Abstract
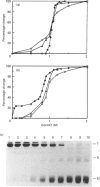
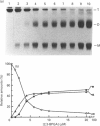
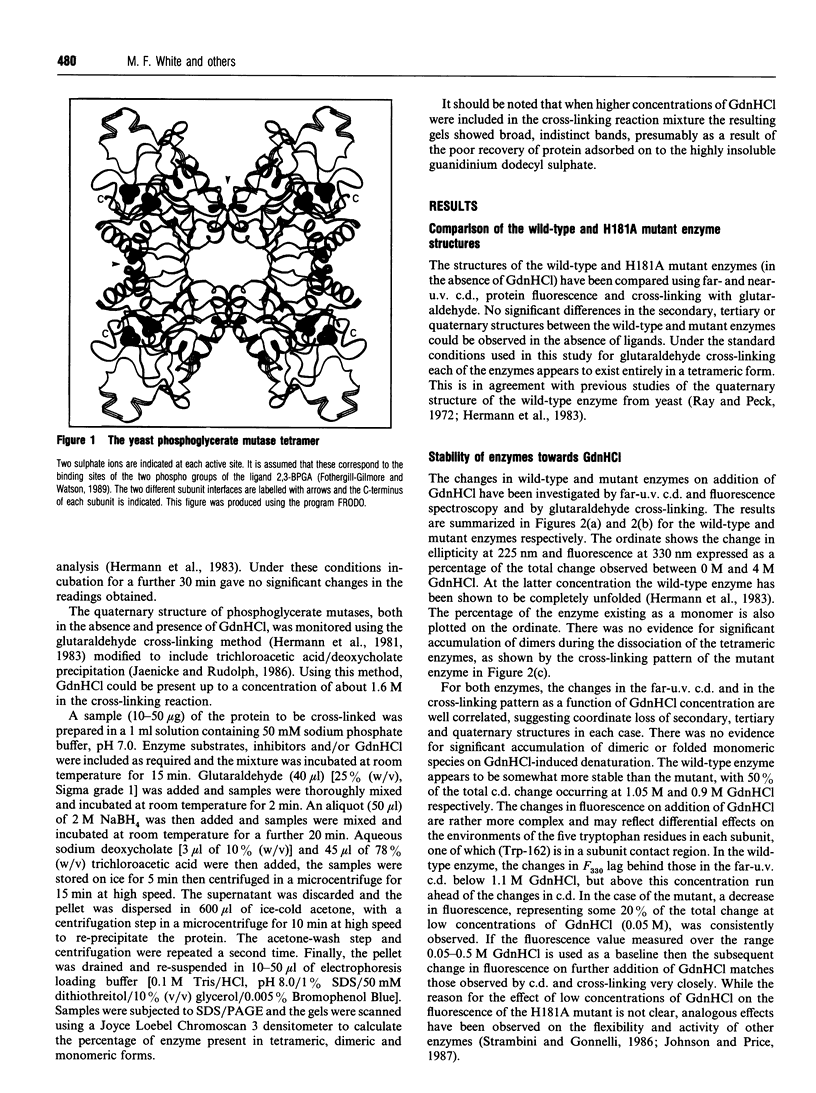
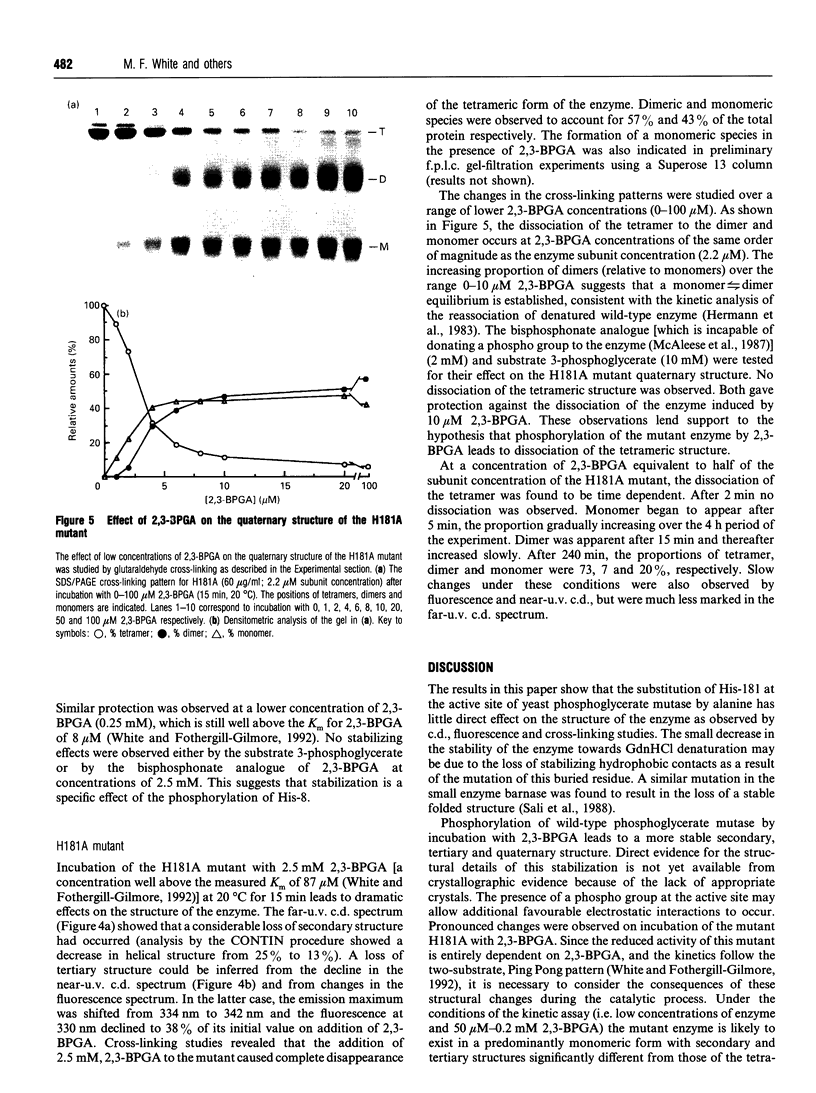
The structure and stability of a mutated yeast phosphoglycerate mutase in which His-181 has been replaced by alanine have been studied. The secondary, tertiary and quaternary structures of the mutant enzyme in the absence of ligands are essentially identical to those of the wild-type enzyme as revealed by c.d., fluorescence and cross-linking studies. The mutant enzyme is slightly less stable than the wild-type enzyme towards denaturation by guanidium chloride (GdnHCl). On addition of cofactor 2,3-bisphosphoglycerate, the wild-type enzyme shows increased stability towards GdnHCl. However, addition of cofactor causes dramatic changes in the structure of the mutant enzyme, leading to dissociation of the tetrameric form to dimeric and monomeric species.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fothergill-Gilmore L. A., Watson H. C. The phosphoglycerate mutases. Adv Enzymol Relat Areas Mol Biol. 1989;62:227–313. doi: 10.1002/9780470123089.ch6. [DOI] [PubMed] [Google Scholar]
- Hermann R., Jaenicke R., Price N. C. Evidence for active intermediates during the reconstitution of yeast phosphoglycerate mutase. Biochemistry. 1985 Apr 9;24(8):1817–1821. doi: 10.1021/bi00329a002. [DOI] [PubMed] [Google Scholar]
- Hermann R., Jaenicke R., Rudolph R. Analysis of the reconstitution of oligomeric enzymes by cross-linking with glutaraldehyde: kinetics of reassociation of lactic dehydrogenase. Biochemistry. 1981 Sep 1;20(18):5195–5201. doi: 10.1021/bi00521a015. [DOI] [PubMed] [Google Scholar]
- Hermann R., Rudolph R., Jaenicke R., Price N. C., Scobbie A. The reconstitution of denatured phosphoglycerate mutase. J Biol Chem. 1983 Sep 25;258(18):11014–11019. [PubMed] [Google Scholar]
- Jaenicke R., Rudolph R. Refolding and association of oligomeric proteins. Methods Enzymol. 1986;131:218–250. doi: 10.1016/0076-6879(86)31043-7. [DOI] [PubMed] [Google Scholar]
- Johnson C. M., Price N. C. Denaturation and renaturation of the monomeric phosphoglycerate mutase from Schizosaccharomyces pombe. Biochem J. 1987 Jul 15;245(2):525–530. doi: 10.1042/bj2450525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAleese S. M., Jutagir V., Blackburn G. M., Fothergill-Gilmore L. A. The bisphosphonomethyl analogue of 2,3-bisphosphoglycerate inhibits yeast but not wheat-germ phosphoglycerate mutase. Biochem J. 1987 Apr 1;243(1):301–304. doi: 10.1042/bj2430301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozaki Y. The preparation of guanidine hydrochloride. Methods Enzymol. 1972;26:43–50. doi: 10.1016/s0076-6879(72)26005-0. [DOI] [PubMed] [Google Scholar]
- Price N. C., Jaenicke R. The quaternary structure of phosphoglycerate mutase from yeast: evidence against dissociation of the tetrameric enzyme at low concentrations. FEBS Lett. 1982 Jul 5;143(2):283–286. doi: 10.1016/0014-5793(82)80117-8. [DOI] [PubMed] [Google Scholar]
- Provencher S. W., Glöckner J. Estimation of globular protein secondary structure from circular dichroism. Biochemistry. 1981 Jan 6;20(1):33–37. doi: 10.1021/bi00504a006. [DOI] [PubMed] [Google Scholar]
- Sali D., Bycroft M., Fersht A. R. Stabilization of protein structure by interaction of alpha-helix dipole with a charged side chain. Nature. 1988 Oct 20;335(6192):740–743. doi: 10.1038/335740a0. [DOI] [PubMed] [Google Scholar]
- Strambini G. B., Gonnelli M. Effects of urea and guanidine hydrochloride on the activity and dynamical structure of equine liver alcohol dehydrogenase. Biochemistry. 1986 May 6;25(9):2471–2476. doi: 10.1021/bi00357a027. [DOI] [PubMed] [Google Scholar]
- White M. F., Fothergill-Gilmore L. A. Development of a mutagenesis, expression and purification system for yeast phosphoglycerate mutase. Investigation of the role of active-site His181. Eur J Biochem. 1992 Jul 15;207(2):709–714. doi: 10.1111/j.1432-1033.1992.tb17099.x. [DOI] [PubMed] [Google Scholar]
- White M. F., Fothergill-Gilmore L. A. Sequence of the gene encoding phosphoglycerate mutase from Saccharomyces cerevisiae. FEBS Lett. 1988 Mar 14;229(2):383–387. doi: 10.1016/0014-5793(88)81161-x. [DOI] [PubMed] [Google Scholar]
- Winn S. I., Watson H. C., Fothergill L. A., Harkins R. N. The active site of yeast phosphoglycerate mutase. Biochem Soc Trans. 1977;5(3):657–659. doi: 10.1042/bst0050657. [DOI] [PubMed] [Google Scholar]