;79(2):325–332. doi: 10.1016/0378-1119(89)90214-x. [DOI] [PubMed] [Google Scholar]
- Freije J. P., Abrahamson M., Olafsson I., Velasco G., Grubb A., López-Otín C. Structure and expression of the gene encoding cystatin D, a novel human cysteine proteinase inhibitor. J Biol Chem. 1991 Oct 25;266(30):20538–20543. [PubMed] [Google Scholar]
- Gauthier F., Fryksmark U., Ohlsson K., Bieth J. G. Kinetics of the inhibition of leukocyte elastase by the bronchial inhibitor. Biochim Biophys Acta. 1982 Jan 18;700(2):178–183. doi: 10.1016/0167-4838(82)90095-4. [DOI] [PubMed] [Google Scholar]
- Gounaris A. D., Brown M. A., Barrett A. J. Human plasma alpha-cysteine proteinase inhibitor. Purification by affinity chromatography, characterization and isolation of an active fragment. Biochem J. 1984 Jul 15;221(2):445–452. doi: 10.1042/bj2210445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb A., Löfberg H. Human gamma-trace, a basic microprotein: amino acid sequence and presence in the adenohypophysis. Proc Natl Acad Sci U S A. 1982 May;79(9):3024–3027. doi: 10.1073/pnas.79.9.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall A., Abrahamson M., Grubb A., Trojnar J., Kania P., Kasprzykowska R., Kasprzykowski F. Cystatin C based peptidyl diazomethanes as cysteine proteinase inhibitors: influence of the peptidyl chain length. J Enzyme Inhib. 1992;6(2):113–123. doi: 10.3109/14756369209040742. [DOI] [PubMed] [Google Scholar]
- Henderson P. J. A linear equation that describes the steady-state kinetics of enzymes and subcellular particles interacting with tightly bound inhibitors. Biochem J. 1972 Apr;127(2):321–333. doi: 10.1042/bj1270321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppsson J. O., Laurell C. B., Franzén B. Agarose gel electrophoresis. Clin Chem. 1979 Apr;25(4):629–638. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lindahl P., Abrahamson M., Björk I. Interaction of recombinant human cystatin C with the cysteine proteinases papain and actinidin. Biochem J. 1992 Jan 1;281(Pt 1):49–55. doi: 10.1042/bj2810049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl P., Alriksson E., Jörnvall H., Björk I. Interaction of the cysteine proteinase inhibitor chicken cystatin with papain. Biochemistry. 1988 Jul 12;27(14):5074–5082. doi: 10.1021/bi00414a019. [DOI] [PubMed] [Google Scholar]
- Machleidt W., Thiele U., Laber B., Assfalg-Machleidt I., Esterl A., Wiegand G., Kos J., Turk V., Bode W. Mechanism of inhibition of papain by chicken egg white cystatin. Inhibition constants of N-terminally truncated forms and cyanogen bromide fragments of the inhibitor. FEBS Lett. 1989 Jan 30;243(2):234–238. doi: 10.1016/0014-5793(89)80135-8. [DOI] [PubMed] [Google Scholar]
- Nicklin M. J., Barrett A. J. Inhibition of cysteine proteinases and dipeptidyl peptidase I by egg-white cystatin. Biochem J. 1984 Oct 1;223(1):245–253. doi: 10.1042/bj2230245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olafsson I., Gudmundsson G., Abrahamson M., Jensson O., Grubb A. The amino terminal portion of cerebrospinal fluid cystatin C in hereditary cystatin C amyloid angiopathy is not truncated: direct sequence analysis from agarose gel electropherograms. Scand J Clin Lab Invest. 1990 Feb;50(1):85–93. doi: 10.1080/00365519009091569. [DOI] [PubMed] [Google Scholar]
- Popović T., Brzin J., Ritonja A., Turk V. Different forms of human cystatin C. Biol Chem Hoppe Seyler. 1990 Jul;371(7):575–580. doi: 10.1515/bchm3.1990.371.2.575. [DOI] [PubMed] [Google Scholar]
- Rawlings N. D., Barrett A. J. FLUSYS: a software package for the collection and analysis of kinetic and scanning data from Perkin-Elmer fluorimeters. Comput Appl Biosci. 1990 Apr;6(2):118–119. doi: 10.1093/bioinformatics/6.2.118. [DOI] [PubMed] [Google Scholar]
- Schägger H., von Jagow G. Tricine-sodium dodecyl sulfate-polyacrylamide gel electrophoresis for the separation of proteins in the range from 1 to 100 kDa. Anal Biochem. 1987 Nov 1;166(2):368–379. doi: 10.1016/0003-2697(87)90587-2. [DOI] [PubMed] [Google Scholar]
- Stubbs M. T., Laber B., Bode W., Huber R., Jerala R., Lenarcic B., Turk V. The refined 2.4 A X-ray crystal structure of recombinant human stefin B in complex with the cysteine proteinase papain: a novel type of proteinase inhibitor interaction. EMBO J. 1990 Jun;9(6):1939–1947. doi: 10.1002/j.1460-2075.1990.tb08321.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A., Kaji H., Nakaya K., Aoki Y., Nakamura Y., Samejima T. Amino acid sequence of derivatives of newborn rat epidermal thiol proteinase inhibitor. Biochem Int. 1985 Oct;11(4):557–564. [PubMed] [Google Scholar]
- Tonnelle C., Colle A., Fougereau M., Manuel Y. Partial amino acid sequence of two forms of human post-gamma-globulin. Biochem Biophys Res Commun. 1979 Feb 14;86(3):613–619. doi: 10.1016/0006-291x(79)91757-1. [DOI] [PubMed] [Google Scholar]
- Wakamatsu N., Kominami E., Takio K., Katunuma N. Three forms of thiol proteinase inhibitor from rat liver formed depending on the oxidation-reduction state of a sulfhydryl group. J Biol Chem. 1984 Nov 25;259(22):13832–13838. [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis using M13-derived vectors: an efficient and general procedure for the production of point mutations in any fragment of DNA. Nucleic Acids Res. 1982 Oct 25;10(20):6487–6500. doi: 10.1093/nar/10.20.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]