Abstract
Substance use disorder is a major concern, with few therapeutic options. Heparan sulfate (HS) and chondroitin sulfate (CS) interact with a plethora of growth factors and their receptors and have profound effects on cellular signaling. Thus, targeting these dynamic interactions might represent a potential novel therapeutic modality. In the present study, we performed mass spectrometry–based glycomic and proteomic analysis to understand the effects of cocaine and methamphetamine (METH) on HS, CS, and the proteome of two brain regions critically involved in drug addiction: the lateral hypothalamus and the striatum. We observed that cocaine and METH significantly alter HS and CS abundances as well as sulfate contents and composition. In particular, repeated METH or cocaine treatments reduced CS 4-O-sulfation and increased CS 6-O-sulfation. Since C4S and C6S exercise differential effects on axon growth, regeneration, and plasticity, these changes likely contribute to drug-induced neural plasticity in these brain regions. Notably, we observed that restoring these alterations by increasing CS 4-0 levels in the lateral hypothalamus by adeno-associated virus delivery of an shRNA to arylsulfatase B (N-acetylgalactosamine-4-sulfatase) ameliorated anxiety and prevented the expression of preference for cocaine in a novelty induced conditioned place preference test during cocaine withdrawal. Finally, proteomics analyses revealed a number of aberrant proteins in METH- and cocaine-treated versus saline-treated mice, including myelin proteolipid protein, calcium/calmodulin-dependent protein kinase type II subunit alpha, synapsin-2, tenascin-R, calnexin, annexin A7, hepatoma-derived growth factor, neurocan, and CSPG5, and oxidative phosphorylation among the top perturbed pathway. Taken together, these data support the role of HS, CS, and associated proteins in stimulants abuse and suggest that manipulation of HSPGs can represent a novel therapeutic strategy.
Keywords: addiction, neurobiology, drug abuse, proteomics, glycomics, mass spectrometry, glycosaminoglycans, proteoglycans, cocaine, methamphetamine, lateral hypothalamus, striatum, heparan sulfate, chondroitin sulfate
Graphical Abstract
Highlights
-
•
Cocaine and METH significantly alter HS and CS abundances and sulfate contents.
-
•
METH/cocaine treatment reduced CS 4-O- and increased CS 6-O-sulfation versus control.
-
•
Increasing CS 4-0 by silencing ARSB improved anxiety and prevented cocaine preference.
-
•
Proteomics data showed a tight clustering of the drug-treated versus control samples.
In Brief
Drug abuse is a major concern with few therapeutic options. Heparan sulfate and chondroitin sulfate interact with growth factors and their receptors and have profound effects on cellular signaling. Thus, targeting these interactions represents a novel therapeutic modality. We performed glycomic and proteomic analysis of cocaine and methamphetamine-treated mouse brains versus control (saline-treated) from lateral hypothalamus and striatum and identified key alterations in chondroitin sulfate and heparan sulfate sulfation patterns and associated proteins and pathways, opening avenues for novel therapeutic strategies for stimulant abuse.
Drug abuse is a major concern, with few therapeutic options (1). Drug-related deaths have grown drastically between 1999 and 2015, and overdose deaths of any kind of drug for Americans 20 to 64 years old increased by 5.5 percent per year (2). About 20.4 million people were diagnosed with substance use disorder, and nearly 71,000 people died of overdose in 2019, with cocaine and methamphetamine (METH) deaths reaching about 15,000 in 2019 (3). This alarming trend drives the need to unravel the neurobiological mechanisms that contribute to drug abuse to develop new therapeutic targets and treatment strategies.
Heparan sulfate (HS) and chondroitin sulfate (CS) are unbranched polysaccharides composed of alternating uronic acid (either glucuronate or iduronate) and GalNAc or GlcNAc disaccharide units, respectively, attached to a serine or threonine residue of the core protein through a characteristic tetrasaccharide linker. HS and CS glycosaminoglycans (GAGs) with their disaccharide units, linkages, modifications, functions; and structural information, including Lawrence code and traditional nomenclature, chemical structures and m/z values are shown in Table 1 and Supplemental Table S1, (4, 5, 6, 7, 8). The interactions between GAGs polysaccharides, growth factors, and growth factor receptors regulate cellular signaling in a spatially and temporally defined manner (9). Thus, the dynamic regulation of HS, CS, and its interaction partners might have potential as a novel therapeutic modality (10).
Table 1.
Type of glycosaminoglycans (GAGs), chondroitin sulfate (CS) and heparan sulfate (HS) unsaturated disaccharides disaccharide unit, linkage, functions, disaccharide Lawrence and traditional nomenclature, m/z, and other description used in the manuscript (4, 5, 6, 7, 8)
| Type of GAG | Disaccharide unit with linkage | Modifications | Functions | Disaccharides | |||
|---|---|---|---|---|---|---|---|
| Chondroitin sulfate (CS) | β-1,3 GalNAc and β-1,4 HexA (Glc or IdoA) | Sulfation at the C2 position of GlcA, C4, and/or C6 position of GalNAc | Cell migration, proliferation and signaling, and extracellular matrix–receptor interaction | Lawrence codes | Traditional names | m/z (z = 1) | Other description |
| D0a0 | ΔHexA-GalNAc | 378 | unsulfated | ||||
| D0a4 | ΔHexA-GalNAc4S | 458 | 4-O sulfated (4S) | ||||
| D0a6 | ΔHexA-GalNAc6S | 458 | 6-O sulfated (6S) | ||||
| D2a4 | ΔHexA2S-GalNAc4S | 538 | 2-O 4-O sulfated | ||||
| D2a6 | ΔHexA2S-GalNAc6S | 538 | 2-O 6-O sulfated | ||||
| D0a10 | ΔHexA-GalNAc4S6S | 538 | 4-O 6-O sulfated | ||||
| D2a10 | ΔHexA2S-GalNAc4S6S | 617 | 2-O 4-O 6-O sulfated | ||||
| Heparin sulfate (HS) | β-1,4 GlcNAc and α-1,4 Hex A (Glc or IdoA) | Epimerization of GlcA to IdoA. Sulfation at 2O-position of IdoA, the N-, 6O- and 3O-position of GlcNS/GlcNAc |
Developmental processes, angiogenesis, and blood coagulation | D0A0 | ΔHexA-GlcNAc (IVA) | 378 | Unsulfated N-acetylated |
| D0A6 | ΔHexA-GlcNAc(6S) (IIA) |
458 | 6-O sulfated N-acetylated |
||||
| D2A0 | ΔHexA(2S)-GlcNAc (III-A) |
458 | 2-O sulfated N-acetylated |
||||
| D2A6 | ΔHexA(2S)-GlcNAc(6S) (IA) |
538 | 2-O, 6-O sulfated N-acetylated |
||||
| D0S0 | ΔHexA-GlcNS (IVS) | 416 | N-sulfated (no O-sulfation) | ||||
| D0S6 | ΔHexA-GlcNS(6S) (IIS) |
496 (247.5; z = 2) | 6-O sulfated N-sulfated |
||||
| D2S0 | ΔHexA(2S)-GlcNS (IIIS) |
496 (247.5; z = 2) | 2-O sulfated N-sulfated |
||||
| D2S6 | ΔHexA(2S)-GlcNS(6S) (IS) |
576 (287.5; z = 2) | 2-O, 6-O sulfated N-sulfated |
||||
GlcA, glucuronic acid; Hex, hexuronic acid; IdoA, iduronic acid.
With the advent of high-throughput genomic and proteomic techniques, a number of studies have generated protein and gene expression profiles for neuroanatomical substrates in rodents, nonhuman primate models of substance use disorder, and human postmortem brain tissue from victims of drug abuse (11). In the present work, we sought to define the effects of drugs of abuse on HS and CS levels and structures.
We previously showed that stimulants like cocaine and METH greatly increase HS content and sulfation levels in the lateral hypothalamus (LH) and that HS contributes to the regulation of cocaine seeking and taking. Accordingly, our previous gene expression studies showcased that the rats with a history of excessive cocaine self-administration had increased expression of the HS proteoglycan syndecan-3 in the LH (12), a brain region involved in motivation for both natural rewards and drug abuse (13, 14). Moreover, syndecan-3 null mice self-administered higher quantities of cocaine than WT mice, and the glial cell line–derived neurotrophic factor, which binds syndecan-3 through its HS chains, significantly increased cocaine intake in syndecan-3 null mice relative to WT mice (12). We showed that the ability of HS-binding neuropeptide glial cell line–derived neurotrophic factor to increase cocaine intake was potentiated by the elimination of HS binding. Additionally, the delivery of heparanase, the endo-β-D-glucuronidase that cleaves HS chains, promoted cocaine self-administration in a manner distinct from the action of syndecan-3, indicating that HS is a resilience factor for cocaine abuse and a novel therapeutic target for cocaine addiction (15).
A fundamental question raised by these results is the mechanism of action of GAGs in drug abuse and its motivation. To address this question, we applied proteomic and glycomic analysis to measure changes in response to cocaine and METH, two widely used stimulants, on HS, CS, and protein levels, respectively, from each of the two brain regions associated with motivation for both natural rewards and drugs of abuse, the LH and the ST (13, 14, 16, 17, 18, 19, 20, 21, 22). We report significant changes in HS and CS disaccharide abundances and percent sulfate levels observed in the drug-treated mice compared to saline-treated mice for both LH and ST. In addition, we observed that both stimulants induced a reduction of CS 4-O-sulfation and an increase in 6-O-sulfation. Notably, adeno-associated virus (AAV) delivery of an shRNA to arylsulfatase B (N-acetylgalactosamine-4-sulfatase, ARSB), to increase CS 4-0 levels in the LH, reduced anxiety and prevented the expression of preference for cocaine in a novelty-induced conditioned place preference test during cocaine withdrawal. Finally, the proteomics data showed a tight and separate clustering of the cocaine and METH-treated samples from the saline-treated samples, indicating the similarity between the drug-treated groups in both brain regions. These data show a number of perturbed proteins and aberrant pathways in stimulant-treated mice. Our results also indicated qualitative changes in glycomic and proteomic maps between the two brain regions. Taken together, our study defines novel relationships among HS, CS, and associated proteins with drug abuse in a mouse model, providing a new focus for the investigation of therapeutics.
Experimental Procedures
Materials
Heparin lyase enzymes I, II, and III and chondroitinase ABC enzyme (CHABC) were purchased from New England Biolabs. Trypsin Gold, mass spectrometry (MS) grade, was purchased from Promega. Trizma base, sodium chloride, Triton X-100, calcium chloride, ammonium bicarbonate, 2,2,2-trifluoroethanol were all purchased from Sigma-Aldrich. EDTA was purchased from Fluka. Ammonium acetate and C-18 zip tips (100 μl), LC-MS grade acetonitrile (ACN), and water were all purchased from Thermo Fisher Scientific. Complete mini protease inhibitor cocktail tablet (EDTA-free) was purchased from Roche Diagnostics.
Experimental Design and Statistical Rationale
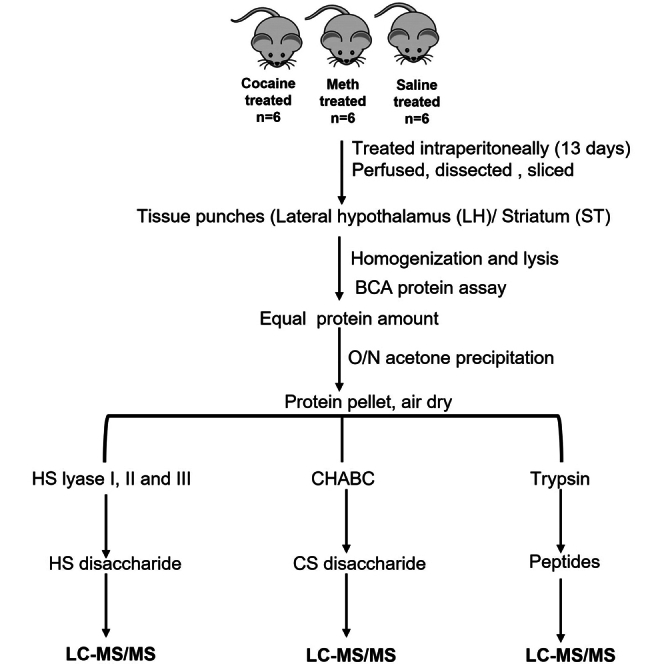
Fresh frozen mouse tissue punches from LH and striatum (ST) were obtained from six cocaine-treated (C1, C2, C3, C4, C5, and C6), six METH-treated (M1, M2, M3, M4, M5, and M6), and six saline-treated (S1, S2, S3, S4, S5, and S6) mice, for a total of n = 18 samples per brain region. All the samples for each brain region were handled for sample processing and acquired on the instrument (for both glycomics and proteomics) at the same time to avoid any sample handling or instrument bias. The two brain regions were processed and acquired about a year apart. For all samples (glycomics and proteomics), two technical LC-MS/MS runs were acquired except for proteomics data for ST brain region. Standard disaccharides, peptide retention time mixtures, and blanks were run routinely in between the samples. In addition, the disaccharide and peptide samples were spiked with internal controls. The total ion current (TIC) levels for all proteomics data files are shown in the Supplemental PDF S1, all TICs were within 2-fold intensity range of 1.8 to 3.04 E9 (for LH; Supplemental Fig. S1A) and 2.0 to 4.00 E9 (For ST; Supplemental Fig. S1B). The spiked internal standard peptide (m/z 496.28; Supplemental Fig. S2) for proteomics and synthetic disaccharide for glycomics (m/z 552; Supplemental Fig. S3) was shown to be consistent concerning signal intensity and retention time for cocaine, METH, and saline samples for LH and ST. Each group cocaine, METH, and saline had n = 6 biological replicate and all the data were tested for multiple corrections using a Benjamin–Hochberg corrected p value cut-off of 0.2.
Mice
Adult male C57BL/6J mice were housed in a climate-controlled vivarium on a 12 h/12 h reverse light/dark cycle (lights on 9:00 AM) with food and water available ad libitum throughout the study. The experimental protocols were performed in accordance with US National Institutes of Health guidelines on animal care and were approved by The Scripps Research Institute Animal Care and Use Committee (15). All reporting in the manuscript follows the recommendations in the ARRIVE guidelines for reporting research work involving live animals (23).
Treatment and Tissue Collection
One cohort of adult male C57BL/6J mice was treated with either cocaine (20 mg/kg/10 ml once per day) or METH (2.5 mg/kg/10 ml twice per day) intraperitoneally for 13 days, as previously reported in (17). After 48 h from the last injection, mice were euthanized by decapitation under heavy isoflurane (5%) anesthesia, brains were immediately harvested, dissected, and brain regions were snap-frozen on dry ice. Another cohort of adult male C57BL/6J mice, previously microinjected with AAV (see below) in the LH, was treated intraperitoneally with cocaine (10 mg/kg/10 ml) for 14 days. After the behavioral experiments were performed, animals were euthanized as described above.
Microinfusion of AAV
AAV vectors (AAV1 serotype) expressing either a bicistronic transcript including the ORFs of mARSB and GFP linked through an IRES (AAV1-mARSB) or expressing GFP alone (AAV1-GFP) from a CMV promoter and antisense to ARSB under the control of U6 promoter with titers in the range of 8.4 × 1012 to 1.0 × 1013 genomic particles per milliliter were prepared according to conventional protocols. Animals were anesthetized for intra-LH virus injections. Briefly, mice were immobilized in a stereotaxic frame in the flat-skull position (Kopf Instruments), and a bilateral stainless-steel injector (33 ga, extended 1 cm below the tip of a 26-ga sleeve tubing) was lowered into the LH (coordinates: −0.94 mm anterior to bregma, ±1.2 mm lateral, and −5.4 mm ventral to skull surface at bregma). The AAV solution was delivered slowly through the injector by a syringe pump (0.5 μl each at a flow rate of 0.05 μl per min, Kd Scientific). The injector remained in place for at least 10 min and then was withdrawn slowly to avoid backflow of the virus. The effects of the AAV microinjections on ARSB expression levels were confirmed by MS (Supplemental Fig. S4) based on CS disaccharide analysis, as indicated below.
Si-RNA and Overexpressing ARSB Experiments Anxiety and Novelty Experiments
Elevated Plus Maze
After 10 days of withdrawal, AAV-microinjected mice were tested in the elevated plus maze (EPM). The EPM consisted of a central platform (5 × 5 cm, W × L) and two open arms (5 × 30 cm, W × L) aligned perpendicularly to two closed transparent arms (5 × 30 cm, W × L) at the height of 40 cm from the ground. Mice were tested individually by being placed in the center of the maze facing an open arm. The spontaneous activity of mice was automatically recorded for 5 min, during which the experimenter left the room. After each experiment, the apparatus was cleaned with 70% ethanol allowing time for evaporation before testing the following mouse. Data were calculated as time spent in the open arms (seconds) and, in agreement with Maccioni et al., 2021 (24), as the percentage of open arm entries (number of entries into open arms divided by the number of entries into open arms + number of entries into closed arms). The analysis was automatically performed by ANY-Maze software. Mice that fell from the maze were discarded from the analysis.
Novelty-Induced Place Conditioning
Competition between novelty and cocaine was tested using a place conditioning procedure. Briefly, mice were treated with cocaine (10 mg/kg) and immediately confined in the cocaine-associated compartment for 15 min daily for 14 days to induce place conditioning. After 14 days from the last cocaine administration, the cocaine-associated compartment was connected to an easily discriminable novel compartment which differed by visual and tactile clues (24, 25, 26) which was used as the novel stimulus (27). Mice were left free to explore the two connected compartments for 5 min. Preference was expressed in terms of time (s) spent in the cocaine-associated or in the novel compartment.
Tissue Lysis of Mouse LH and ST
Fresh frozen mouse tissue punches: (LH and ST processed at 1 year apart) cocaine-treated; C1, C2, C3, C4, C5, and C6, METH-treated; M1, M2, M3, M4, M5, and M6, and saline-treated; S1, S2, S3, S4, S5, and S6) were lysed described (15) with buffer containing 50 mM Tris–HCl (pH = 7.4), 150 mM NaCl, 2 mM EDTA, protease inhibitor cocktail tablet, and 0.5% Triton X-100. Tissues were homogenized and incubated for 30 min on ice. This was followed by centrifugation at 12,000 RPM, 4 °C for 30 min. The supernatant was collected into separate tubes, and the protein concentration of each sample was determined using the Pierce bicinchoninic acid assay (Thermo Fisher Scientific). The samples were stored at −20 °C until further use. The methodology is shown in Supplemental Figure S5.
Enzyme Digestion for HS and Size-Exclusion Chromatography
The HS release and size-exclusion chromatography (SEC) were carried out as previously described (15). Briefly, an equal amount of protein for each sample was taken, and a 5-fold volume of acetone was added and incubated at −20 °C overnight. The samples were centrifuged at 20,000×g, 4 °C for 30 min. The pellets were washed with equal volumes of acetone: water (6:1) and centrifuged again. The supernatant was discarded, and the pellet was allowed to dry in the air. The protein pellet from each sample was resuspended in a volume of 100 μl 20 mM Tris–HCl buffer pH 7.4, in the presence of 5 mM CaCl2, 5 mM ammonium acetate, 10 mU each of heparin lyase I, heparin lyase II, and heparin lyase III (NEB) (8) were added, and the mixture sonicated briefly. The mixture was incubated at 37 °C overnight using Eppendorf thermomixer at a speed of 400 RPM. The digested solution was added directly onto a 3 kDa cut-off filtration unit (Amicon, 0.5 ml) and centrifuged at 14,000g for 20 min. The filter was washed with 100 μl water, centrifuged again, and the HS disaccharides were collected as a flow-through. The flow-through was dried by vacuum centrifugation and desalted using an SEC column (Superdex peptide PC 3.2/30, GE Healthcare), using 25 mM ammonium acetate in 5% ACN (pH = 4.4) as the mobile phase at an isocratic flow 0.04 ml/min for 60 min. The HS disaccharides eluted between 35 to 45 min and were detected using UV absorbance at 232 nm. The cleaned HS disaccharide samples were dried by vacuum centrifugation and stored at −20 °C until analyzed using LC-MS/MS.
Enzyme Digestion for CS and SEC
As indicated above, an equal amount of protein for each sample was taken, a 5-fold volume of acetone was added, and incubated at −20 °C overnight. The samples were centrifuged at 20,000×g, 4 °C for 30 min to obtain a protein pellet which was air dried. The protein pellet from each sample was resuspended in a volume of 100 μl 20 mM Tris–HCl buffer pH7.4 in the presence of 5 mM ammonium acetate and 20 mU of CHABC (NEB) (20) and sonicated briefly. The workup for CS disaccharides was the same as for HS disaccharides (above).
Enzyme Digestion for Peptides and C-18 Zip-Tip Cleanup
As indicated above, an equal amount of protein for each sample was taken, and five times acetone was added and further incubated at −20 °C overnight and centrifuged at 20,000g, 4 °C, for 30 min, to obtain a protein pellet which was air dried. The protein pellet from each sample was resuspended in 100 μl of 50% 2,2,2-trifluoroethanol/50% 50 mM ammonium bicarbonate and sonicated briefly to dissolve the pellet and incubated at 60 °C for 2 h on an Eppendorf thermomixer at a speed of 400 RPM. After 2 h, the solution was cooled, and 100 μl of 50 mM ammonium bicarbonate and 5 mM DTT was added and incubated at 60 °C for 30 min on an Eppendorf thermomixer at a speed of 400 RPM. A volume of 5 μl of 200 mM iodoacetic acid (10 mM) was added to the solution and incubated in the dart at room temperature for 30 min. The solution was diluted to a total volume of 500 μl, and 1:30 trypsin enzyme by weight was added, and the solution was incubated overnight at 37 °C using an Eppendorf thermomixer at a speed of 400 RPM. The trypsinization was stopped with 1% formic acid, and the solution was dried by vacuum centrifugation (SPD1010 Speedvac system, Thermo Savant). The dried sample was resuspended in 2% ACN/water/0.1% TFA and passed through C-18 zip tips (Thermo Fisher Scientific); the cleaned peptides were eluted using 60% ACN/water/0.1% TFA and dried by vacuum centrifugation. The cleaned peptides were further stored at −20 °C until analyzed using LC-MS/MS.
LC-MS/MS Analysis for HS and CS Disaccharides (Glycomics)
HS and CS disaccharides were analyzed using negative ionization mode electrospray LC-MS as previously described (15). Disaccharides were separated using a 1.9 μm, 0.3 × 150 mm GlycanPac AXH-1 (Thermo Fisher Scientific) column mounted on an Agilent 1200 LC (Agilent Technologies). A 20-min isocratic method was used (85% B) and a flow rate of 7 μl/min (HS) and 5 μl/min (CS). Solvent A was 50 mM ammonium formate pH 4.4 in 10% ACN, and solvent B was 95% ACN/5% water. MS analyses were performed using an Agilent 6520 Q-TOF (Agilent Technologies) using electrospray ionization. An 800 fmol quantity of ΔHexA2S-GlcNCoEt(6S) (Iduron) was added to all the samples as an internal standard before LC-MS analyses. The targeted tandem mass spectrometry (MS/MS) analysis was performed to differentiate between HS and CS disaccharide isoforms (Table 1 and Supplemental Table S1) with a collisional-induced dissociation. A fixed collision energy of 20 was used. The targeted list for HS was m/z 458 (charge; z = 1) and m/z 247.5 (z = 2), and for CS was m/z 458 (z = 1). The relative and absolute abundance were determined using standard curves as previously described (28, 29, 30).
LC-MS/MS Analysis Peptide (Proteomics)
Nano-LC-MS/MS separation was performed using a nanoAcquity high-performance liquid chromatography (Waters Technology) and Q-Exactive mass spectrometer (Thermo Fisher Scientific). A 50 fmol retention time calibration mixture (Pierce) was added to all samples as an internal standard prior to LC-MS analysis. Reversed-phased C-18 analytical (BEH C18, 150 μm × 100 mm) and trapping (180 μm × 20 mm) columns from Waters technology were used with a 120 min method with a gradient from 2 to 98% ACN in 97 min, using 99% water/1% ACN/0.1% formic acid as mobile phase A and 99% ACN/1% water/0.1% formic acid as mobile phase B at a flow rate of 0.5 μl/min as previously described in (30). Data-dependent acquisition MS/MS was acquired in the positive ionization mode for the top ten most abundant precursor ions. Full MS scans were acquired from m/z 350 to 2000 with 70,000 resolution using an automatic gain control target of 1e6 and a maximum injection time of 100 ms. Dynamic exclusion (12 s) was enabled. The minimum threshold for precursor selection was set to 5 × 104. Precursor ions were fragmented using 2 micro scans at a resolution of 17,500 with a maximum injection time of 50 ms and an automatic gain control value of 2 e5 using higher energy collision-induced dissociation with a step-up normalized collision energy of 27, 35.
Protein Identification, PEAKS Label-free Quantification, and Data Analysis
The raw LC-MS/MS data were converted into mzXML format using ProteoWizard msConvert (31). The data were searched using PeaksDB and PeaksPTM using Peaks Studio version 8.0 (Bioinformatics Solutions, Inc) against the Uniprot/Swissprot database for Mus musculus (house mouse) with a 1% false discovery rate (FDR) and at least two unique peptides. A 10-ppm error tolerance for the precursor (MS1) and 0.02 Da mass error tolerance for fragment ions (MS2) were specified. A maximum of three missed cleavages per peptide was allowed for the database search, permitting nontryptic cleavage at one end. Trypsin was specified as the enzyme and carbamidomethylation as a fixed modification. A peaksPTM search was queued after the peaksDB search, using advanced settings of a larger set of variable modifications, including hydroxylation P, oxidation M, hydroxylation K, hydroxylation-Hex K, hydroxylation-Hex-Hex K, HexNAc ST, HexHexNAc ST, phosphorylation STY, ubiquitination K, deamidation N, methoxy K, and nitrotyrosine Y. The final protein list generated was a combination of peaksDB and peaksPTM searches. The label-free quantification was achieved using PEAKS Studio Quantification, a label-free module with a setting of mass error tolerance of 10 ppm and a retention time shift tolerance of 2.0 min. For data filtering for label-free peptide quantification following parameters were used: significance 15, fold change 1, quality 0, average area 1E4, charges from 1 to 10, detected in at least one of 18 samples. The protein quantification following settings was used: significance 0, fold change 1, at least two unique peptides, significance method PEAKS. All Abundances were normalized using TICs by the software relative to saline sample 1 (S1).
Glycomics Data Analysis Using Excel Spreadsheet
Student's t-tests were performed with two-tailed distribution using Microsoft Excel to test for alterations in the obtained HS and CS disaccharide profiles. Different concentrations (500 fmol, 1 pmol, 2 pmol, 5 pmol, and 10 pmol) of eight HS standard unsaturated disaccharides (D0A0, D2A0, D0A6, D2A6, D0S0, D2S0, D0S6, and D2S6) (Iduron), and four CS unsaturated disaccharides (D0a0, D0a4, D0a6, and D0a10); nomenclature, m/z, Lawrence codes and its designation, and other description for the disaccharides are shown in Table 1 and Supplemental Table S1 (4, 5, 6, 7, 8) were run on LC-MS as triplicates, for plotting an MS standard curve. The MS/MS standard curve was plotted for different ratios of HS isoforms (D2A0/D0A6 and D2S0/D0S6) and CS isoforms (D0a4/D0a6) as previously described (8). The area under the curve for extracted ion chromatograms (EICs) for HS and CS disaccharides from each sample was obtained from the raw LC-MS/MS data using qualitative analysis software (version B.06; Agilent Technologies). The obtained abundances for each disaccharide were first normalized to a spiked internal control (as indicated above) and then further normalized to a standard curve to obtain an absolute abundance of HS or CS disaccharides (fmol). A relative abundance was then calculated for each disaccharide from the absolute abundance. For differentiating the isoforms, EIC (MS/MS) for diagnostic ions, as indicated in (32) for each CS and HS disaccharide isoform, was extracted, and abundance was obtained from manual area calculation. The obtained abundance was then used to calculate the % relative abundance of each isoform which was further normalized using the MS/MS standard curve to obtain the % absolute abundance of each HS or CS isoform.
Statistical Analysis
In order to assess the variability in proteins and GAG disaccharides among the different treatments, we employed several statistical and data visualization methods. We generated heatmaps, hierarchical clustering dendrograms, and principal component analysis (PCA) plots for all LH and ST data and then again for each pair of treatments. We also performed t tests to determine significantly differentially expressed proteins for each drug treatment against saline, using a Benjamin–Hochberg corrected p value cut-off of 0.2. Protein data was standardized by logging and normalizing in order to fit every sample's protein expression into a normal distribution with mean 0 and SD 1 for sample comparison. GAG data were similarly standardized but without the logging step due to the disaccharide expression already following a normal distribution. All statistical analyses and data visualizations were performed using R (33).To test the significance of the effects of AAV microinjections in mice’s performance in the EPM, one-way ANOVA followed by Tukey post hoc test was used for the total distance, for the percentage of time spent in the open arms and for the percentage of open arms entries. One-way ANOVA followed by Fisher post hoc was used for the latency to the first open arm entry. Two-way ANOVA followed by Tukey post hoc test was used to verify the statistical effects of AAV microinjections in mice’s preference for the cocaine-associated compartment or the novelty-associated one.
Functional Analysis (Proteomics)
To understand the biological deregulation of aberrantly expressed proteins, the differentially quantified proteins from peaks output were used and further analyzed by in-house and online functional annotation and bioinformatics tools for protein gene ontology (GO) annotation, and pathway analysis, including IPA software (Ingenuity Systems), DAVID Bioinformatics resource 6.8 (https://david.ncifcrf.gov/), and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways (http://www.genome.jp/kegg/pathway.html). The scores computed by IPA for each network are derived from a p value and indicate the likelihood of the proteins being found together in that network by random chance (34). The PCA, clustering plots, and differentially expressed protein lists were obtained from the in-house software PEAKSviz (https://jdhogan.shinyapps.io/peaksviz/).
Results
Brain Region–Specific Changes (HS, CS Disaccharide, and Proteins)
Two brain regions, the LH and ST were used for this study. The saline samples from each brain region were used to determine the qualitative brain region–specific changes in HS and CS disaccharides and proteins. The HS and CS disaccharide abundances in the two brain regions with the difference in relative abundance (%) in LH versus ST are summarized in Supplemental Table S2. Interestingly, D0A6 and D0A0 HS disaccharides were the most abundant HS disaccharide in LH and ST, respectively, and D0A0 was notably lower in LH versus ST. The elevation of D0A6 in LH was an unusual observation, as most brain studies report D0A0 (similar to our ST data) as the most abundant disaccharide (30). To rule out any analytical bias related to sample processing or LC-MS instrument performance, we compared the HS disaccharide profiles of HSBK (heparan sulfate sodium salt from the bovine kidney), a standard control that was processed and acquired on the instrument with both brain region samples at separate times. The EIC for D0A0 and D2A0/D0A6 (Supplemental Fig. S6) for HSBK for both batches showed similar EICs with no significant difference as observed in our samples, ruling out any analytical bias in our data. Sulfated disaccharides (D2A0, D0A6, D2S0, D0S6, D2S6) were higher for LH than ST, pointing toward a higher HS sulfation in LH than ST, which was also confirmed by HS content (%). For CS disaccharides, unsulfated (D0a0) disaccharide was observed to be higher, while sulfated (D0a4/a6) disaccharides were observed to be lower in LH versus ST, pointing toward lower CS sulfation in LH versus ST contrary to HS sulfation. However, the total CS content (%) was similar in both brain regions. The total CS abundance (fmol) was decreased for LH versus ST Importantly, both brain regions showed a higher CS 4-O sulfation (D0a4) than 6-O sulfation (D0a6).
For proteomics data, a Venn diagram (Supplemental Fig. S7) was constructed using proteins (unique peptide ≥2) observed in saline samples for LH and ST, and a GO and pathway analysis of common and exclusive proteins to each brain region was performed using DAVID. Most proteins in LH were localized in the cytoplasm, whereas most proteins in ST belonged to nonmembrane-bound organelles. The majority of common proteins in both brain regions were localized in mitochondrion. Oxidative phosphorylation was among the top KEGG pathways for ST exclusive and common (LH and ST) proteins, while regulation of actin cytoskeleton was among the top pathway for LH exclusive proteins. It is important to stress that these qualitative brain region–specific trends need further validation with a larger cohort and a dedicated brain region–specific study.
HS Disaccharide Analysis for LH and ST
HS disaccharides were enzymatically released from tissue lysis of the LH and ST, purified using SEC, and analyzed using a GlycanPac AXH-1 column ESInegative ion MS/MS-based quantitative glycomics. Eight HS unsaturated disaccharides four N-acetylated (D0A0;unsulfated, D2A0;2-O sulfated, D0A6; 6-O sulfated, D2A6; and 2-O, 6-O sulfated), and four N-sulfated (D0S0; no O-sulfation, D2S0; 2-O sulfated, D0S6; 6-O sulfated, and D2S6; 2-O, 6-O sulfated) (Table 1) were observed for cocaine (C1-C6), METH (M1, M3-M6; M2 sample was removed for being an outlier sample), and saline (S1-S6) in LH (Supplemental File S1A), while only seven HS disaccharides (D0A0, D2A0, D0A6, D0S0, D2S0, D0S6, and D2S6; D2A6 was absent, or present below detectable limits) were observed for cocaine (C1-C6), METH (M1-M6), and saline (S1-S6) in ST (Supplemental File S2A). GlcN-3-0 sulfated and saturated HS disaccharides were not observed as they were below the detectable limit of the instrument used. The disaccharide abundances were normalized to a spiked internal disaccharide (ΔHexA2S-GlcNCoEt(6S)) and MS standard curve. An MS standard curve for different concentrations of eight standard HS disaccharides (D0A0, D2A0, D0A6, D2A6, D0S0, D2S0, D0S6, and D2S6) was plotted for both LH (Supplemental File S1B) and ST samples (Supplemental File S2B). In addition, an MS/MS standard curve was plotted for different ratios of HS isoforms (D2A0/D0A6 and D2S0/D0S6) for both LH (Supplemental File S1C) and ST samples (Supplemental File S2C).
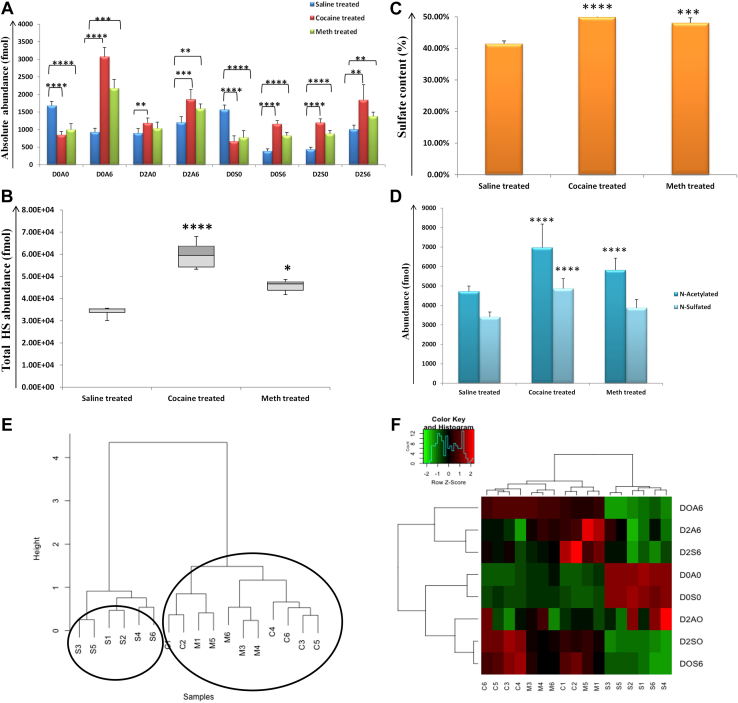
For LH, significant changes in the abundances (fmol) of different HS disaccharides were observed for cocaine, METH, and saline-treated samples. Importantly, unsulfated D0A0 (N-acetylated) was observed to decrease for drug-treated samples (cocaine; p = 1.4 E-07 and METH; p = 0.0001) when compared to saline-treated samples. In addition, D0S0 (N-sulfated; no O-sulfation) HS disaccharide was decreased for cocaine (p = 7.5 E-07) and METH (p = 0.0001) compared to saline (Fig. 1A). Interestingly, D0A6 (N-acetylated, 6-O-sulfated) HS disaccharide was the most abundant HS disaccharide and was increased for drug-treated samples (cocaine versus saline; p = 4.46 E-09, METH versus saline; p = 0.0001). In fact, D0A6 was significantly higher for cocaine-treated than METH-treated samples (p = 0.0003). D2A0 (2-O sulfated), an isoform of D0A6, was also significantly higher for only cocaine (p = 0.004) than saline. The disulfated (D2A6, D2S0, D0S6) and trisulfated (D2S6) HS disaccharides were elevated for cocaine and METH versus saline, indicating a higher sulfation pattern in the drug-treated samples.
Fig. 1.
Heparan sulfate (HS) disaccharides analysis for lateral hypothalamus (LH).A, absolute abundances in fmol for eight HS disaccharides (D0A0, D2A0, D0A6, D2A6, D0S0, D2S0, D0S6, and D2S6) for saline (blue color), cocaine (red color), and METH (green color) in LH. B, total HS disaccharide abundance (fmol) for saline, cocaine, and METH-treated samples in LH. C, sulfate content (%) for saline, cocaine, and METH-treated samples in LH. D, N-acetylated and N-sulfated HS disaccharide abundance (fmol) for saline-, cocaine-, and METH-treated samples in LH. (∗=p ≥ 0.05, ∗∗= p ≥ 0.001, ∗∗∗= p ≥ 0.0001, ∗∗∗∗= p ≥ 0.00001) (mean ± SD). E, unsupervised hierarchical clustering of the entire HS disaccharide data for saline, cocaine, and METH samples. F, heatmap representation of relative quantification for HS disaccharides (red: increased and green: decreased abundance). METH, methamphetamine.
The total HS abundance (fmol) (Fig. 1B) and sulfate content (%) (Fig. 1C) were significantly higher for cocaine (p = 1.1 E-05, and p = 3.8 E-06, respectively) and METH (p = 0.02, and p = 0.0002, respectively) than saline in LH. Among the drug samples, cocaine was observed to have significantly higher HS abundance (fmol) (p = 0.007) than METH in LH. A comparison of N-acetylated (D0A0, D2A0, D0A6, D2A6) and N-sulfated (D0S0, D2S0, D0S6, D2S6) HS disaccharides revealed N-acetylated form to be more abundant than N-sulfated for all the samples, and a significant increase in N-acetylated HS disaccharides for both cocaine (p = 4.13 E-06) and METH (p = 0.01) versus saline and N-sulfated HS disaccharides for cocaine versus METH (p = 0.006) and saline (p = 8.3 E−05) was observed, Figure 1D. Unsupervised hierarchical clustering of entire HS disaccharide data for the samples in LH showed a tight clustering of drug samples, while the saline samples were clustered separately, Figure 1E. A heatmap representation of relative quantification for HS disaccharides also illustrated the decrease in unsulfated (D0A0, D0S0) and increase in sulfated (D0A6, D2A6, D2S0, D0S6, and D2S6) disaccharides (Fig. 1F) in drug versus saline-treated samples.
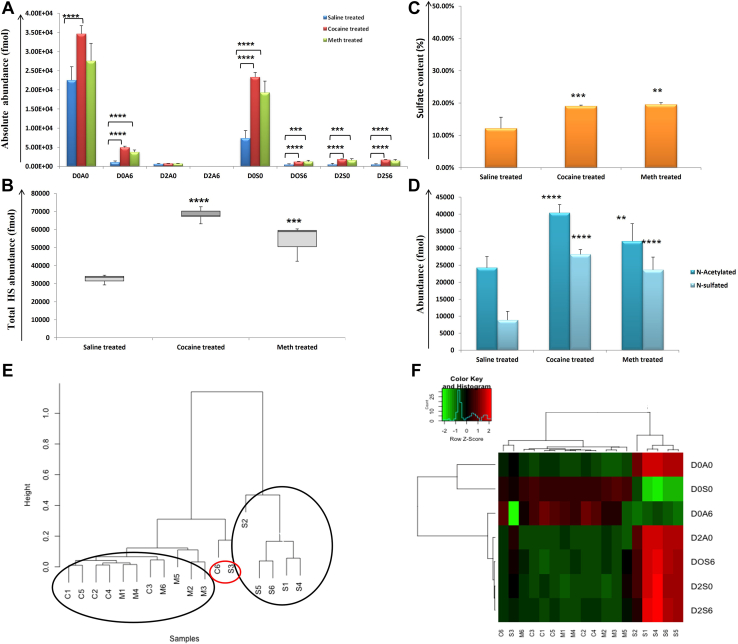
By contrast, for ST, unsulfated D0A0 (N-acetylated) was the most abundant HS disaccharide for all the samples and was increased for cocaine (p = 2.7 E-05) and METH (p = 0.05) versus saline samples. D0S0 (N-sulfated; no O-sulfation) HS disaccharide was the second most abundant HS disaccharide, which was also increased for cocaine (p = 1.1E-08) and METH (p = 1.06 E-05) versus saline samples (Fig. 2A). D2A6 disaccharide was absent (or below detectable limits) for ST samples. In agreement with LH, sulfated HS disaccharides were significantly elevated in the drug compared to saline samples for ST. In addition, the total HS abundance (fmol) (Fig. 2B) and sulfate content (%) (Fig. 2C) were significantly higher for cocaine (p = 4.0 E-09, and p = 0.0006, respectively) and METH (p = 0.0001, and p = 0.009, respectively) versus saline-treated samples. The N-acetylated were more abundant than N-sulfated HS disaccharides for cocaine, METH, and saline samples. In addition, both N-acetylated and N-sulfated HS disaccharides were significantly higher for cocaine (p = 2.02 E-0-6, p = 1.1 E−08, respectively) and METH (p = 0.01, p = 1.1 E-05, respectively) than saline samples (Fig. 2D). Unsupervised hierarchical clustering of entire HS disaccharide data in ST showed a tight clustering of drug samples, while the saline samples were clustered separately, Figure 2E. A heatmap representation of relative quantification for HS disaccharides showed differences in HS disaccharides between drug versus saline samples, Figure 2F.
Fig. 2.
Heparan sulfate (HS) disaccharides analysis for striatum (ST).A, absolute abundance in fmol for seven HS disaccharides (D0A0, D2A0, D0A6, D0S0, D2S0, D0S6, and D2S6) (D2A6 was not detected) for saline (blue color), cocaine (red color), and METH (green color) in ST. B, total HS disaccharide abundance (fmol) for saline-, cocaine-, and METH-treated samples in ST. C, sulfate content (%) for saline-, cocaine-, and METH-treated samples in ST. D, N-acetylated and N-sulfated HS disaccharide abundance (fmol) for saline-, cocaine-, and METH-treated samples in ST. (∗=p ≥ 0.05, ∗∗= p ≥ 0.001, ∗∗∗= p ≥ 0.0001, ∗∗∗∗= p ≥ 0.00001) (mean ± SD). E, unsupervised hierarchical clustering of the entire HS disaccharide data for saline, cocaine, and METH samples. F, heatmap representation of relative quantification for HS disaccharides (red: increased and green: decreased abundance). METH, methamphetamine.
CS Disaccharide Analysis for LH and ST
CS disaccharides were released enzymatically from tissue lysis of the LH and ST, purified using SEC), and analyzed using GlycanPac AXH-1 column ESI-negative ion-MS/MS-based quantitative glycomics. Four CS unsaturated disaccharides D0a0 (unsulfated), D0a4 (4-O sulfated), D0a6 (6-O sulfated), D0a10 (4-O and 6-O sulfated) (or D2a6/D2a4; nondifferentiated isoforms with same m/z 538; used as D0a10 throughout the manuscript) (Table 1) were observed for cocaine (C1-C6), METH (M1-M6), and saline (S1-S6) in LH (Supplemental File S3A) and ST (Supplemental File S4A). The trisulfated CS disaccharide D2a10 and saturated CS disaccharides were not observed and/or were below the detectable limit. The CS disaccharides were normalized to a spiked internal disaccharide (ΔHexA2S-GlcNCoEt (6S)) and MS standard curve to obtain an absolute abundance of CS disaccharides. An MS standard curve for different concentrations of four HS disaccharides D0a0, D0a4, D0a6, D0a10) was plotted for both LH (Supplemental File S3B-i) and ST samples (Supplemental File S4B-i). In addition, an MS/MS standard curve was plotted for different ratios of CS isoforms (D0a4/a6) for both LH (Supplemental File S3B-ii) and ST samples (Supplemental File S4B-ii).
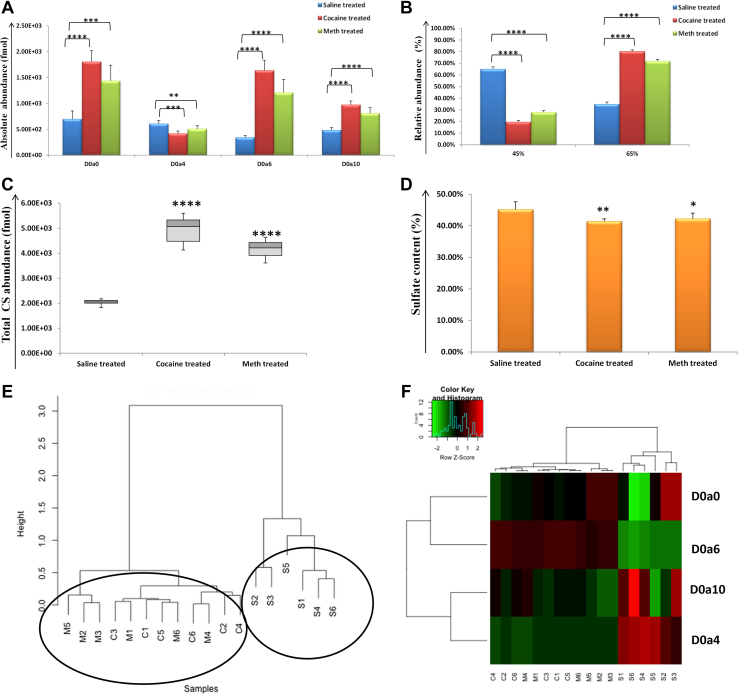
For LH, unsulfated D0a0 was the most abundant CS disaccharide for all the samples, and it was significantly elevated for cocaine (C; p = 1.2 E-06) and METH (M; p = 3.3 E-04) versus saline (S)-treated samples (Fig. 3A). Interestingly, when compared to D0a4 (4-O-sulfation) and D0a6 (6-O-sulfation), D0a6 was more abundant in drug-treated samples, while D0a4 was more abundant for saline samples. In addition, a significant increase in D0a6 and a concomitant decrease of D0a4 was observed for cocaine (p = 1.9 E-08 and p = 0.0001, respectively) and METH (p = 8.4 E-06 and p= 0.01, respectively) when compared to saline samples. The disulfated CS disaccharide D0a10 was also significantly increased for cocaine (p = 6.8 E-08) and METH (p = 5.8 E-05) relative to saline samples. The unique differential expression of 4-O (4S) and 6-0 (6S) sulfation was also illustrated by the relative abundance (%) of 4S versus 6S, where 6S was relatively higher, while 4S was significantly lower for cocaine (p= 2.1 E-13) and METH (p= 2.8 E-12) than saline samples (Fig. 3B). Unsupervised hierarchical clustering of entire CS disaccharide data in LH showed a tight clustering of drug samples, while the saline samples were clustered separately, Figure 3E. A heatmap representation of relative quantification for CS disaccharides also highlighted the increased D0a6 and increased D0a4, Figure 3F, in drug-treated versus saline samples. The total CS abundance (fmol) was observed to increase for drug-treated samples (cocaine; p = 6.6 E-07, and METH; p = 4.6 E-07) compared to saline samples in LH (Fig. 3C), whereas sulfate content (%) was observed to decrease for drug-treated samples (cocaine; p = 0.004, and METH; p = 0.03) compared to saline samples (Fig. 3D).
Fig. 3.
Chondroitin sulfate (CS) disaccharides analysis for lateral hypothalamus (LH).A, absolute abundance in fmol for four CS disaccharides (D0a0, D2a4, D0a6, and D0a10) for saline (blue color), cocaine (red color), and METH (green color) in LH. B, relative abundance (%) for 4-0 sulfation (4S) and 6-0 sulfation (6S) for saline-, cocaine-, and METH-treated samples in LH. C, Total CS abundance in fmol for saline-, cocaine-, and METH-treated samples in LH. D, sulfate content (%) for saline-, cocaine-, and METH-treated samples in LH. (∗=p ≥ 0.05, ∗∗= p ≥ 0.001, ∗∗∗= p ≥ 0.0001, ∗∗∗∗= p ≥ 0.00001) (mean ± SD). E, unsupervised hierarchical clustering of the entire CS disaccharide data for saline, cocaine, and METH samples. F, heatmap representation of relative quantification for CS disaccharides (red: increased and green: decreased abundance). METH, methamphetamine.
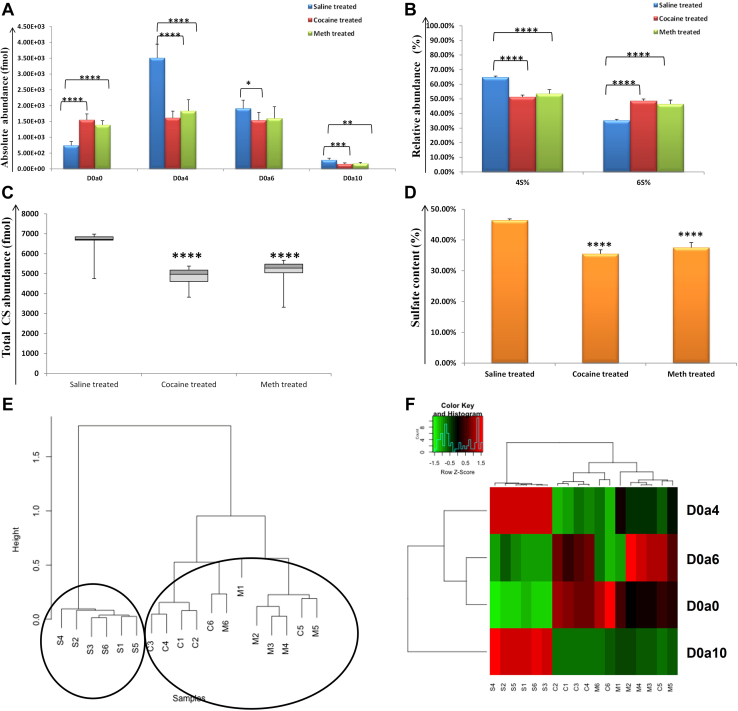
For ST, D0a4 was the most abundant CS disaccharide, similar to LH, and was significantly decreased for cocaine (p = 2.38 E-06) and METH (p = 2.6 E-05) compared to saline samples. However, unlike LH, D0a6 was also decreased for cocaine (p = 0.03) and METH (p = 0.1; not significant) versus saline samples (Fig. 4A). The unsulfated D0a0 was increased for cocaine (p = 4.8 E-06) and METH (p = 6.1 E-06) when compared to saline-treated samples, and the disulfated D0a10 was also significantly decreased for cocaine (p = 0.0009), and METH (p = 0.002) relative to saline-treated samples in ST. The unique differential expression of 4-O (4S) and 6-0 (6S) sulfation was also illustrated by the relative abundance (%) of 4S versus 6S, where 6S was relatively higher, while 4S was lower for cocaine (p = 8.8 E−10) and METH (p= 2.5 E-06) than saline samples in ST (Fig. 4B). The total CS abundance (fmol) was observed to decrease for drug-treated samples (cocaine; p = 4.8 E-06, and METH; p = 6.1 E-06) compared to saline samples in ST, unlike LH (Fig. 4C). While similar to LH, sulfate content (%) was observed to decrease for drug-treated samples (cocaine; p = 2.78 E-09, and METH; p = 0.04) compared to saline samples (Fig. 4D). Unsupervised hierarchical clustering of entire CS disaccharide data in ST showed a tight clustering of drug samples, while the saline samples were clustered separately, Figure 4E. A heatmap representation of relative quantification for CS disaccharides also showed increased D0a6 and increased D0a4, Figure 4F, in drug-treated versus saline-treated samples.
Fig. 4.
Chondroitin sulfate (CS) disaccharides analysis for striatum (ST).A, absolute abundance in fmol for four CS disaccharides (D0a0, D2a4, D0a6, and D0a10) for saline (blue color), cocaine (red color), and METH (green color) in ST. B, relative abundance (%) for 4-0 sulfation (4S) and 6-0 sulfation (6S) for saline-, cocaine-, and METH-treated samples in ST. C, total CS abundance in fmol for saline-, cocaine-, and METH-treated samples in ST. D, sulfate content (%) for saline-, cocaine-, and METH-treated samples in ST. (∗=p ≥ 0.05, ∗∗= p ≥ 0.001, ∗∗∗= p ≥ 0.0001, ∗∗∗∗= p ≥ 0.00001) (mean ± SD). E, unsupervised hierarchical clustering of the entire CS disaccharide data for saline, cocaine, and METH samples. F, heatmap representation of relative quantification for CS disaccharides (red: increased and green: decreased abundance). METH, methamphetamine.
Proteomics Analysis for LH and ST
Two different drug treatments, cocaine and METH, and a saline treatment as control were performed as six biological sets for each (cocaine C1-C6, METH M1-M6, and saline S1-S6), for two brain regions LH and ST, and were analyzed by reversed-phase nano-LC-MS/MS. For LH, two technical replicates and for ST, one technical replicate for each biological sample were acquired and processed using PEAKS software v8.0. About 900 to 1000 and 700 to 800 proteins with two unique peptides for LH (Supplemental File S5) and ST (Supplemental File S6), respectively, were observed for combined six biological (and technical replicates) for each treatment after PEAKS PTM analysis. A list of proteoglycans and their interacting and binding partners identified in the proteomics data for cocaine, METH, and saline from both LH and ST are shown in Supplemental Table S3.
A Venn diagram (http://bioinfogp.cnb.csic.es/tools/venny/) was constructed for a combined protein list from six biological replicates for each cocaine, METH, and saline for LH, and ST (Supplemental Fig. S8, A and B), to visualize common and exclusive proteins between the three groups. Interestingly, the majority of proteins 766 for LH and 607 for ST were common between the three groups. Some unique proteins were observed for cocaine (67), METH (84), and saline (69) for LH, but no unique proteins were present for cocaine and saline, and only METH (117) showed some unique proteins for ST. A GO annotation analysis of the complete protein list from all samples from LH and ST was performed using DAVID Bioinformatics resource 6.8 (https://david.ncifcrf.gov/). Supplemental Fig. S8 shows the top, C, GO cellular components; D, GO biological process; E, GO molecular function; F, KEGG pathway analysis; for LH; left panel, and ST; right panel. Among the top GO annotations (based on higher % protein) were cytoplasm as the cellular component, transport as the biological process, protein binding as the molecular function, and metabolic pathways for KEGG pathway analysis. This was true for all three groups and both brain regions. Protein binding and metabolic pathways have been reported among top molecular function and KEGG pathway analysis, respectively, in the prefrontal cortex after repeated cocaine exposure (35).
Using the label-free quantification data from PEAKS Studio Quantification label-free module for LH (Supplemental File S7A) and ST (Supplemental File S7B), and an R program (33), an unsupervised clustering and PCA plots for complete proteomics data for six biological replicates of cocaine (C1-C6), METH (M1-M6), and saline (S1-S6) for both LH (Supplemental Fig. S9, A and B) and ST (Supplemental Fig. S9, C and D) were plotted. As shown in Supplemental Fig. S9, a tight clustering of drug-treated biological samples, that is, cocaine and METH, were observed, while saline samples were clustered separately. In addition, saline biological replicates had higher intra-similarity as than drug biological replicates, and among biological replicates for drug treatments, cocaine samples were sparser than METH samples. In addition, ST showed much closer clustering for drug samples than LH.
A t test was performed on the label-free quantified data to obtain differentially expressed proteins; a Benjamin–Hochberg corrected p value cut-off of 0.2 was used to determine significant proteins. A pairwise t test (cocaine versus saline and METH versus saline) was performed to obtain increased and decreased abundance proteins for LH (Supplemental File S8-i) cocaine versus saline, ii) METH versus saline) and ST (Supplemental File S9-i) cocaine versus saline, ii) METH versus saline). Using filtering parameters such as Bonferroni value of <1 and an FDR <0.02, 54 (33 increased, and 21 decreased) and 96 (41 increased, and 55 decreased), differentially expressed proteins were observed for cocaine versus saline and METH versus saline, respectively in LH, and 376 (202 increased, and 174 decreased) and 403 (232 increased, and 171 decreased) differentially expressed proteins were observed for cocaine versus saline and METH versus saline, respectively in ST. The top ten increased and decreased abundance proteins with their respective t values (value from the t test which is similar to the difference between the means of the two groups or the log of the fold change. A negative t,value represents the lower expression, while a positive t value represents higher expression in the first group, for cocaine versus saline and METH versus saline for both LH (A) and ST (B) are shown in Table 2. Interestingly, a number of common differentially expressed proteins were observed for both drug versus saline, including increased cytochrome c oxidase subunit 6A1, ubiquitin thioesterase, histone H1.4, and NADH dehydrogenase (ubiquinone) 1 beta subcomplex subunit 6, and decreased tenascin-R (TENR), synapsin-2 (SYN2), and synaptophysin in LH, and increased DnaJ homolog subfamily C member 5, ubiquitin-2, and prefoldin subunit 2, and decreased myelin proteolipid protein (MYPR), syntaxin-binding protein 1, and Ras-related protein in ST. Some proteins had an opposite expression in the two brain regions, for example, Thy-1 membrane glycoprotein, which was decreased for METH in LH but was increased for cocaine in ST when compared to saline samples. Intriguingly, CSPGs, neurocan (NCAN), and CSPG5 were observed among increased abundance proteins for both cocaine and METH versus saline in ST, Table 2, and Supplemental File S9.
Table 2.
Differentially (increased abundance-i and decreased abundance-ii) proteins for lateral hypothalamus (LH) and striatum (ST) for pairwise comparison, that is, cocaine versus saline (left panel) and methamphetamine (METH) versus saline (right panel)
| Protein accession | Protein name | t | Protein accession | Protein name | t |
|---|---|---|---|---|---|
| Lateral hypothalamus (LH) | |||||
| i) Top ten increased abundance cocaine versus saline | i) Top ten increased abundance METH versus saline | ||||
| P43024|CX6A1_MOUSE | Cytochrome c oxidase subunit 6A1, mitochondrial | 11.7 | P43024|CX6A1_MOUSE | Cytochrome c oxidase subunit 6A1, mitochondrial | 24.9 |
| P97450|ATP5J_MOUSE | ATP synthase-coupling factor 6, mitochondrial | 11.2 | Q7TQI3|OTUB1_MOUSE | Ubiquitin thioesterase OTUB1 | 17.2 |
| Q7TQI3|OTUB1_MOUSE | Ubiquitin thioesterase OTUB1 | 9.8 | P43274|H14_MOUSE | Histone H1.4 | 15.8 |
| Q3UIU2|NDUB6_MOUSE | NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 6 | 9.5 | O35658|C1QBP_MOUSE | Complement component 1 Q subcomponent-binding protein, mitochondrial | 13.3 |
| Q9CPQ1|COX6C_MOUSE | Cytochrome c oxidase subunit 6C | 9.4 | Q8CCT4|TCAL5_MOUSE | Transcription elongation factor A protein-like 5 | 11.7 |
| P02088|HBB1_MOUSE | Hemoglobin subunit beta-1 | 8.4 | Q9CQJ8|NDUB9_MOUSE | NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 9 | 11.3 |
| P43274|H14_MOUSE | Histone H1.4 | 8.5 | Q91WS0|CISD1_MOUSE | CDGSH iron-sulfur domain-containing protein 1 | 11.2 |
| Q9CQZ6|NDUB3_MOUSE | NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 3 | 8.4 | O55126|NIPS2_MOUSE | Protein NipSnap homolog 2 | 11.1 |
| O08749|DLDH_MOUSE | Dihydrolipoyl dehydrogenase, mitochondrial | 8.2 | Q3UIU2|NDUB6_MOUSE | NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 6 | 10.7 |
| Q9CQH3|NDUB5_MOUSE | NADH dehydrogenase [ubiquinone] 1 beta subcomplex subunit 5, mitochondrial | 8.1 | Q8K1M6|DNM1L_MOUSE | Dynamin-1–like protein | 10.5 |
| ii) Top ten decreased abundance cocaine versus saline | ii) Top ten decreased abundance METH versus saline | ||||
| Q9JIA1|LGI1_MOUSE | Leucine-rich glioma-inactivated protein 1 | −10.6 | Q91VD9|NDUS1_MOUSE | NADH-ubiquinone oxidoreductase 75 kDa subunit, mitochondrial | −10.6 |
| P56695|WFS1_MOUSE | Wolframin | −9.2 | P04104|K2C1_MOUSE | Keratin, type II cytoskeletal 1 | −10.4 |
| Q80TL4|PHF24_MOUSE | PHD finger protein 24 | −7.7 | P16330|CN37_MOUSE | 2′,3′-cyclic-nucleotide 3′-phosphodiesterase | −9.9 |
| Q9DBG3|AP2B1_MOUSE | AP-2 complex subunit beta | −7.2 | P68372|TBB4B_MOUSE | Tubulin beta-4B chain | −9.5 |
| P11798|KCC2A_MOUSE | Calcium/calmodulin-dependent protein kinase type II subunit alpha | −6.8 | P17182|ENOA_MOUSE | Alpha-enolase | −9.1 |
| Q60829|PPR1B_MOUSE | Protein phosphatase 1 regulatory subunit 1B | −6.6 | P01831|THY1_MOUSE | Thy-1 membrane glycoprotein | −9.1 |
| Q9JME5|AP3B2_MOUSE | AP-3 complex subunit beta-2 | −6.2 | P18242|CATD_MOUSE | Cathepsin D | −8.1 |
| Q5SQX6|CYFP2_MOUSE | Cytoplasmic FMR1-interacting protein 2 | −5.8 | Q7TMM9|TBB2A_MOUSE | Tubulin beta-2A chain | −7.9 |
| Q62277|SYPH_MOUSE | Synaptophysin | −5.7 | P48962|ADT1_MOUSE | ADP/ATP translocase 1 | −7.6 |
| Q9Z1S5|SEPT3_MOUSE | Neuronal-specific septin-3 | −5.7 | P01942|HBA_MOUSE | Hemoglobin subunit alpha | −7.5 |
| Striatum (ST) | |||||
| i) Top ten increased abundance cocaine versus saline | i) Top ten increased abundance METH versus saline | ||||
| P60904|DNJC5_MOUSE | DnaJ homolog subfamily C member 5 | 28.9 | P60904|DNJC5_MOUSE | DnaJ homolog subfamily C member 5 | 30.4 |
| Q9WV98|TIM9_MOUSE | Mitochondrial import inner membrane translocase subunit Tim9 | 22.3 | Q9QZM0|UBQL2_MOUSE | Ubiquilin-2 | 28.1 |
| P63323|RS12_MOUSE | 40S ribosomal protein S12 | 21.5 | O70591|PFD2_MOUSE | Prefoldin subunit 2 | 24.9 |
| P01831|THY1_MOUSE | Thy-1 membrane glycoprotein | 20.8 | Q9WV98|TIM9_MOUSE | Mitochondrial import inner membrane translocase subunit Tim9 | 23.5 |
| Q9CWM4|PFD1_MOUSE | Prefoldin subunit 1 | 20.4 | Q9CWM4|PFD1_MOUSE | Prefoldin subunit 1 | 20.3 |
| P56212|ARP19_MOUSE | cAMP-regulated phosphoprotein 19 | 20.0 | P63323|RS12_MOUSE | 40S ribosomal protein S12 | 19.8 |
| O70591|PFD2_MOUSE | Prefoldin subunit 2 | 17.8 | P56212|ARP19_MOUSE | cAMP-regulated phosphoprotein 19 | 19.1 |
| Q9QZM0|UBQL2_MOUSE | Ubiquilin-2 | 17.5 | Q9D1X0|NOL3_MOUSE | Nucleolar protein 3 | 18.8 |
| Q8R317|UBQL1_MOUSE | Ubiquilin-1 | 17.1 | P55066|NCAN_MOUSE | Neurocan core protein | 18.7 |
| Q9D8Z2|TRIA1_MOUSE | TP53-regulated inhibitor of apoptosis 1 | 16.3 | P63158|HMGB1_MOUSE | High mobility group protein B1 | 17.8 |
| ii) Top ten decreased abundance cocaine versus saline | Top ten decreased abundance METH versus saline | ||||
| P60202|MYPR_MOUSE | Myelin proteolipid protein | −21.5 | Q9R1Q9|VAS1_MOUSE | V-type proton ATPase subunit S1 | −32.2 |
| P52480|KPYM_MOUSE | Pyruvate kinase PKM | −18.9 | Q9D6M3|GHC1_MOUSE | Mitochondrial glutamate carrier 1 | −23.5 |
| Q61885|MOG_MOUSE | Myelin-oligodendrocyte glycoprotein | −17.8 | Q9CQQ7|AT5F1_MOUSE | ATP synthase F(0) complex subunit B1, mitochondrial | −23.5 |
| P68368|TBA4A_MOUSE | Tubulin alpha-4A chain | −17.4 | P60202|MYPR_MOUSE | Myelin proteolipid protein | −20.4 |
| P63011|RAB3A_MOUSE | Ras-related protein Rab-3A | −16.3 | P63011|RAB3A_MOUSE | Ras-related protein Rab-3A | −19.1 |
| O08599|STXB1_MOUSE | Syntaxin-binding protein 1 | −15.8 | O08599|STXB1_MOUSE | Syntaxin-binding protein 1 | −18.9 |
| Q9D0K2|SCOT1_MOUSE | Succinyl-CoA:3-ketoacid coenzyme A transferase 1, mitochondrial | −15.6 | Q8VDN2|AT1A1_MOUSE | ATP synthase F(0) complex subunit B1, mitochondrial | −18.4 |
| O88569|ROA2_MOUSE | Heterogeneous nuclear ribonucleoproteins A2/B1 | −15.0 | P52480|KPYM_MOUSE | Pyruvate kinase PKM | −18.2 |
| P06151|LDHA_MOUSE | L-lactate dehydrogenase A chain | −14.7 | P62827|RAN_MOUSE | GTP-binding nuclear protein Ran | −17.6 |
| P62806|H4_MOUSE | Histone H4 | −14.5 | Q9DBG3|AP2B1_MOUSE | AP-2 complex subunit beta | −17.1 |
A negative t value represents the lower expression, while a positive t value represents the higher expression in the first group. t = t value, a value from the t test which is similar to the difference between the means of the two groups or the log of the fold change.
A three-way ANOVA was also performed to obtain a list of differentially expressed proteins in the three treatments: cocaine, METH, and saline (LH; Supplemental File S8-iii, and ST; Supplemental File S9-iii). For LH, 97 differentially expressed proteins were observed using a Bonferroni value of <1 and an FDR< 0.2, including cytochrome c oxidase subunit 6A1, ubiquitin thioesterase, NADH dehydrogenase 1 beta subcomplex subunit 6, NADH dehydrogenase [ubiquinone] iron-sulfur protein 1 mitochondrial, intercellular adhesion molecule 5, calcium/calmodulin-dependent protein kinase type II subunit alpha and beta, cytochrome c oxidase subunit 6C, synaptophysin, SYN2, calnexin (CALX), hepatoma-derived growth factor (HDGF), neurogranin, neurofilament medium polypeptide, beta-hexosaminidase subunit beta, and TENR. For ST, 451 differentially expressed proteins were observed, including calcium/calmodulin-dependent protein kinase type II subunit alpha, DnaJ homolog subfamily C member 5, ubiquitin-2, MYPR, cytochrome c oxidase subunit 2, CALX, APO-E, APO-D, alpha-synuclein (SYUA), microtubule-associated protein tau (TAU), SYN2, amyloid-beta A4 protein (A4), calreticulin, annexin A6 and A7, and HDGF. Figure 5 shows a box plot representation of the logged and scaled abundances of some of the important proteins for the three treatments in LH and ST.
Fig. 5.
Box plots for differentially expressed proteins. A box-plot representation of differentially expressed proteins after ANOVA analysis for cocaine, methamphetamine, and saline in the lateral hypothalamus (LH) (first and second row) and striatum (ST) (third and fourth row). The y-axis represents logged and scaled abundance of each protein.
Proteomics data for the two brain regions were used to determine characteristic features defined by proteins that were differentially expressed in the same direction (both with t value >0 or t value <0) and FDR <0.2 in both brain regions LH and ST for cocaine versus saline and METH versus saline comparisons. Using this criterion, 66 and 135 common differentially expressed proteins were observed for both brain regions for cocaine versus saline (Supplemental File S10-i) and METH versus saline (Supplemental File S10-ii), respectively, and 43 proteins (Supplemental File S10-iii), including, MYPR, KCC2A, SYN2, TENR, CALX, ANXA7, and HDGF were found to overlap for both drug treatments (cocaine and METH) versus saline, common to both brain regions (LH and ST). A KEGG pathway analysis of these 43 common proteins using DAVID revealed previously reported oxidative phosphorylation as the top aberrant pathway for drug abuse, as shown in Supplemental Fig. S10. An ingenuity pathway analysis (IPA) on the 43 proteins illustrated neurological disease (p = 2.19 E-02-8.53 E-07; 24 molecules), organismal injury and abnormalities (p = 2.31 e-02-8.53 e07; 42 molecules), and developmental disorders (p = 2.12 E-02-3.20E-05; 9 molecules) among top diseases and disorders (IPA summary, Supplemental PDF S2). Among the top networks were cellular assembly and organization, cellular function and maintenance, and cellular movement (IPA score 47), Supplemental Fig. S11. The network was overlayed with diseases and biological functions (nervous system and development function, neurological disease, developmental disorder, and psychological disorder; pink border) and canonical pathways (opioid signaling pathway, clathrin-mediated endocytosis signaling, synaptic long-term potentiation, and synaptogenesis signaling pathway).
Increasing CS 4-0 Levels in the LH Ameliorates Anxiety and Prevents the Expression of Preference for Cocaine in a Novelty-Induced Conditioned Place Preference Test during Withdrawal
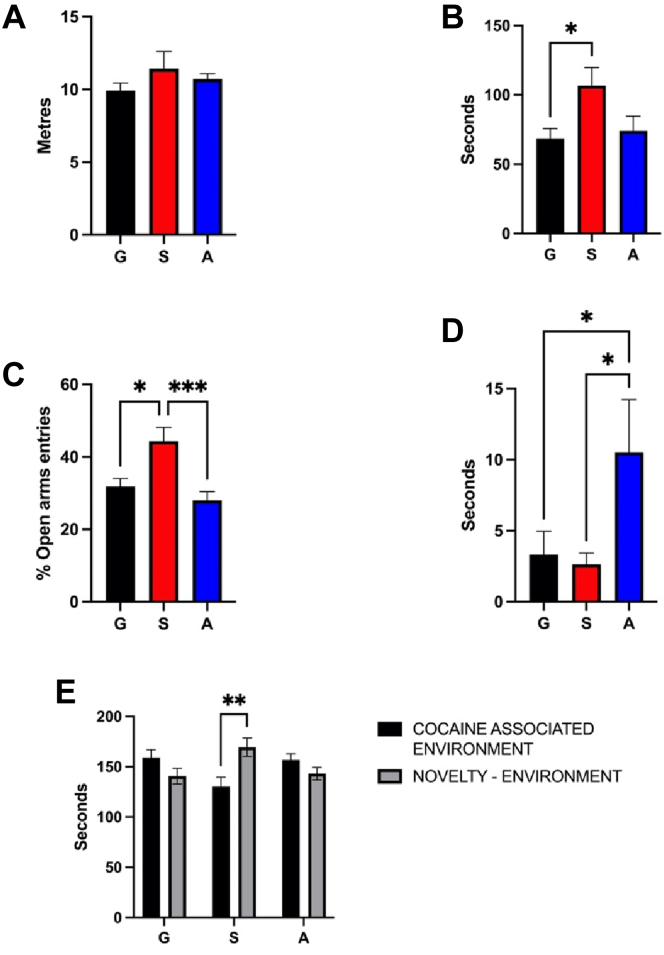
We used AAV to overexpress ARSB (N-acetylgalactosamine-4-sulfatase) or an shRNA to ARSB, while an empty AAV was used for control. Figure 6, A–D represents the effects of overexpressing or downregulating LH ARSB by shRNA in mice’s performance at the EPM after 10 days of cocaine withdrawal following an intraperitoneal treatment with cocaine (10 mg/kg/10 ml) for 14 days Figure 6A represents the total activity of the control group (G), the shRNA ARSB group (S), and the upregulated ARSB group (A) during the test. One-Way ANOVA did not reveal any significant effect (p > 0.05), showing that mice were not sedate nor hyperactive, hence validating the test. Anxiety-related parameters were significantly different. Accordingly, one-way ANOVA revealed a significant difference between the groups in the time spent in the open arms (Fig. 6B), in the percentage of entries in the open arms (Fig. 6C), and in the latency to the first entry in the open arms (Fig. 6D) (F2,42 = 3.88, p < 0.05; F2,42 = 8.73, p < 0.001; F2,42 = 3.36, p < 0.05). Moreover, Tukey post hoc test revealed that S-group spent significantly more time in the open arms when compared to the G-group (p < 0.05) and had a significantly higher percentage of entries in the open arms than G (p < 0.05) and A (p < 0.001). Furthermore, Fisher post hoc revealed that A had a significantly higher latency to the first entry in the open arms than G (p < 0.05) and S (p < 0.05). In addition, Figure 6E represents the effects of overexpressing or downregulating LH ARSB in mice’s preference between cocaine and novelty in a novelty-induced place preference test during cocaine withdrawal. Two-way ANOVA revealed a significant effect of Group x Novelty interaction (F2,90 = 8.34, p < 0.001). Moreover, Tukey post hoc revealed that S significantly spent more time exploring the novelty during withdrawal (p < 0.01) when compared to G and A. Altogether, these results point out how restoring cocaine-induced reduction of C4-S0, through silencing ARSB RNA, reduced anxiety and preference for cocaine in S mice during cocaine withdrawal. To verify the efficiency of AAV injections, we used MS CS disaccharide analysis to capture the changes in 4-O and 6-O sulfation for overexpressing ARSB (N-acetylgalactosamine-4-sulfatase) or to knock it down through shARSB and GFP as control (Supplemental Fig. S4). A significant increase in CS 4-0 levels in the LH by AAV delivery of an shRNA to ARSB was observed.
Fig. 6.
Effects of downregulating by shRNA (S), overexpressing (A) ARSB, and control group (G) in mice’s anxiety in the elevated plus maze and in mice’s preference for cocaine over novelty during cocaine withdrawal.A, the total activity of the control group (G), the shRNA ARSB group (S), and the upregulated ARSB group (A) during the test. B, one-way ANOVA revealed a significant difference between the groups in the time spent in the open arms. C, the percentage of entries in the open arms. D, the latency to the first entry in the open arms (F2,42 = 3.88, p < 0.05; F2,42 = 8.73, p < 0.001; F2,42 = 3.36, p < 0.05). Tukey post hoc revealed that S spent significantly more time in the open arms when compared to the control (G) (p < 0.05) and had a significantly higher percentage of entries in the open arms than G (p < 0.05) and A (p < 0.001). Moreover, Fisher post hoc revealed that A had a significantly higher latency to the first entry in the open arms than G (p < 0.05) and S (p < 0.05). In addition, two-way ANOVA revealed a significant effect of Group x Novelty interaction (F2,90 = 8.34, p < 0.001) in mice’s preference between cocaine and novelty. E, Tukey post hoc revealed that S significantly spent more time exploring the novelty (p < 0.01). ARSB, arylsulfatase B.
Discussion
Aberration in protein and gene expression in neuropsychiatry has been well documented, but only limited studies have utilized MS-based proteomics to profile altered proteins related to drug abuse while profiling of glycoconjugates (HS and CS) remains unexplored. This study provides in-depth profiling of HS and CS disaccharides, as well as of the proteome of two essential brain regions involved in drug abuse, the LH, and the ST harvested from mice treated with two classic brain stimulants, cocaine and METH. Comprehensive glycomics and proteomics profiles of drugs versus saline treatments were generated, underpinning the structural aberration of these biomolecules associated with these drugs of abuse.
We found that both stimulants induce significant changes in HS and CS disaccharide profiles when for drug treatment compared to saline treatment. A key observation was an increase in total HS abundance and sulfate content in both brain regions indicating a potential increase in heparan sulfate proteoglycans (HSPGs) and sulfotransferase enzymes (or a decrease in sulfatase) associated to the treatment with both drugs. Accordingly, our previous study showed an increase in gene expression of HSPG syndecan-3 in LH after excessive cocaine self-administration (12). In aggrement with our findings, another study showed downregulation of Sulf1, the extracellular 6-O-endosulfatases in mice nucleus accumbens after treatment with cocaine (36). The same study also reported a decrease in NDST4, a bifunctional enzyme that catalyzes N-deacetylation and N-sulfation in HS, congruent with our observation of an increase in HS N-acetylation and decreases in N-sulfation in drug treatments (36).
Another interesting observation was an increase in total CS abundance for LH and a decrease in CS sulfate content for LH and ST in drug-treated versus saline samples. Conversely, CS sulfation was observed to increase with ethanol treatment in astrocytes (37). A major finding of this study was a significant increase in CS 6-O-sulfation and a concomitant decrease in CS 4-O-sulfation on drug treatments observed in both brain regions, indicating a probable enzymatic aberration in sulfotransferase or sulfatase enzymes leading to biosynthetic or degradation pathways for CS 4-O and 6-O sulfation.
To explore this, we overexpressed ARSB (A) or downregulated it by shRNA (S), an enzyme responsible for the degradation of CS 4-0 sulfation. A significant increase in CS 4-0 levels in the LH by AAV delivery of an shRNA to ARSB was observed (Supplemental Fig. S4). Notably, the downregulation of ARSB decreased anxiety in mice and increased their preference for novelty over cocaine during withdrawal (Fig. 6), suggesting that restoring the levels of CS 4-0 altered by the treatment with cocaine can reduce the severity of withdrawal manifestations. A previous study on astrocytes reported inhibition of ARSB on treatment with ethanol, triggering the increase in 4-0 sulfation and inhibiting astrocyte-mediated neurite outgrowth (37). A similar increase in 4-0 sulfation was reported in brain injury (38). Notably, 6-0 sulfation has been reported to correlate with axonal inhibition and cortical plasticity (39, 40). Together with the present results, these observations support that CS sulfation patterns likely contribute to drug-induced neural plasticity in brain regions like the LH (14).
Chondroitin sulfate proteoglycans (CSPGs) are spatio-temporarily expressed during brain development and regulate critical processes, such as neuronal migration, neurite growth, axon outgrowth, synaptogenesis, and synaptic maturation (41, 42, 43). Elevated levels of CSPGs are present in brain extracellular matrix structures, including perineuronal nets (PNNs), where they stabilize synaptic connections and regulate neural plasticity (44, 45). PNNs are widely detected in both LH and ST in mice, and they have been associated with drug addiction (46). Intriguingly, PNNs in the LH have an active role in regulating cue-induced reinstatement of cocaine-seeking behavior, and their removal via chondroitinase (CHABC) administration abolished the acquisition of cocaine-induced conditioned place preference and attenuated the acquisition of cocaine self-administration (47, 48). Moreover, the removal of CS GAG chains with CHABC enzyme restores plasticity in adult rat visual cortex (49). CSPGs are also known to impede axonal regeneration and treatment with CHABC enhanced axonal growth (50). Together, these studies document the essential roles of CS and their sulfation pattern in axonal guidance and synaptic plasticity, the two key processes associated with the drug of abuse (51, 52, 53, 54).
Our proteomics data revealed a number of aberrant proteins in drug-treated versus saline samples, which have been previously reported in psychostimulant brain studies (35, 55, 56, 57, 58, 59, 60), and some unique proteins not reported earlier, including CSPG5, TENR, HSPG, and CALR. Specifically, two CSPGs, NCAN and CSPG5, were increased by both drug treatments in ST. A previous study on ethanol treatment also illustrated an increase in NCAN that inhibited astrocyte-mediated neurite outgrowth (37). In addition, some of the CSPG-interacting proteins, including HDGF and ANXA7, were increased, while TENR and annexin A6 were decreased with drug treatment compared to saline. Upregulation of annexin A7, an annexin family of calcium-dependent phospholipid binding proteins has been previously reported in the ST with cocaine treatment (55). Taken together, these data support that aberration in both GAG and proteoglycan regulation play a role in the pathogenesis of drug abuse.
Several proteins involved in neurodegenerative disorders such as Alzheimer's and Parkinson's disease, including Tau, APO-E, APO-D, A4, and SYUA, were observed to be increased by the treatment with both stimulants in ST for drug-treated relative to saline samples. Several studies have documented the link between drug abuse and neurodegeneration, including a previous study that suggested dysregulation of iron homeostasis in cocaine-addicted brains that leads to cell death, a phenomenon also observed in neurodegeneration (61). Intriguingly, proteoglycans, through their GAG chains, are known to play essential roles in the aggregation of these proteins in neurodegenerative disorders (62, 63, 64). Thus, there could be an association between aberrant GAGs and neurodegenerative proteins observed in our study. The role of drug-induced neurodegeneration, suggested by the present results, is a finding that warrants further exploration.
In conclusion, little is known about aberration in HS, CS, and proteins in the LH and ST with drug abuse. Here, we highlighted unique alterations in specific HS and CS disaccharides and some key proteins after cocaine and METH exposure which may serve as novel markers and therapeutic targets for substance use disorder. In addition, the altered pathways and associated molecular mechanisms, as reported here, will strengthen our fundamental knowledge of the neurobiology of addiction. The expression patterns reported by this study and the functional roles discussed herein need to be further characterized to unravel the biological implication of drug abuse in the brain.
Analytical Considerations and Future Directions
Due to highly complex GAG structures, proteoglycans (PGs) are not identified effectively with MS and require further fractionation and removal of GAG structures to achieve deep sequencing of PGs (65). Thus, it is unsurprising that we do not observe many PGs in the proteomics data and only observe two differentially expressed CSPGs in the ST. At the same time, a number of GAG disaccharides are differentially expressed at the glycomics level. Altered peptides are generally present at a very low level and require further processing and/or targeted experiments. We, therefore, in the future, aim to achieve in-depth profiles of proteoglycans by immunoprecipitating CS-PG and HS-PGs, removing high molecular weight GAG chains to minimize complexity and further analyzing them by MS to determine the altered expression of PGs in the drug abuse.
Data Availability
The datasets generated and/or analyzed during the current study are available in the ProteomeXchange Consortium via the PRIDE (66) partner repository with the dataset identifier PXD038576 and 10.6019/PXD038576.
Reviewer account details are:
Username: reviewer_pxd038576@ebi.ac.uk
Password: 8Z0BFNBp
Supplemental data
Conflict of interest
The authors declare no competing interests.
Acknowledgments
Funding and additional information
This work was supported by NIH grants P41GM104603, R21HL131554, U01CA221234, DA053801, DA036241, and DA048882. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Author contributions
M. K. S., R. M., T. K., V. R.-C., and J. C. data curation; J. Z., V. C., and P. P. S. methodology; M. S. investigation; M. K. S., R. M., J. D. H., T. K., and J. C. formal analysis; M. S. writing–original draft; M. K. S., J. D. H., T. K., V. R.-C., J. C., J. Z., and P. P. S. writing–review and editing; J. D. H. software; V. R.-C., J. Z., and P. P. S. conceptualization; J. Z. and P. P. S. funding acquisition.
Contributor Information
Joseph Zaia, Email: jzaia@bu.edu.
Pietro Paolo Sanna, Email: psanna@scripps.edu.
Supplemental Data
References
- 1.Volkow N.D. Personalizing the treatment of substance use disorders. Am. J. Psychiatry. 2020;177:113–116. doi: 10.1176/appi.ajp.2019.19121284. [DOI] [PubMed] [Google Scholar]
- 2.Shiels M.S., Freedman N.D., Thomas D. Berrington de Gonzalez A: Trends in U.S. Drug Overdose Deaths in Non-Hispanic Black, Hispanic, and Non-Hispanic White Persons, 2000–2015. Ann. Intern. Med. 2018;168:453–455. doi: 10.7326/M17-1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McCance-Katz E.F. vol. 97. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2019. pp. 78–82. (The National Survey on Drug Use and Health: 2017). [Google Scholar]
- 4.Sethi M.K., Zaia J. Extracellular matrix proteomics in schizophrenia and Alzheimer's disease. Anal. Bioanal. Chem. 2017;409:379–394. doi: 10.1007/s00216-016-9900-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tóth G., Sugár S., Pál D., Fügedi K.D., Drahos L., Schlosser G., et al. Glycosaminoglycan analysis of FFPE tissues from Prostate cancer and benign prostate hyperplasia patients reveals altered regulatory functions and independent markers for survival. Cancers. 2022;14:4867. doi: 10.3390/cancers14194867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saad O.M., Leary J.A. Delineating mechanisms of dissociation for isomeric heparin disaccharides using isotope labeling and ion trap tandem mass spectrometry. J. Am. Soc. Mass Spectrom. 2004;15:1274–1286. doi: 10.1016/j.jasms.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Lawrence R., Lu H., Rosenberg R.D., Esko J.D., Zhang L. Disaccharide structure code for the easy representation of constituent oligosaccharides from glycosaminoglycans. Nat. Methods. 2008;5:291–292. doi: 10.1038/nmeth0408-291. [DOI] [PubMed] [Google Scholar]
- 8.Shao C., Shi X., White M., Huang Y., Hartshorn K., Zaia J. Comparative glycomics of leukocyte glycosaminoglycans. FEBS J. 2013;280:2447–2461. doi: 10.1111/febs.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bernfield M., Gotte M., Park P.W., Reizes O., Fitzgerald M.L., Lincecum J., et al. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 1999;68:729–777. doi: 10.1146/annurev.biochem.68.1.729. [DOI] [PubMed] [Google Scholar]
- 10.Lindahl U., Kjellen L. Pathophysiology of heparan sulphate: many diseases, few drugs. J. Intern. Med. 2013;273:555–571. doi: 10.1111/joim.12061. [DOI] [PubMed] [Google Scholar]
- 11.Hemby S.E. Assessment of genome and proteome profiles in cocaine abuse. Prog. Brain Res. 2006;158:173–195. doi: 10.1016/S0079-6123(06)58009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen J., Repunte-Canonigo V., Kawamura T., Lefebvre C., Shin W., Howell L.L., et al. Hypothalamic proteoglycan syndecan-3 is a novel cocaine addiction resilience factor. Nat. Commun. 2013;4:1955. doi: 10.1038/ncomms2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Aston-Jones G., Smith R.J., Sartor G.C., Moorman D.E., Massi L., Tahsili-Fahadan P., et al. Lateral hypothalamic orexin/hypocretin neurons: a role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed S.H., Lutjens R., van der Stap L.D., Lekic D., Romano-Spica V., Morales M., et al. Gene expression evidence for remodeling of lateral hypothalamic circuitry in cocaine addiction. Proc. Natl. Acad. Sci. U. S. A. 2005;102:11533–11538. doi: 10.1073/pnas.0504438102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J., Kawamura T., Sethi M.K., Zaia J., Repunte-Canonigo V., Sanna P.P. Heparan sulfate: resilience factor and therapeutic target for cocaine abuse. Sci. Rep. 2017;7 doi: 10.1038/s41598-017-13960-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yager L.M., Garcia A.F., Wunsch A.M., Ferguson S.M. The ins and outs of the striatum: role in drug addiction. Neuroscience. 2015;301:529–541. doi: 10.1016/j.neuroscience.2015.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Burton A.C., Bissonette G.B., Vazquez D., Blume E.M., Donnelly M., Heatley K.C., et al. Previous cocaine self-administration disrupts reward expectancy encoding in ventral striatum. Neuropsychopharmacology. 2018;43:2350–2360. doi: 10.1038/s41386-018-0058-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Volkow N.D., Wang G.-J., Telang F., Fowler J.S., Logan J., Childress A.-R., et al. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. J. Neurosci. 2006;26:6583. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chang L., Alicata D., Ernst T., Volkow N. Structural and metabolic brain changes in the striatum associated with methamphetamine abuse. Addiction. 2007;102:16–32. doi: 10.1111/j.1360-0443.2006.01782.x. [DOI] [PubMed] [Google Scholar]
- 20.Kita T., Matsunari Y., Saraya T., Shimada K., O'Hara K., Kubo K., et al. Methamphetamine-induced striatal dopamine release, behavior changes and neurotoxicity in BALB/c mice. Int. J. Dev. Neurosci. 2000;18:521–530. doi: 10.1016/s0736-5748(00)00022-8. [DOI] [PubMed] [Google Scholar]
- 21.Ito C., Onodera K., Yamatodani A., Watanabe T., Sato M. The effect of methamphetamine on histamine release in the rat hypothalamus. Psych. Clin. Neurosci. 1997;51:79–81. doi: 10.1111/j.1440-1819.1997.tb02911.x. [DOI] [PubMed] [Google Scholar]
- 22.Zanos P., Wright S.R., Georgiou P., Yoo J.H., Ledent C., Hourani S.M., et al. Chronic methamphetamine treatment induces oxytocin receptor up-regulation in the amygdala and hypothalamus via an adenosine A2A receptor-independent mechanism. Pharmacol. Biochem. Behav. 2014;119:72–79. doi: 10.1016/j.pbb.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Kilkenny C., Browne W.J., Cuthill I.C., Emerson M., Altman D.G. Improving bioscience research reporting: the ARRIVE guidelines for reporting animal research. PLoS Biol. 2010;8 doi: 10.1371/journal.pbio.1000412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maccioni R., Cottiglia F., Maccioni E., Talani G., Sanna E., Bassareo V., et al. The biologically active compound of Withania somnifera (L.) Dunal, docosanyl ferulate, is endowed with potent anxiolytic properties but devoid of typical benzodiazepine-like side effects. J. Psychopharmacol. 2021;35:1277–1284. doi: 10.1177/02698811211008588. [DOI] [PubMed] [Google Scholar]
- 25.Porru S., Maccioni R., Bassareo V., Peana A.T., Salamone J.D., Correa M., et al. Effects of caffeine on ethanol-elicited place preference, place aversion and ERK phosphorylation in CD-1 mice. J. Psychopharmacol. 2020;34:1357–1370. doi: 10.1177/0269881120965892. [DOI] [PubMed] [Google Scholar]
- 26.Maccioni R., Serra M., Marongiu J., Cottiglia F., Maccioni E., Bassareo V., et al. Effects of docosanyl ferulate, a constituent of Withania somnifera, on ethanol- and morphine-elicited conditioned place preference and ERK phosphorylation in the accumbens shell of CD1 mice. Psychopharmacology. 2022;239:795–806. doi: 10.1007/s00213-022-06069-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crawford C.A., Der-Ghazarian T., Britt C.E., Varela F.A., Kozanian O.O. Novelty-induced conditioned place preference, sucrose preference, and elevated plus maze behavior in adult rats after repeated exposure to methylphenidate during the preweanling period. Behav. Brain Res. 2013;246:29–35. doi: 10.1016/j.bbr.2013.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao C., Shi X., Phillips J.J., Zaia J. Mass spectral profiling of glycosaminoglycans from histological tissue surfaces. Anal. Chem. 2013;85:10984–10991. doi: 10.1021/ac402517s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turiak L., Shao C., Meng L., Khatri K., Leymarie N., Wang Q., et al. Workflow for combined proteomics and glycomics profiling from histological tissues. Anal. Chem. 2014;86:9670–9678. doi: 10.1021/ac5022216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raghunathan R., Polinski N.K., Klein J.A., Hogan J.D., Shao C., Khatri K., et al. Glycomic and proteomic changes in aging brain nigrostriatal pathway. Mol. Cell Proteomics. 2018;17:1778–1787. doi: 10.1074/mcp.RA118.000680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adusumilli R., Mallick P. Data conversion with ProteoWizard msConvert. Methods Mol. Biol. 2017;1550:339–368. doi: 10.1007/978-1-4939-6747-6_23. [DOI] [PubMed] [Google Scholar]
- 32.Zaia J. Mass spectrometry and glycomics. OMICS. 2010;14:401–418. doi: 10.1089/omi.2009.0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.R: a Language and Environment for Statistical Computing. R Foundation for Statistical Computing; Vienna, Austria: 2024. [Google Scholar]
- 34.Long F., Liu H., Hahn C., Sumazin P., Zhang M.Q., Zilberstein A. Genome-wide prediction and analysis of function-specific transcription factor binding sites. In silico Biol. 2004;4:395–410. [PubMed] [Google Scholar]
- 35.Guan X., Guan Y. Proteomic profile of differentially expressed proteins in the medial prefrontal cortex after repeated cocaine exposure. Neuroscience. 2013;236:262–270. doi: 10.1016/j.neuroscience.2013.01.039. [DOI] [PubMed] [Google Scholar]
- 36.Lo Iacono L., Valzania A., Visco-Comandini F., Viscomi M.T., Felsani A., Puglisi-Allegra S., et al. Regulation of nucleus accumbens transcript levels in mice by early-life social stress and cocaine. Neuropharmacology. 2016;103:183–194. doi: 10.1016/j.neuropharm.2015.12.011. [DOI] [PubMed] [Google Scholar]
- 37.Zhang X., Bhattacharyya S., Kusumo H., Goodlett C.R., Tobacman J.K., Guizzetti M. Arylsulfatase B modulates neurite outgrowth via astrocyte chondroitin-4-sulfate: dysregulation by ethanol. Glia. 2014;62:259–271. doi: 10.1002/glia.22604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bhattacharyya S., Zhang X., Feferman L., Johnson D., Tortella F.C., Guizzetti M., et al. Decline in arylsulfatase B and Increase in chondroitin 4-sulfotransferase combine to increase chondroitin 4-sulfate in traumatic brain injury. J. Neurochem. 2015;134:728–739. doi: 10.1111/jnc.13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Properzi F., Carulli D., Asher R.A., Muir E., Camargo L.M., van Kuppevelt T.H., et al. Chondroitin 6-sulphate synthesis is up-regulated in injured CNS, induced by injury-related cytokines and enhanced in axon-growth inhibitory glia. Eur. J. Neurosci. 2005;21:378–390. doi: 10.1111/j.1460-9568.2005.03876.x. [DOI] [PubMed] [Google Scholar]
- 40.Miyata S., Komatsu Y., Yoshimura Y., Taya C., Kitagawa H. Persistent cortical plasticity by upregulation of chondroitin 6-sulfation. Nat. Neurosci. 2012;15:414–422. doi: 10.1038/nn.3023. [DOI] [PubMed] [Google Scholar]
- 41.Laabs T., Carulli D., Geller H.M., Fawcett J.W. Chondroitin sulfate proteoglycans in neural development and regeneration. Curr. Opin. Neurobiol. 2005;15:116–120. doi: 10.1016/j.conb.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 42.Maeda N., Fukazawa N., Ishii M. Chondroitin sulfate proteoglycans in neural development and plasticity. Front. Biosci. 2010;15:626–644. doi: 10.2741/3637. [DOI] [PubMed] [Google Scholar]
- 43.Klausmeyer A., Conrad R., Faissner A., Wiese S. Influence of glial-derived matrix molecules, especially chondroitin sulfates, on neurite growth and survival of cultured mouse embryonic motoneurons. J. Neurosci. Res. 2011;89:127–141. doi: 10.1002/jnr.22531. [DOI] [PubMed] [Google Scholar]
- 44.Miao Q.L., Ye Q., Zhang X.H. Perineuronal net, CSPG receptor and their regulation of neural plasticity. Sheng Li Xue Bao. 2014;66:387–397. [PubMed] [Google Scholar]
- 45.Pantazopoulos H., Berretta S. In sickness and in Health: perineuronal nets and synaptic plasticity in psychiatric disorders. Neural Plast. 2016;2016 doi: 10.1155/2016/9847696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Slaker M., Blacktop J.M., Sorg B.A. Caught in the net: perineuronal nets and addiction. Neural Plast. 2016;2016 doi: 10.1155/2016/7538208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Blacktop J.M., Todd R.P., Sorg B.A. Role of perineuronal nets in the anterior dorsal lateral hypothalamic area in the acquisition of cocaine-induced conditioned place preference and self-administration. Neuropharmacology. 2017;118:124–136. doi: 10.1016/j.neuropharm.2017.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Blacktop J.M., Sorg B.A. Perineuronal nets in the lateral hypothalamus area regulate cue-induced reinstatement of cocaine-seeking behavior. Neuropsychopharmacology. 2019;44:850–858. doi: 10.1038/s41386-018-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pizzorusso T., Medini P., Berardi N., Chierzi S., Fawcett J.W., Maffei L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science. 2002;298:1248–1251. doi: 10.1126/science.1072699. [DOI] [PubMed] [Google Scholar]
- 50.Bradbury E.J., Moon L.D., Popat R.J., King V.R., Bennett G.S., Patel P.N., et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 2002;416:636–640. doi: 10.1038/416636a. [DOI] [PubMed] [Google Scholar]
- 51.Jones S., Bonci A. Synaptic plasticity and drug addiction. Curr. Opin. Pharmacol. 2005;5:20–25. doi: 10.1016/j.coph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 52.Winder D.G., Egli R.E., Schramm N.L., Matthews R.T. Synaptic plasticity in drug reward circuitry. Curr. Mol. Med. 2002;2:667–676. doi: 10.2174/1566524023361961. [DOI] [PubMed] [Google Scholar]
- 53.Bahi A., Dreyer J.L. Cocaine-induced expression changes of axon guidance molecules in the adult rat brain. Mol. Cell. Neurosci. 2005;28:275–291. doi: 10.1016/j.mcn.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 54.Tapocik J.D., Luu T.V., Mayo C.L., Wang B.D., Doyle E., Lee A.D., et al. Neuroplasticity, axonal guidance and micro-RNA genes are associated with morphine self-administration behavior. Addict. Biol. 2013;18:480–495. doi: 10.1111/j.1369-1600.2012.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vicente-Rodríguez M., Herradón G., Ferrer-Alcón M., Uribarri M., Pérez-García C. Chronic cocaine use causes changes in the striatal proteome depending on the endogenous expression of pleiotrophin. Chem. Res. Toxicol. 2015;28:1443–1454. doi: 10.1021/acs.chemrestox.5b00130. [DOI] [PubMed] [Google Scholar]
- 56.Reynolds J.L., Mahajan S.D., Bindukumar B., Sykes D., Schwartz S.A., Nair M.P.N. Proteomic analysis of the effects of cocaine on the enhancement of HIV-1 replication in normal human astrocytes (NHA) Brain Res. 2006;1123:226–236. doi: 10.1016/j.brainres.2006.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lull M.E., Erwin M.S., Morgan D., Roberts D.C., Vrana K.E., Freeman W.M. Persistent proteomic alterations in the medial prefrontal cortex with abstinence from cocaine self-administration. Proteomics Clin. Appl. 2009;3:462–472. doi: 10.1002/prca.200800055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yang L., Sun Z.S., Zhu Y.P. Proteomic analysis of rat prefrontal cortex in three phases of morphine-induced conditioned place preference. J. Proteome Res. 2007;6:2239–2247. doi: 10.1021/pr060649o. [DOI] [PubMed] [Google Scholar]
- 59.Faure J.J., Hattingh S.M., Stein D.J., Daniels W.M. Proteomic analysis reveals differentially expressed proteins in the rat frontal cortex after methamphetamine treatment. Metab. Brain Dis. 2009;24:685–700. doi: 10.1007/s11011-009-9167-0. [DOI] [PubMed] [Google Scholar]
- 60.Vicente-Rodríguez M., Gramage E., Herradón G., Pérez-García C. Phosphoproteomic analysis of the striatum from pleiotrophin knockout and midkine knockout mice treated with cocaine reveals regulation of oxidative stress-related proteins potentially underlying cocaine-induced neurotoxicity and neurodegeneration. Toxicology. 2013;314:166–173. doi: 10.1016/j.tox.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 61.Ersche K.D., Acosta-Cabronero J., Jones P.S., Ziauddeen H., van Swelm R.P.L., Laarakkers C.M.M., et al. Disrupted iron regulation in the brain and periphery in cocaine addiction. Transl. Psych. 2017;7 doi: 10.1038/tp.2016.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Papy-Garcia D., Christophe M., Huynh M.B., Fernando S., Ludmilla S., Sepulveda-Diaz J.E., et al. Glycosaminoglycans, protein aggregation and neurodegeneration. Curr. Protein Pept. Sci. 2011;12(3):258–268. doi: 10.2174/138920311795860188. [DOI] [PubMed] [Google Scholar]
- 63.Lehri-Boufala S., Ouidja M.O., Barbier-Chassefiere V., Henault E., Raisman-Vozari R., Garrigue-Antar L., et al. New roles of glycosaminoglycans in alpha-synuclein aggregation in a cellular model of Parkinson disease. PLoS one. 2015;10 doi: 10.1371/journal.pone.0116641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.van Horssen J., Wesseling P., van den Heuvel L.P., de Waal R.M., Verbeek M.M. Heparan sulphate proteoglycans in Alzheimer's disease and amyloid-related disorders. Lancet Neurol. 2003;2:482–492. doi: 10.1016/s1474-4422(03)00484-8. [DOI] [PubMed] [Google Scholar]
- 65.Klein J.A., Meng L., Zaia J. Deep sequencing of complex proteoglycans: a novel strategy for high coverage and site-specific identification of glycosaminoglycan-linked peptides. Mol. Cell Proteomics. 2018;17:1578–1590. doi: 10.1074/mcp.RA118.000766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Perez-Riverol Y., Bai J., Bandla C., Garcia-Seisdedos D., Hewapathirana S., Kamatchinathan S., et al. The PRIDE database resources in 2022: a hub for mass spectrometry-based proteomics evidences. Nucleic Acids Res. 2022;50:D543–D552. doi: 10.1093/nar/gkab1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available in the ProteomeXchange Consortium via the PRIDE (66) partner repository with the dataset identifier PXD038576 and 10.6019/PXD038576.
Reviewer account details are:
Username: reviewer_pxd038576@ebi.ac.uk
Password: 8Z0BFNBp