Abstract
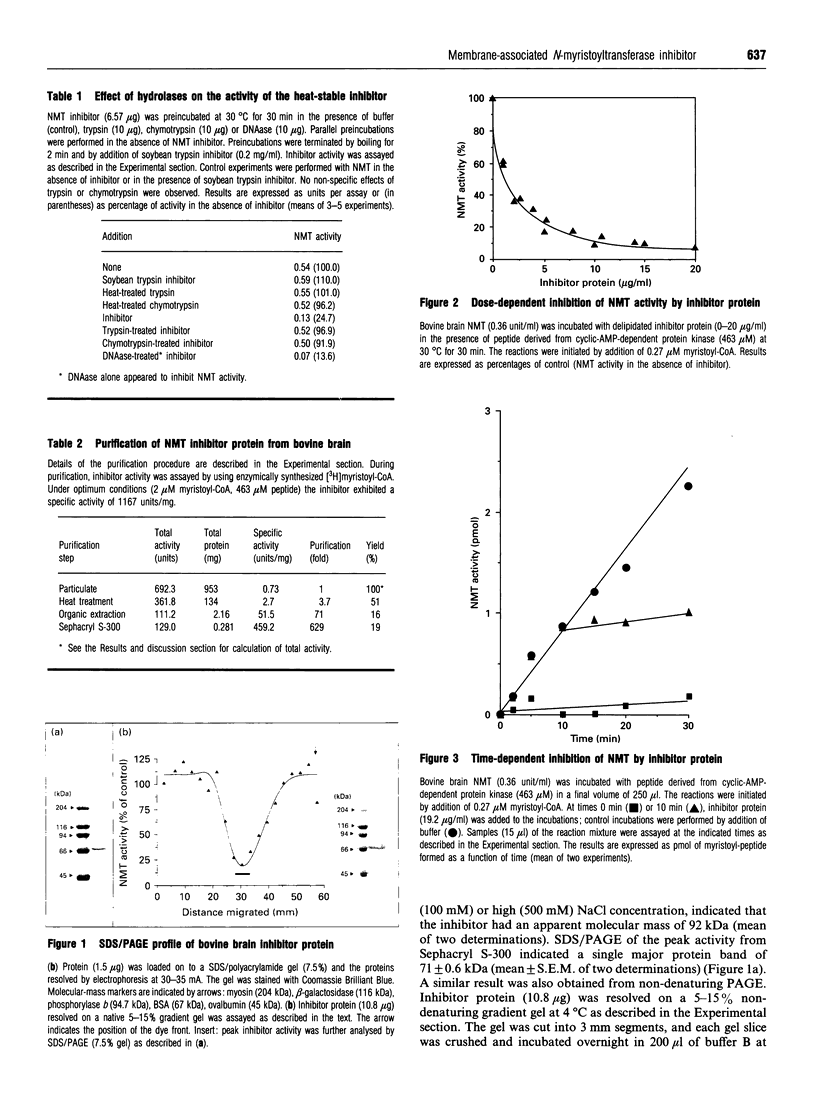
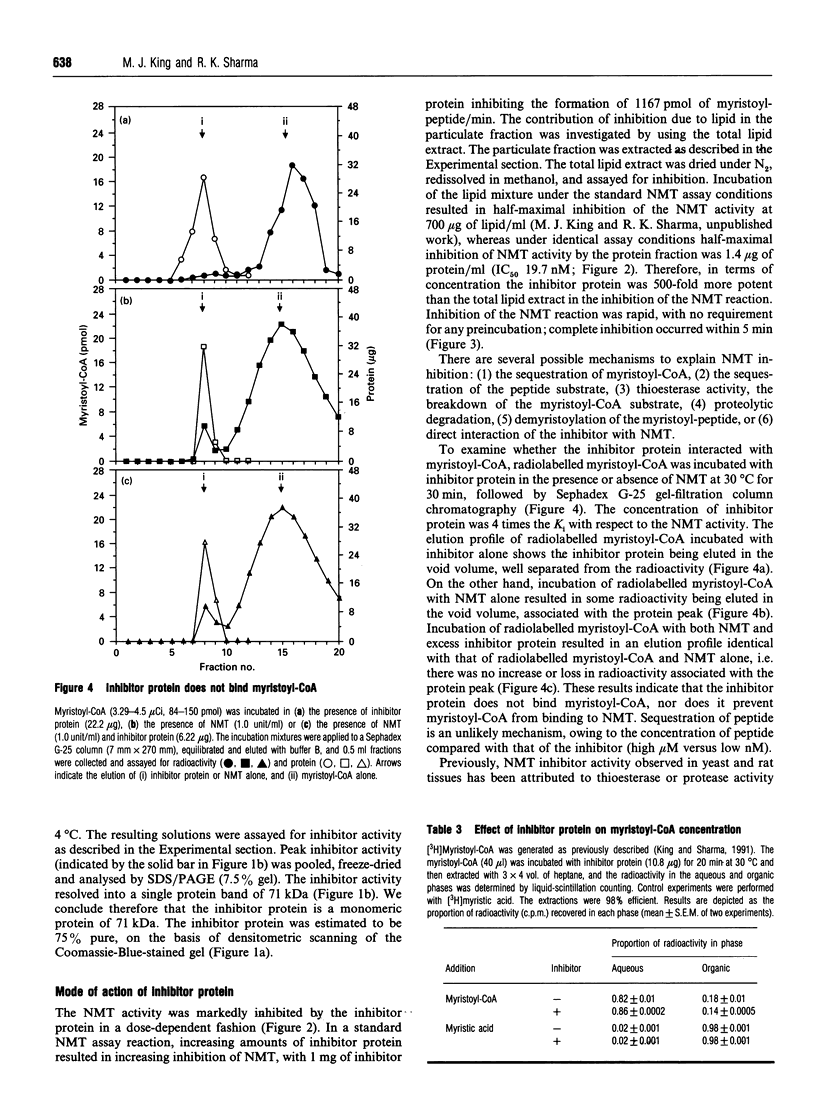
N-Myristoyl-CoA: protein N-myristoyltransferase (NMT) is the enzyme that catalyses the covalent transfer of myristic acid from myristoyl-CoA to the N-terminal glycine residue of a protein substrate. Subcellular fractionation of bovine brain indicates that NMT activity was located in both the cytosolic and the particulate fraction of the cell. Removal of the particulate fraction resulted in a 2-fold enhancement of NMT activity. Reconstitution of the particulate fraction and cytosolic fraction resulted in inhibition of the elevated cytosolic NMT activity. These results indicated the existence of putative inhibitor(s) activity of NMT located in the particulate fraction of bovine brain. The inhibitor was stable to heat and was identified as a protein, on the basis of its susceptibility to the proteases trypsin and chymotrypsin. Protease degradation first required the delipidation of the particulate fraction. The inhibitor was purified to near-homogeneity by heat treatment, solvent extraction and Sephacryl S-300 gelfiltration column chromatography. The inhibitor was purified 630-fold from the particulate fraction with a 20% yield. The protein inhibitor had an apparent molecular mass of 92 kDa by gel filtration and 71 kDa by SDS/PAGE, indicating the protein is monomeric. The inhibitor did not interact directly with myristoyl-CoA and possessed no protease, thioesterase or demyristoylase activity. Purified inhibitor protein inhibited the formation of 1167 pmol of myristoyl-peptide/min per mg of protein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken A., Cohen P., Santikarn S., Williams D. H., Calder A. G., Smith A., Klee C. B. Identification of the NH2-terminal blocking group of calcineurin B as myristic acid. FEBS Lett. 1982 Dec 27;150(2):314–318. doi: 10.1016/0014-5793(82)80759-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bryant M., Ratner L. Myristoylation-dependent replication and assembly of human immunodeficiency virus 1. Proc Natl Acad Sci U S A. 1990 Jan;87(2):523–527. doi: 10.1073/pnas.87.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buss J. E., Kamps M. P., Sefton B. M. Myristic acid is attached to the transforming protein of Rous sarcoma virus during or immediately after synthesis and is present in both soluble and membrane-bound forms of the protein. Mol Cell Biol. 1984 Dec;4(12):2697–2704. doi: 10.1128/mcb.4.12.2697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr S. A., Biemann K., Shoji S., Parmelee D. C., Titani K. n-Tetradecanoyl is the NH2-terminal blocking group of the catalytic subunit of cyclic AMP-dependent protein kinase from bovine cardiac muscle. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6128–6131. doi: 10.1073/pnas.79.20.6128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deichaite I., Casson L. P., Ling H. P., Resh M. D. In vitro synthesis of pp60v-src: myristylation in a cell-free system. Mol Cell Biol. 1988 Oct;8(10):4295–4301. doi: 10.1128/mcb.8.10.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover C. J., Goddard C., Felsted R. L. N-myristoylation of p60src. Identification of a myristoyl-CoA:glycylpeptide N-myristoyltransferase in rat tissues. Biochem J. 1988 Mar 1;250(2):485–491. doi: 10.1042/bj2500485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heuckeroth R. O., Towler D. A., Adams S. P., Glaser L., Gordon J. I. 11-(Ethylthio)undecanoic acid. A myristic acid analogue of altered hydrophobicity which is functional for peptide N-myristoylation with wheat germ and yeast acyltransferase. J Biol Chem. 1988 Feb 15;263(5):2127–2133. [PubMed] [Google Scholar]
- Ingebritsen T. S. Phosphotyrosyl-protein phosphatases. II. Identification and characterization of two heat-stable protein inhibitors. J Biol Chem. 1989 May 5;264(13):7754–7759. [PubMed] [Google Scholar]
- Iozzo R. V., Hacobian N. Myristoylation of heparan sulfate proteoglycan and proteins occurs post-translationally in human colon carcinoma cells. Biochem Biophys Res Commun. 1990 Oct 30;172(2):905–912. doi: 10.1016/0006-291x(90)90761-b. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Buss J. E., Sefton B. M. Mutation of NH2-terminal glycine of p60src prevents both myristoylation and morphological transformation. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4625–4628. doi: 10.1073/pnas.82.14.4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King M. J., Sharma R. K. N-myristoyl transferase assay using phosphocellulose paper binding. Anal Biochem. 1991 Dec;199(2):149–153. doi: 10.1016/0003-2697(91)90082-5. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- McIlhinney R. A., McGlone K. A simplified assay for the enzyme responsible for the attachment of myristic acid to the N-terminal glycine residue of proteins, myristoyl-CoA: glycylpeptide N-myristoyltransferase. Biochem J. 1989 Oct 15;263(2):387–391. doi: 10.1042/bj2630387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlhinney R. A., McGlone K. Characterisation of a myristoyl CoA:glycylpeptide N-myristoyl transferase activity in rat brain: subcellular and regional distribution. J Neurochem. 1990 Jan;54(1):110–117. doi: 10.1111/j.1471-4159.1990.tb13289.x. [DOI] [PubMed] [Google Scholar]
- McIlhinney R. A., McGlone K. Evidence for a non-myristoylated pool of the 80 kDa protein kinase C substrate of rat brain. Biochem J. 1990 Nov 1;271(3):681–685. doi: 10.1042/bj2710681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N., Spizz G. Fatty acylation of cellular proteins. Temporal and subcellular differences between palmitate and myristate acylation. J Biol Chem. 1986 Feb 15;261(5):2458–2466. [PubMed] [Google Scholar]
- Paige L. A., Chafin D. R., Cassady J. M., Geahlen R. L. Detection of myristoyl CoA:protein N-myristoyltransferase activity by ion-exchange chromatography. Anal Biochem. 1989 Sep;181(2):254–258. doi: 10.1016/0003-2697(89)90239-x. [DOI] [PubMed] [Google Scholar]
- Pillai S., Baltimore D. Myristoylation and the post-translational acquisition of hydrophobicity by the membrane immunoglobulin heavy-chain polypeptide in B lymphocytes. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7654–7658. doi: 10.1073/pnas.84.21.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towler D. A., Adams S. P., Eubanks S. R., Towery D. S., Jackson-Machelski E., Glaser L., Gordon J. I. Myristoyl CoA:protein N-myristoyltransferase activities from rat liver and yeast possess overlapping yet distinct peptide substrate specificities. J Biol Chem. 1988 Feb 5;263(4):1784–1790. [PubMed] [Google Scholar]
- Towler D. A., Eubanks S. R., Towery D. S., Adams S. P., Glaser L. Amino-terminal processing of proteins by N-myristoylation. Substrate specificity of N-myristoyl transferase. J Biol Chem. 1987 Jan 25;262(3):1030–1036. [PubMed] [Google Scholar]
- Towler D., Glaser L. Protein fatty acid acylation: enzymatic synthesis of an N-myristoylglycyl peptide. Proc Natl Acad Sci U S A. 1986 May;83(9):2812–2816. doi: 10.1073/pnas.83.9.2812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayasarathy C., Bhat N. R., Avadhani N. G. Intramitochondrial fatty acylation of a cytoplasmic imported protein in animal cells. J Biol Chem. 1989 May 15;264(14):7772–7775. [PubMed] [Google Scholar]
- Weaver T. A., Panganiban A. T. N myristoylation of the spleen necrosis virus matrix protein is required for correct association of the Gag polyprotein with intracellular membranes and for particle formation. J Virol. 1990 Aug;64(8):3995–4001. doi: 10.1128/jvi.64.8.3995-4001.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Silva A. M., Klein C. A rapid posttranslational myristylation of a 68-kD protein in D. discoideum. J Cell Biol. 1990 Aug;111(2):401–407. doi: 10.1083/jcb.111.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]



