Abstract
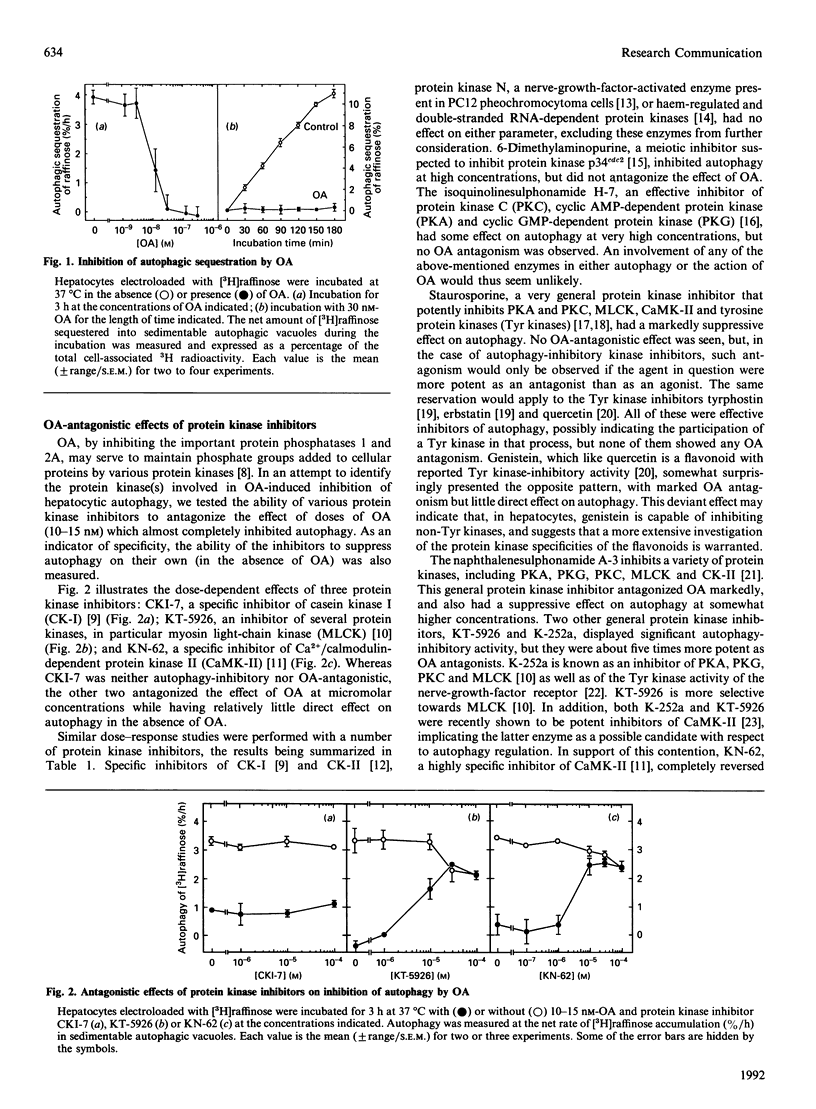
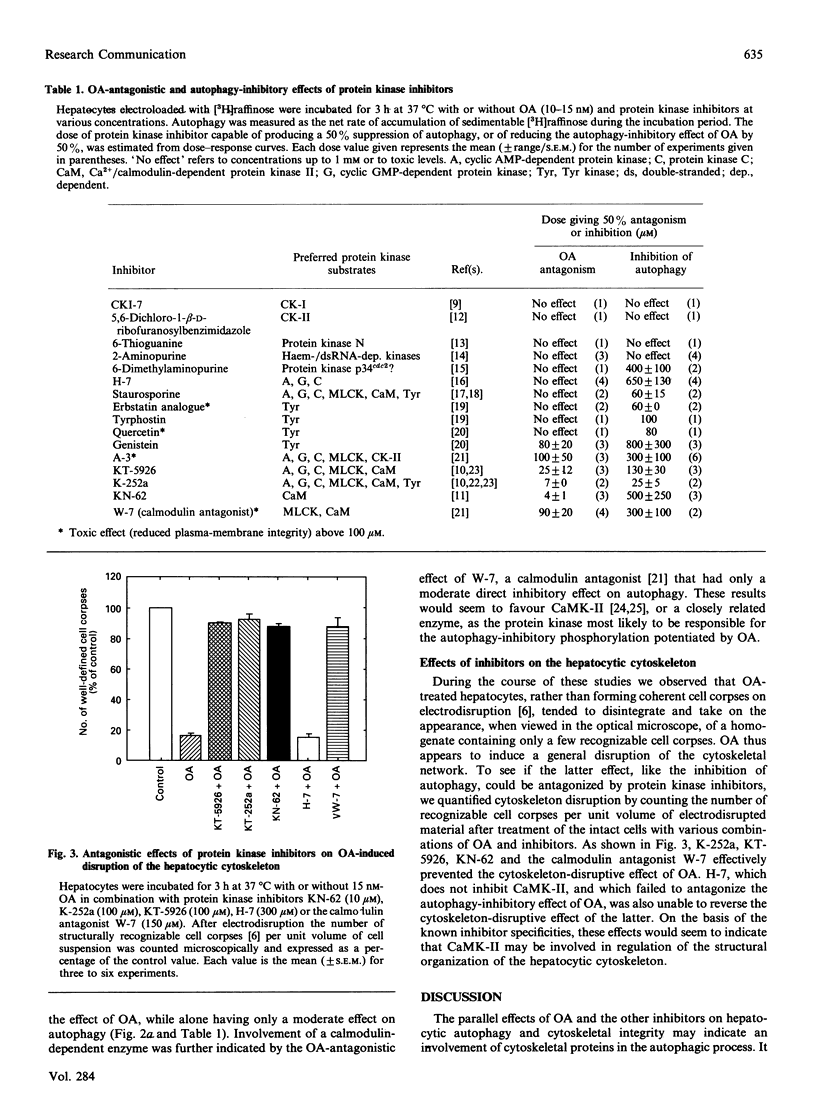
The protein phosphatase inhibitor okadaic acid suppressed autophagy completely in isolated rat hepatocytes, as measured by the sequestration of electroinjected [3H]raffinose into sedimentable autophagic vacuoles. Okadaic acid was effectively antagonized by the general protein kinase inhibitors K-252a and KT-5926, the calmodulin antagonist W-7, and by KN-62, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II (CaMK-II). These inhibitors also antagonized a cytoskeleton-disruptive effect of okadaic acid, manifested as the disintegration of cell corpses after breakage of the plasma membrane. CaMK-II, or a closely related enzyme, would thus seem to play a role in the control of autophagy as well as in the control of cytoskeletal organization.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama T., Ishida J., Nakagawa S., Ogawara H., Watanabe S., Itoh N., Shibuya M., Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem. 1987 Apr 25;262(12):5592–5595. [PubMed] [Google Scholar]
- Baitinger C., Alderton J., Poenie M., Schulman H., Steinhardt R. A. Multifunctional Ca2+/calmodulin-dependent protein kinase is necessary for nuclear envelope breakdown. J Cell Biol. 1990 Nov;111(5 Pt 1):1763–1773. doi: 10.1083/jcb.111.5.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg M. M., Sternberg D. W., Parada L. F., Chao M. V. K-252a inhibits nerve growth factor-induced trk proto-oncogene tyrosine phosphorylation and kinase activity. J Biol Chem. 1992 Jan 5;267(1):13–16. [PubMed] [Google Scholar]
- Bialojan C., Takai A. Inhibitory effect of a marine-sponge toxin, okadaic acid, on protein phosphatases. Specificity and kinetics. Biochem J. 1988 Nov 15;256(1):283–290. doi: 10.1042/bj2560283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bøe R., Gjertsen B. T., Vintermyr O. K., Houge G., Lanotte M., Døskeland S. O. The protein phosphatase inhibitor okadaic acid induces morphological changes typical of apoptosis in mammalian cells. Exp Cell Res. 1991 Jul;195(1):237–246. doi: 10.1016/0014-4827(91)90523-w. [DOI] [PubMed] [Google Scholar]
- Chijiwa T., Hagiwara M., Hidaka H. A newly synthesized selective casein kinase I inhibitor, N-(2-aminoethyl)-5-chloroisoquinoline-8-sulfonamide, and affinity purification of casein kinase I from bovine testis. J Biol Chem. 1989 Mar 25;264(9):4924–4927. [PubMed] [Google Scholar]
- Colbran R. J., Schworer C. M., Hashimoto Y., Fong Y. L., Rich D. P., Smith M. K., Soderling T. R. Calcium/calmodulin-dependent protein kinase II. Biochem J. 1989 Mar 1;258(2):313–325. doi: 10.1042/bj2580313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson J. E., Paatero G. I., Meriluoto J. A., Codd G. A., Kass G. E., Nicotera P., Orrenius S. Rapid microfilament reorganization induced in isolated rat hepatocytes by microcystin-LR, a cyclic peptide toxin. Exp Cell Res. 1989 Nov;185(1):86–100. doi: 10.1016/0014-4827(89)90039-6. [DOI] [PubMed] [Google Scholar]
- Falconer I. R., Yeung D. S. Cytoskeletal changes in hepatocytes induced by Microcystis toxins and their relation to hyperphosphorylation of cell proteins. Chem Biol Interact. 1992 Jan;81(1-2):181–196. doi: 10.1016/0009-2797(92)90033-h. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Balkow K., Hunt T., Jackson R. J., Trachsel H. Phosphorylation of initiation factor elF-2 and the control of reticulocyte protein synthesis. Cell. 1977 May;11(1):187–200. doi: 10.1016/0092-8674(77)90330-0. [DOI] [PubMed] [Google Scholar]
- Fujita-Yamaguchi Y., Kathuria S. Characterization of receptor tyrosine-specific protein kinases by the use of inhibitors. Staurosporine is a 100-times more potent inhibitor of insulin receptor than IGF-I receptor. Biochem Biophys Res Commun. 1988 Dec 30;157(3):955–962. doi: 10.1016/s0006-291x(88)80967-7. [DOI] [PubMed] [Google Scholar]
- Gordon P. B., Seglen P. O. Autophagic sequestration of [14C]sucrose, introduced into rat hepatocytes by reversible electro-permeabilization. Exp Cell Res. 1982 Nov;142(1):1–14. doi: 10.1016/0014-4827(82)90402-5. [DOI] [PubMed] [Google Scholar]
- Grinde B. Autophagy and lysosomal proteolysis in the liver. Experientia. 1985 Sep 15;41(9):1089–1095. doi: 10.1007/BF01951685. [DOI] [PubMed] [Google Scholar]
- Hashimoto Y., Nakayama T., Teramoto T., Kato H., Watanabe T., Kinoshita M., Tsukamoto K., Tokunaga K., Kurokawa K., Nakanishi S. Potent and preferential inhibition of Ca2+/calmodulin-dependent protein kinase II by K252a and its derivative, KT5926. Biochem Biophys Res Commun. 1991 Nov 27;181(1):423–429. doi: 10.1016/s0006-291x(05)81436-6. [DOI] [PubMed] [Google Scholar]
- Haystead T. A., Sim A. T., Carling D., Honnor R. C., Tsukitani Y., Cohen P., Hardie D. G. Effects of the tumour promoter okadaic acid on intracellular protein phosphorylation and metabolism. Nature. 1989 Jan 5;337(6202):78–81. doi: 10.1038/337078a0. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Inagaki M., Kawamoto S., Itoh H., Saitoh M., Hagiwara M., Takahashi J., Hidaka H. Naphthalenesulfonamides as calmodulin antagonists and protein kinase inhibitors. Mol Pharmacol. 1986 Jun;29(6):577–581. [PubMed] [Google Scholar]
- Ishihara H., Martin B. L., Brautigan D. L., Karaki H., Ozaki H., Kato Y., Fusetani N., Watabe S., Hashimoto K., Uemura D. Calyculin A and okadaic acid: inhibitors of protein phosphatase activity. Biochem Biophys Res Commun. 1989 Mar 31;159(3):871–877. doi: 10.1016/0006-291x(89)92189-x. [DOI] [PubMed] [Google Scholar]
- Lamb N. J., Fernandez A., Watrin A., Labbé J. C., Cavadore J. C. Microinjection of p34cdc2 kinase induces marked changes in cell shape, cytoskeletal organization, and chromatin structure in mammalian fibroblasts. Cell. 1990 Jan 12;60(1):151–165. doi: 10.1016/0092-8674(90)90725-t. [DOI] [PubMed] [Google Scholar]
- MacNicol M., Jefferson A. B., Schulman H. Ca2+/calmodulin kinase is activated by the phosphatidylinositol signaling pathway and becomes Ca2(+)-independent in PC12 cells. J Biol Chem. 1990 Oct 25;265(30):18055–18058. [PubMed] [Google Scholar]
- Miura G. A., Robinson N. A., Geisbert T. W., Bostian K. A., White J. D., Pace J. G. Comparison of in vivo and in vitro toxic effects of microcystin-LR in fasted rats. Toxicon. 1989;27(11):1229–1240. doi: 10.1016/0041-0101(89)90031-7. [DOI] [PubMed] [Google Scholar]
- Nakanishi S., Yamada K., Iwahashi K., Kuroda K., Kase H. KT5926, a potent and selective inhibitor of myosin light chain kinase. Mol Pharmacol. 1990 Apr;37(4):482–488. [PubMed] [Google Scholar]
- Neant I., Guerrier P. 6-Dimethylaminopurine blocks starfish oocyte maturation by inhibiting a relevant protein kinase activity. Exp Cell Res. 1988 May;176(1):68–79. doi: 10.1016/0014-4827(88)90121-8. [DOI] [PubMed] [Google Scholar]
- Nishiwaki-Matsushima R., Nishiwaki S., Ohta T., Yoshizawa S., Suganuma M., Harada K., Watanabe M. F., Fujiki H. Structure-function relationships of microcystins, liver tumor promoters, in interaction with protein phosphatase. Jpn J Cancer Res. 1991 Sep;82(9):993–996. doi: 10.1111/j.1349-7006.1991.tb01933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta Y., Ohba T., Fukunaga K., Miyamoto E. Serum and growth factors rapidly elicit phosphorylation of the Ca2+/calmodulin-dependent protein kinase II in intact quiescent rat 3Y1 cells. J Biol Chem. 1988 Aug 15;263(23):11540–11547. [PubMed] [Google Scholar]
- Schulman H., Lou L. L. Multifunctional Ca2+/calmodulin-dependent protein kinase: domain structure and regulation. Trends Biochem Sci. 1989 Feb;14(2):62–66. doi: 10.1016/0968-0004(89)90045-5. [DOI] [PubMed] [Google Scholar]
- Seglen P. O., Bohley P. Autophagy and other vacuolar protein degradation mechanisms. Experientia. 1992 Feb 15;48(2):158–172. doi: 10.1007/BF01923509. [DOI] [PubMed] [Google Scholar]
- Seglen P. O., Gordon P. B., Holen I., Høyvik H. Hepatocytic autophagy. Biomed Biochim Acta. 1991;50(4-6):373–381. [PubMed] [Google Scholar]
- Seglen P. O., Gordon P. B., Tolleshaug H., Høyvik H. Use of [3H]raffinose as a specific probe of autophagic sequestration. Exp Cell Res. 1986 Jan;162(1):273–277. doi: 10.1016/0014-4827(86)90446-5. [DOI] [PubMed] [Google Scholar]
- Seglen P. O. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29–83. doi: 10.1016/s0091-679x(08)61797-5. [DOI] [PubMed] [Google Scholar]
- Suganuma M., Fujiki H., Suguri H., Yoshizawa S., Hirota M., Nakayasu M., Ojika M., Wakamatsu K., Yamada K., Sugimura T. Okadaic acid: an additional non-phorbol-12-tetradecanoate-13-acetate-type tumor promoter. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1768–1771. doi: 10.1073/pnas.85.6.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokumitsu H., Chijiwa T., Hagiwara M., Mizutani A., Terasawa M., Hidaka H. KN-62, 1-[N,O-bis(5-isoquinolinesulfonyl)-N-methyl-L-tyrosyl]-4-phenylpiperazi ne, a specific inhibitor of Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 1990 Mar 15;265(8):4315–4320. [PubMed] [Google Scholar]
- Twomey B., Muid R. E., Nixon J. S., Sedgwick A. D., Wilkinson S. E., Dale M. M. The effect of new potent selective inhibitors of protein kinase C on the neutrophil respiratory burst. Biochem Biophys Res Commun. 1990 Sep 28;171(3):1087–1092. doi: 10.1016/0006-291x(90)90795-o. [DOI] [PubMed] [Google Scholar]
- Vallano M. L., Goldenring J. R., Lasher R. S., Delorenzo R. J. Association of calcium/calmodulin-dependent kinase with cytoskeletal preparations: phosphorylation of tubulin, neurofilament, and microtubule-associated proteins. Ann N Y Acad Sci. 1986;466:357–374. doi: 10.1111/j.1749-6632.1986.tb38406.x. [DOI] [PubMed] [Google Scholar]
- Volonté C., Rukenstein A., Loeb D. M., Greene L. A. Differential inhibition of nerve growth factor responses by purine analogues: correlation with inhibition of a nerve growth factor-activated protein kinase. J Cell Biol. 1989 Nov;109(5):2395–2403. doi: 10.1083/jcb.109.5.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaish P., Gazit A., Gilon C., Levitzki A. Blocking of EGF-dependent cell proliferation by EGF receptor kinase inhibitors. Science. 1988 Nov 11;242(4880):933–935. doi: 10.1126/science.3263702. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Fukunaga K., Tanaka E., Miyamoto E. Ca2+- and calmodulin-dependent phosphorylation of microtubule-associated protein 2 and tau factor, and inhibition of microtubule assembly. J Neurochem. 1983 Oct;41(4):1119–1125. doi: 10.1111/j.1471-4159.1983.tb09060.x. [DOI] [PubMed] [Google Scholar]
- Yamauchi T., Fujisawa H. Phosphorylation of microtubule-associated protein 2 by calmodulin-dependent protein kinase (Kinase II) which occurs only in the brain tissues. Biochem Biophys Res Commun. 1982 Dec 15;109(3):975–981. doi: 10.1016/0006-291x(82)92035-6. [DOI] [PubMed] [Google Scholar]
- Zandomeni R. O. Kinetics of inhibition by 5,6-dichloro-1-beta-D-ribofuranosylbenzimidazole on calf thymus casein kinase II. Biochem J. 1989 Sep 1;262(2):469–473. doi: 10.1042/bj2620469. [DOI] [PMC free article] [PubMed] [Google Scholar]


