Abstract
The Ca(2+)- and phospholipid-binding protein, annexin V, has been shown by an immune assay to represent 0.4% of total cell protein in cultured chick-embryo fibroblasts. Immunofluorescent localization studies indicate that in primary cultures the protein is abundant in the cytoplasm of the cells and also extends into the nucleus. Nuclear staining is no longer detectable, however, in approx. 25% of the cells following sub-culture. Sub-populations of annexin V are associated with cytoskeletal structures and with the inner face of the plasma membrane in a Ca(2+)-independent manner. In addition, we report results indicating the secretion of annexin V from this cell type.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrigo A. P., Darlix J. L., Spahr P. F. A cellular protein phosphorylated by the avian sarcoma virus transforming gene product is associated with ribonucleoprotein particles. EMBO J. 1983;2(3):309–315. doi: 10.1002/j.1460-2075.1983.tb01424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
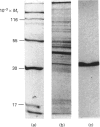
- Boustead C. M., Brown R., Walker J. H. Isolation, characterization and localization of annexin V from chicken liver. Biochem J. 1993 Apr 15;291(Pt 2):601–608. doi: 10.1042/bj2910601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boustead C. M., Walker J. H., Geisow M. J. Isolation and characterization of two novel calcium-dependent phospholipid-binding proteins from bovine lung. FEBS Lett. 1988 Jun 20;233(2):233–238. doi: 10.1016/0014-5793(88)80433-2. [DOI] [PubMed] [Google Scholar]
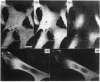
- Burgoyne R. D., Geisow M. J. The annexin family of calcium-binding proteins. Review article. Cell Calcium. 1989 Jan;10(1):1–10. doi: 10.1016/0143-4160(89)90038-9. [DOI] [PubMed] [Google Scholar]
- Christmas P., Callaway J., Fallon J., Jones J., Haigler H. T. Selective secretion of annexin 1, a protein without a signal sequence, by the human prostate gland. J Biol Chem. 1991 Feb 5;266(4):2499–2507. [PubMed] [Google Scholar]
- Courtneidge S., Ralston R., Alitalo K., Bishop J. M. Subcellular location of an abundant substrate (p36) for tyrosine-specific protein kinases. Mol Cell Biol. 1983 Mar;3(3):340–350. doi: 10.1128/mcb.3.3.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton M. R., Moss S. E., Crumpton M. J. Diversity in the lipocortin/calpactin family. Cell. 1988 Oct 7;55(1):1–3. doi: 10.1016/0092-8674(88)90002-5. [DOI] [PubMed] [Google Scholar]
- Davidson F. F., Dennis E. A., Powell M., Glenney J. R., Jr Inhibition of phospholipase A2 by "lipocortins" and calpactins. An effect of binding to substrate phospholipids. J Biol Chem. 1987 Feb 5;262(4):1698–1705. [PubMed] [Google Scholar]
- Drust D. S., Creutz C. E. Aggregation of chromaffin granules by calpactin at micromolar levels of calcium. Nature. 1988 Jan 7;331(6151):88–91. doi: 10.1038/331088a0. [DOI] [PubMed] [Google Scholar]
- Funakoshi T., Hendrickson L. E., McMullen B. A., Fujikawa K. Primary structure of human placental anticoagulant protein. Biochemistry. 1987 Dec 15;26(25):8087–8092. doi: 10.1021/bi00399a011. [DOI] [PubMed] [Google Scholar]
- Geisow M. J., Walker J. H., Boustead C., Taylor W. Annexins--new family of Ca2+-regulated-phospholipid binding protein. Biosci Rep. 1987 Apr;7(4):289–298. doi: 10.1007/BF01121450. [DOI] [PubMed] [Google Scholar]
- Gerke V., Weber K. Identity of p36K phosphorylated upon Rous sarcoma virus transformation with a protein purified from brush borders; calcium-dependent binding to non-erythroid spectrin and F-actin. EMBO J. 1984 Jan;3(1):227–233. doi: 10.1002/j.1460-2075.1984.tb01789.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giambanco I., Pula G., Ceccarelli P., Bianchi R., Donato R. Immunohistochemical localization of annexin V (CaBP33) in rat organs. J Histochem Cytochem. 1991 Sep;39(9):1189–1198. doi: 10.1177/39.9.1833446. [DOI] [PubMed] [Google Scholar]
- Glenney J. R., Jr, Tack B., Powell M. A. Calpactins: two distinct Ca++-regulated phospholipid- and actin-binding proteins isolated from lung and placenta. J Cell Biol. 1987 Mar;104(3):503–511. doi: 10.1083/jcb.104.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haigler H. T., Schlaepfer D. D., Burgess W. H. Characterization of lipocortin I and an immunologically unrelated 33-kDa protein as epidermal growth factor receptor/kinase substrates and phospholipase A2 inhibitors. J Biol Chem. 1987 May 15;262(14):6921–6930. [PubMed] [Google Scholar]
- Huber R., Römisch J., Paques E. P. The crystal and molecular structure of human annexin V, an anticoagulant protein that binds to calcium and membranes. EMBO J. 1990 Dec;9(12):3867–3874. doi: 10.1002/j.1460-2075.1990.tb07605.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jindal H. K., Chaney W. G., Anderson C. W., Davis R. G., Vishwanatha J. K. The protein-tyrosine kinase substrate, calpactin I heavy chain (p36), is part of the primer recognition protein complex that interacts with DNA polymerase alpha. J Biol Chem. 1991 Mar 15;266(8):5169–5176. [PubMed] [Google Scholar]
- Johnston P. A., Perin M. S., Reynolds G. A., Wasserman S. A., Südhof T. C. Two novel annexins from Drosophila melanogaster. Cloning, characterization, and differential expression in development. J Biol Chem. 1990 Jul 5;265(19):11382–11388. [PubMed] [Google Scholar]
- Kaetzel M. A., Hazarika P., Diaz-Munoz M., Dubinsky W., Hamilton S. L., Dedman J. R. Annexins: a subcellular localization and reconstitution approach to elucidate cellular function. Biochem Soc Trans. 1990 Dec;18(6):1108–1110. doi: 10.1042/bst0181108. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Klee C. B. Ca2+-dependent phospholipid- (and membrane-) binding proteins. Biochemistry. 1988 Sep 6;27(18):6645–6653. doi: 10.1021/bi00418a001. [DOI] [PubMed] [Google Scholar]
- Kretsinger R. H. Structure and evolution of calcium-modulated proteins. CRC Crit Rev Biochem. 1980;8(2):119–174. doi: 10.3109/10409238009105467. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lehto V. P., Virtanen I., Paasivuo R., Ralston R., Alitalo K. The p36 substrate of tyrosine-specific protein kinases co-localizes with non-erythrocyte alpha-spectrin antigen, p230, in surface lamina of cultured fibroblasts. EMBO J. 1983;2(10):1701–1705. doi: 10.1002/j.1460-2075.1983.tb01645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer-Fogy I., Reutelingsperger C. P., Pieters J., Bodo G., Stratowa C., Hauptmann R. Cloning and expression of cDNA for human vascular anticoagulant, a Ca2+-dependent phospholipid-binding protein. Eur J Biochem. 1988 Jul 1;174(4):585–592. doi: 10.1111/j.1432-1033.1988.tb14139.x. [DOI] [PubMed] [Google Scholar]
- Middleton C. A., Brown A. F., Brown R. M., Karavanova I. D., Roberts D. J., Vasiliev J. M. The polarization of fibroblasts in early primary cultures is independent of microtubule integrity. J Cell Sci. 1989 Sep;94(Pt 1):25–32. doi: 10.1242/jcs.94.1.25. [DOI] [PubMed] [Google Scholar]
- Peters R., Lang I., Scholz M., Schulz B., Kayne F. Fluorescence microphotolysis to measure nucleocytoplasmic transport in vivo and in vitro. Biochem Soc Trans. 1986 Oct;14(5):821–822. doi: 10.1042/bst0140821. [DOI] [PubMed] [Google Scholar]
- Radke K., Carter V. C., Moss P., Dehazya P., Schliwa M., Martin G. S. Membrane association of a 36,000-dalton substrate for tyrosine phosphorylation in chicken embryo fibroblasts transformed by avian sarcoma viruses. J Cell Biol. 1983 Nov;97(5 Pt 1):1601–1611. doi: 10.1083/jcb.97.5.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas E., Pollard H. B., Haigler H. T., Parra C., Burns A. L. Calcium-activated endonexin II forms calcium channels across acidic phospholipid bilayer membranes. J Biol Chem. 1990 Dec 5;265(34):21207–21215. [PubMed] [Google Scholar]
- Römisch J., Pâques E. P. Annexins: calcium-binding proteins of multi-functional importance? Med Microbiol Immunol. 1991;180(3):109–126. doi: 10.1007/BF00206115. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D. D., Haigler H. T. Expression of annexins as a function of cellular growth state. J Cell Biol. 1990 Jul;111(1):229–238. doi: 10.1083/jcb.111.1.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer D. D., Mehlman T., Burgess W. H., Haigler H. T. Structural and functional characterization of endonexin II, a calcium- and phospholipid-binding protein. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6078–6082. doi: 10.1073/pnas.84.17.6078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semich R., Gerke V., Robenek H., Weber K. The p36 substrate of pp60src kinase is located at the cytoplasmic surface of the plasma membrane of fibroblasts; an immunoelectron microscopic analysis. Eur J Cell Biol. 1989 Dec;50(2):313–323. [PubMed] [Google Scholar]
- Simons K., Virta H. Perforated MDCK cells support intracellular transport. EMBO J. 1987 Aug;6(8):2241–2247. doi: 10.1002/j.1460-2075.1987.tb02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smallwood M., Keen J. N., Bowles D. J. Purification and partial sequence analysis of plant annexins. Biochem J. 1990 Aug 15;270(1):157–161. doi: 10.1042/bj2700157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Südhof T. C. Calelectrins are a ubiquitous family of Ca2+-binding proteins purified by Ca2+-dependent hydrophobic affinity chromatography by a mechanism distinct from that of calmodulin. Biochem Biophys Res Commun. 1984 Aug 30;123(1):100–107. doi: 10.1016/0006-291x(84)90385-1. [DOI] [PubMed] [Google Scholar]
- Südhof T. C., Ebbecke M., Walker J. H., Fritsche U., Boustead C. Isolation of mammalian calelectrins: a new class of ubiquitous Ca2+-regulated proteins. Biochemistry. 1984 Mar 13;23(6):1103–1109. doi: 10.1021/bi00301a010. [DOI] [PubMed] [Google Scholar]
- Tait J. F., Sakata M., McMullen B. A., Miao C. H., Funakoshi T., Hendrickson L. E., Fujikawa K. Placental anticoagulant proteins: isolation and comparative characterization four members of the lipocortin family. Biochemistry. 1988 Aug 23;27(17):6268–6276. doi: 10.1021/bi00417a011. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. H. Isolation from cholinergic synapses of a protein that binds to membranes in a calcium-dependent manner. J Neurochem. 1982 Sep;39(3):815–823. doi: 10.1111/j.1471-4159.1982.tb07965.x. [DOI] [PubMed] [Google Scholar]
- Walker J. H., Kristjansson G. I., Stadler H. Identification of a synaptic vesicle antigen (Mr 86,000) conserved between Torpedo and rat. J Neurochem. 1986 Mar;46(3):875–881. doi: 10.1111/j.1471-4159.1986.tb13053.x. [DOI] [PubMed] [Google Scholar]
- Woolgar J. A., Boustead C. M., Walker J. H. Characterization of annexins in mammalian brain. J Neurochem. 1990 Jan;54(1):62–71. doi: 10.1111/j.1471-4159.1990.tb13283.x. [DOI] [PubMed] [Google Scholar]