Abstract
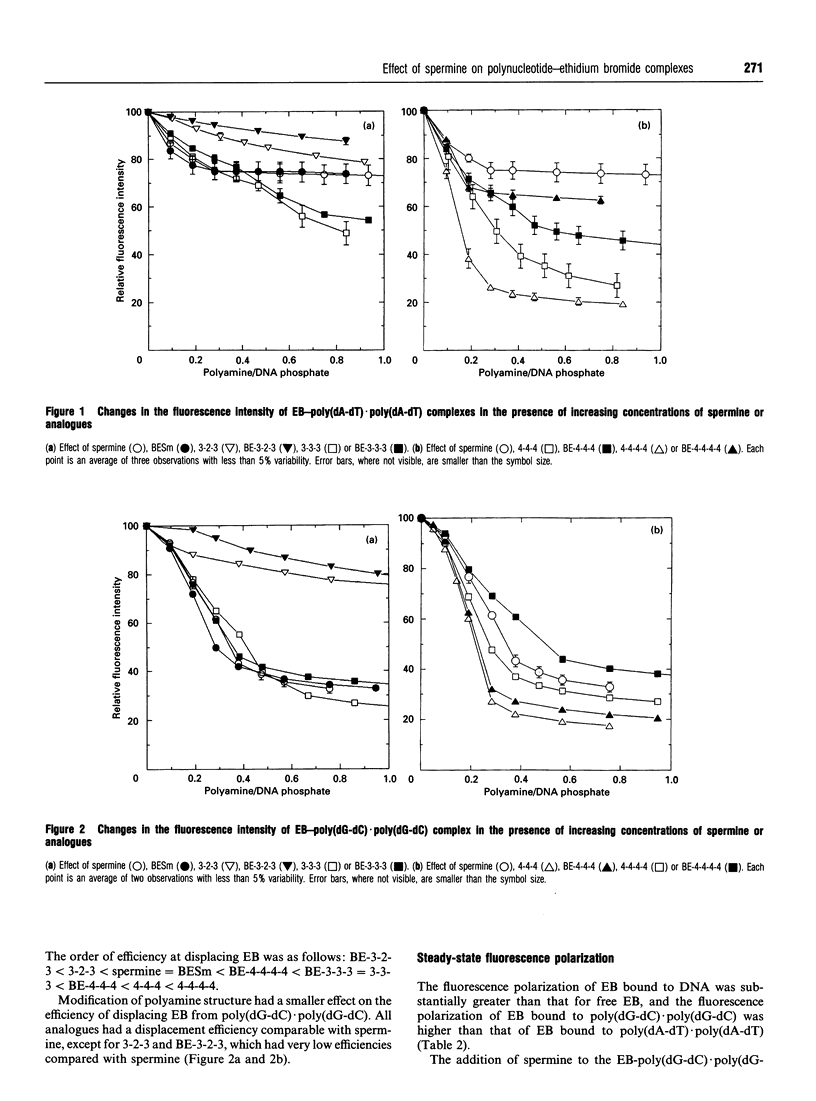
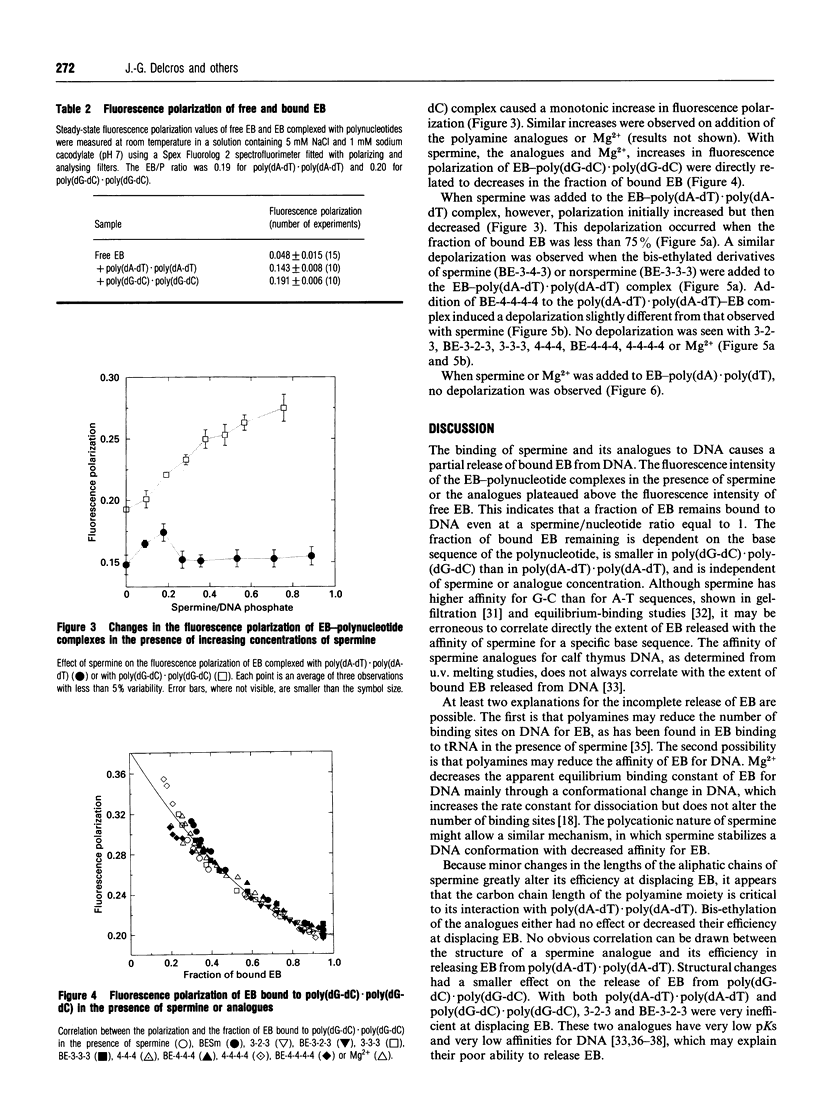
The interactions of spermine and polyamine analogues with synthetic polynucleotides of various base sequences complexed with ethidium bromide (EB) were investigated using measurements of fluorescence intensity and steady-state fluorescence polarization. Spermine and polyamine analogues displaced some but not all of the EB bound to poly(dA-dT).poly(dA-dT) or poly(dG-dC).poly(dG-dC), suggesting that polyamines may stabilize these polynucleotides in a conformation with reduced affinity for EB. Modifications of the aliphatic backbone of spermine have pronounced effects on its ability to displace EB from poly(dA-dT).poly(dA-dT) but not from poly-(dG-dC).poly(dG-dC). Spermine and some but not all of the polyamine analogues caused fluorescence depolarization when they interacted with the complex of EB and poly(dA-dT).poly-(dA-dT). Neither spermine nor any of the analogues, however, induced fluorescence depolarization in the complex of EB with poly(dG-dC).poly(dG-dC) or poly(dA).poly(dT). This suggests that spermine and some spermine analogues induce structural changes specific to alternating A-T sequences.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baguley B. C., Falkenhaug E. M. The interaction of ethidium with synthetic double-stranded polynucleotides at low ionic strength. Nucleic Acids Res. 1978 Jan;5(1):161–171. doi: 10.1093/nar/5.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu H. S., Feuerstein B. G., Deen D. F., Lubich W. P., Bergeron R. J., Samejima K., Marton L. J. Correlation between the effects of polyamine analogues on DNA conformation and cell growth. Cancer Res. 1989 Oct 15;49(20):5591–5597. [PubMed] [Google Scholar]
- Basu H. S., Pellarin M., Feuerstein B. G., Deen D. F., Bergeron R. J., Marton L. J. Effect of N1,N14-bis(ethyl)homospermine on the growth of U-87 MG and SF-126 human brain tumor cells. Cancer Res. 1990 Jun 1;50(11):3137–3140. [PubMed] [Google Scholar]
- Basu H. S., Schwietert H. C., Feuerstein B. G., Marton L. J. Effects of variation in the structure of spermine on the association with DNA and the induction of DNA conformational changes. Biochem J. 1990 Jul 15;269(2):329–334. doi: 10.1042/bj2690329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu H. S., Shafer R. H., Marton L. J. A stopped-flow H-D exchange kinetic study of spermine-polynucleotide interactions. Nucleic Acids Res. 1987 Jul 24;15(14):5873–5886. doi: 10.1093/nar/15.14.5873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S. A natural source of fluorescence depolarization in dye-DNA complexes. J Theor Biol. 1977 Aug 7;67(3):459–470. doi: 10.1016/0022-5193(77)90049-2. [DOI] [PubMed] [Google Scholar]
- Basu S. Binding and interaction of acridine oragne with intraphage DNA. Biochim Biophys Acta. 1971 Nov 29;254(1):48–59. doi: 10.1016/0005-2787(71)90113-4. [DOI] [PubMed] [Google Scholar]
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresloff J. L., Crothers D. M. Equilibrium studies of ethidium--polynucleotide interactions. Biochemistry. 1981 Jun 9;20(12):3547–3553. doi: 10.1021/bi00515a038. [DOI] [PubMed] [Google Scholar]
- Cain B. F., Baguley B. C., Denny W. A. Potenial antitumor agents. 28. Deoxyribonucleic acid polyintercalating agents. J Med Chem. 1978 Jul;21(7):658–668. doi: 10.1021/jm00205a013. [DOI] [PubMed] [Google Scholar]
- Chattoraj D. K., Gosule L. C., Schellman A. DNA condensation with polyamines. II. Electron microscopic studies. J Mol Biol. 1978 May 25;121(3):327–337. doi: 10.1016/0022-2836(78)90367-4. [DOI] [PubMed] [Google Scholar]
- Chen H. H., Rau D. C., Charney E. The flexibility of alternating dA-dT sequences. J Biomol Struct Dyn. 1985 Feb;2(4):709–719. doi: 10.1080/07391102.1985.10506318. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Dickerson R. E. Structure of a B-DNA dodecamer. III. Geometry of hydration. J Mol Biol. 1981 Sep 25;151(3):535–556. doi: 10.1016/0022-2836(81)90009-7. [DOI] [PubMed] [Google Scholar]
- Ellerton N. F., Isenberg I. Fluorescence polarization study of DNA--proflavine complexes. Biopolymers. 1969;8(6):767–786. doi: 10.1002/bip.1969.360080607. [DOI] [PubMed] [Google Scholar]
- Feuerstein B. G., Marton L. J., Keniry M. A., Wade D. L., Shafer R. H. New DNA polymorphism: evidence for a low salt, left-handed form of poly(dG-m5dC). Nucleic Acids Res. 1985 Jun 11;13(11):4133–4141. doi: 10.1093/nar/13.11.4133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein B. G., Pattabiraman N., Marton L. J. Molecular dynamics of spermine-DNA interactions: sequence specificity and DNA bending for a simple ligand. Nucleic Acids Res. 1989 Sep 12;17(17):6883–6892. doi: 10.1093/nar/17.17.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein B. G., Pattabiraman N., Marton L. J. Molecular mechanics of the interactions of spermine with DNA: DNA bending as a result of ligand binding. Nucleic Acids Res. 1990 Mar 11;18(5):1271–1282. doi: 10.1093/nar/18.5.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein B. G., Pattabiraman N., Marton L. J. Spermine-DNA interactions: a theoretical study. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5948–5952. doi: 10.1073/pnas.83.16.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feuerstein B. G., Williams L. D., Basu H. S., Marton L. J. Implications and concepts of polyamine-nucleic acid interactions. J Cell Biochem. 1991 May;46(1):37–47. doi: 10.1002/jcb.240460107. [DOI] [PubMed] [Google Scholar]
- Genest D., Mirau P. A., Kearns D. R. Investigation of DNA dynamics and drug-DNA interaction by steady state fluorescence anisotropy. Nucleic Acids Res. 1985 Apr 11;13(7):2603–2615. doi: 10.1093/nar/13.7.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gosule L. C., Schellman J. A. DNA condensation with polyamines I. Spectroscopic studies. J Mol Biol. 1978 May 25;121(3):311–326. doi: 10.1016/0022-2836(78)90366-2. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Sakamoto I., Goto N., Kashiwagi K., Honma R., Hirose S. Interaction between polyamines and nucleic acids or phospholipids. Arch Biochem Biophys. 1982 Dec;219(2):438–443. doi: 10.1016/0003-9861(82)90175-8. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minyat E. E. The transitions between left- and right-handed forms of poly(dG-dC). Nucleic Acids Res. 1981 Sep 25;9(18):4783–4798. doi: 10.1093/nar/9.18.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain S. C., Sobell H. M. Visualization of drug-nucleic acid interactions at atomic resolution. VII. Structure of an ethidium/dinucleoside monophosphate crystalline complex, ethidium: uridylyl(3'-5') adenosine. J Biomol Struct Dyn. 1984 Mar;1(5):1161–1177. doi: 10.1080/07391102.1984.10507510. [DOI] [PubMed] [Google Scholar]
- Jain S. C., Sobell H. M. Visualization of drug-nucleic acid interactions at atomic resolution. VIII. Structures of two ethidium/dinucleoside monophosphate crystalline complexes containing ethidium: cytidylyl(3'-5') guanosine. J Biomol Struct Dyn. 1984 Mar;1(5):1179–1194. doi: 10.1080/07391102.1984.10507511. [DOI] [PubMed] [Google Scholar]
- Jones R. L., Zon G., Krishnamoorthy C. R., Wilson W. D. Sequence-dependent cooperative interactions in A/T-containing oligo- and polydeoxyribonucleotides. Biochemistry. 1986 Nov 18;25(23):7431–7439. doi: 10.1021/bi00371a027. [DOI] [PubMed] [Google Scholar]
- Latha P. K., Brahmachari S. K. A novel structural transition in poly(dG-Me5dC):Z in equilibrium B in equilibrium Z. FEBS Lett. 1985 Mar 25;182(2):315–318. doi: 10.1016/0014-5793(85)80323-9. [DOI] [PubMed] [Google Scholar]
- LePecq J. B., Paoletti C. A fluorescent complex between ethidium bromide and nucleic acids. Physical-chemical characterization. J Mol Biol. 1967 Jul 14;27(1):87–106. doi: 10.1016/0022-2836(67)90353-1. [DOI] [PubMed] [Google Scholar]
- Lybrand T., Kollman P. Molecular mechanical calculations on the interaction of ethidium cation with double-helical DNA. Biopolymers. 1985 Oct;24(10):1863–1879. doi: 10.1002/bip.360241003. [DOI] [PubMed] [Google Scholar]
- Majumder K., Brahmachari S. K. Sequence specificity in spermine-induced structural changes in CG-oligomers. Biochem Int. 1989 Feb;18(2):455–465. [PubMed] [Google Scholar]
- Marquet R., Houssier C. Different binding modes of spermine to A-T and G-C base pairs modulate the bending and stiffening of the DNA double helix. J Biomol Struct Dyn. 1988 Oct;6(2):235–246. doi: 10.1080/07391102.1988.10507710. [DOI] [PubMed] [Google Scholar]
- Pegg A. E. Polyamine metabolism and its importance in neoplastic growth and a target for chemotherapy. Cancer Res. 1988 Feb 15;48(4):759–774. [PubMed] [Google Scholar]
- Pegg A. E. Recent advances in the biochemistry of polyamines in eukaryotes. Biochem J. 1986 Mar 1;234(2):249–262. doi: 10.1042/bj2340249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum G. E., Bloomfield V. A. Structural and electrostatic effects on binding of trivalent cations to double-stranded and single-stranded poly[d (AT)]. Biopolymers. 1990 Jan;29(1):13–27. doi: 10.1002/bip.360290105. [DOI] [PubMed] [Google Scholar]
- Porschke D. Structure and dynamics of double helices in solution: modes of DNA bending. J Biomol Struct Dyn. 1986 Dec;4(3):373–389. doi: 10.1080/07391102.1986.10506356. [DOI] [PubMed] [Google Scholar]
- Reinhardt C. G., Krugh T. R. A comparative study of ethidium bromide complexes with dinucleotides and DNA: direct evidence for intercalation and nucleic acid sequence preferences. Biochemistry. 1978 Nov 14;17(23):4845–4854. doi: 10.1021/bi00616a001. [DOI] [PubMed] [Google Scholar]
- Sakai T. T., Torget R., I J., Freda C. E., Cohen S. S. The binding of polyamines and of ethidium bromide to tRNA. Nucleic Acids Res. 1975 Jul;2(7):1005–1022. doi: 10.1093/nar/2.7.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slobodyansky E., Stellwagen J., Stellwagen N. C. CD of ethidium bromide complexes with normal and electrophoretically anomalous DNA restriction fragments. Biopolymers. 1988 Jul;27(7):1107–1126. doi: 10.1002/bip.360270706. [DOI] [PubMed] [Google Scholar]
- Stewart K. D. The effect of structural changes in a polyamine backbone on its DNA-binding properties. Biochem Biophys Res Commun. 1988 May 16;152(3):1441–1446. doi: 10.1016/s0006-291x(88)80447-9. [DOI] [PubMed] [Google Scholar]
- Tabor C. W., Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Thomas T. J., Messner R. P. Structural specificity of polyamines in left-handed Z-DNA formation. Immunological and spectroscopic studies. J Mol Biol. 1988 May 20;201(2):463–467. doi: 10.1016/0022-2836(88)90155-6. [DOI] [PubMed] [Google Scholar]
- WEBER G. Polarization of the fluorescence of macromolecules. I. Theory and experimental method. Biochem J. 1952 May;51(2):145–155. doi: 10.1042/bj0510145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson W. D., Wang Y. H., Krishnamoorthy C. R., Smith J. C. Intercalators as probes of DNA conformation: propidium binding to alternating and non-alternating polymers containing guanine. Chem Biol Interact. 1986 Apr;58(1):41–56. doi: 10.1016/s0009-2797(86)80085-0. [DOI] [PubMed] [Google Scholar]
- Wilson W. D., Wang Y. H., Krishnamoorthy C. R., Smith J. C. Poly(dA).poly(dT) exists in an unusual conformation under physiological conditions: propidium binding to poly(dA).poly(dT) and poly[d(A-T)].poly[d(A-T)]. Biochemistry. 1985 Jul 16;24(15):3991–3999. doi: 10.1021/bi00336a029. [DOI] [PubMed] [Google Scholar]
- Winkle S. A., Crooks P. A. Equilibrium binding of spermine and histamine to salmon sperm DNA and poly(dGdC). J Pharm Pharmacol. 1988 Nov;40(11):809–811. doi: 10.1111/j.2042-7158.1988.tb05179.x. [DOI] [PubMed] [Google Scholar]


