Abstract
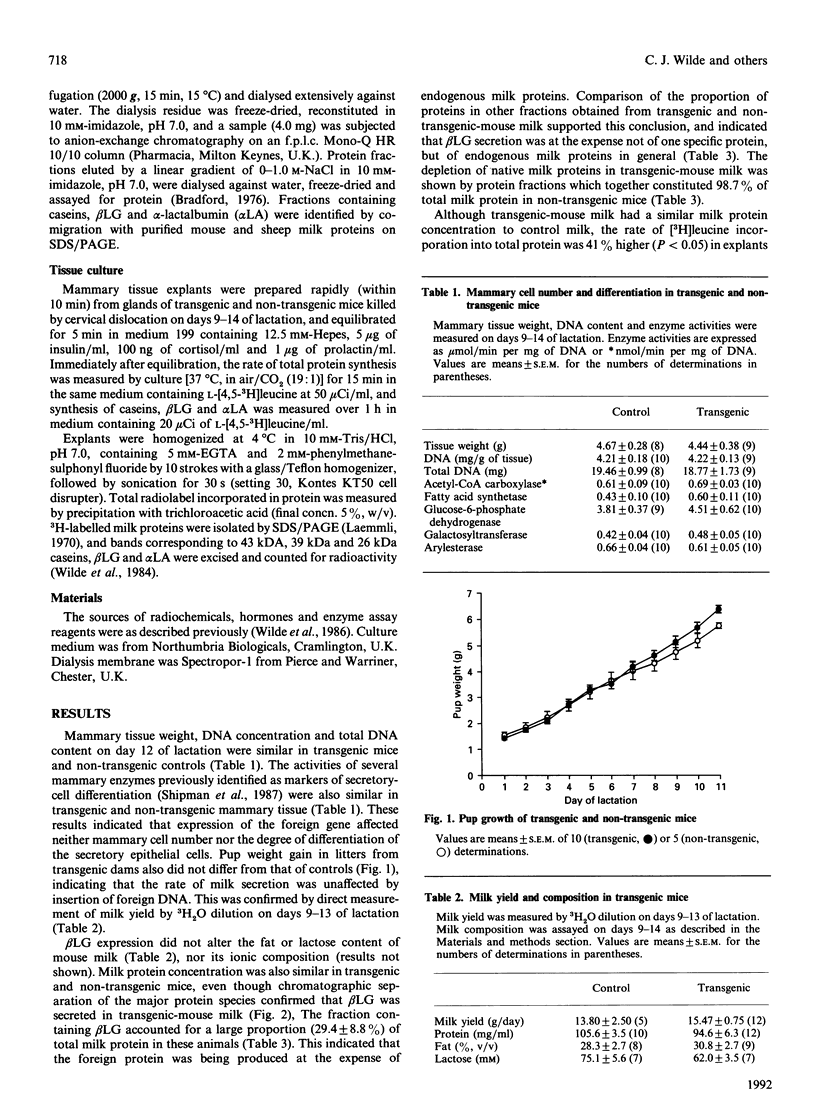
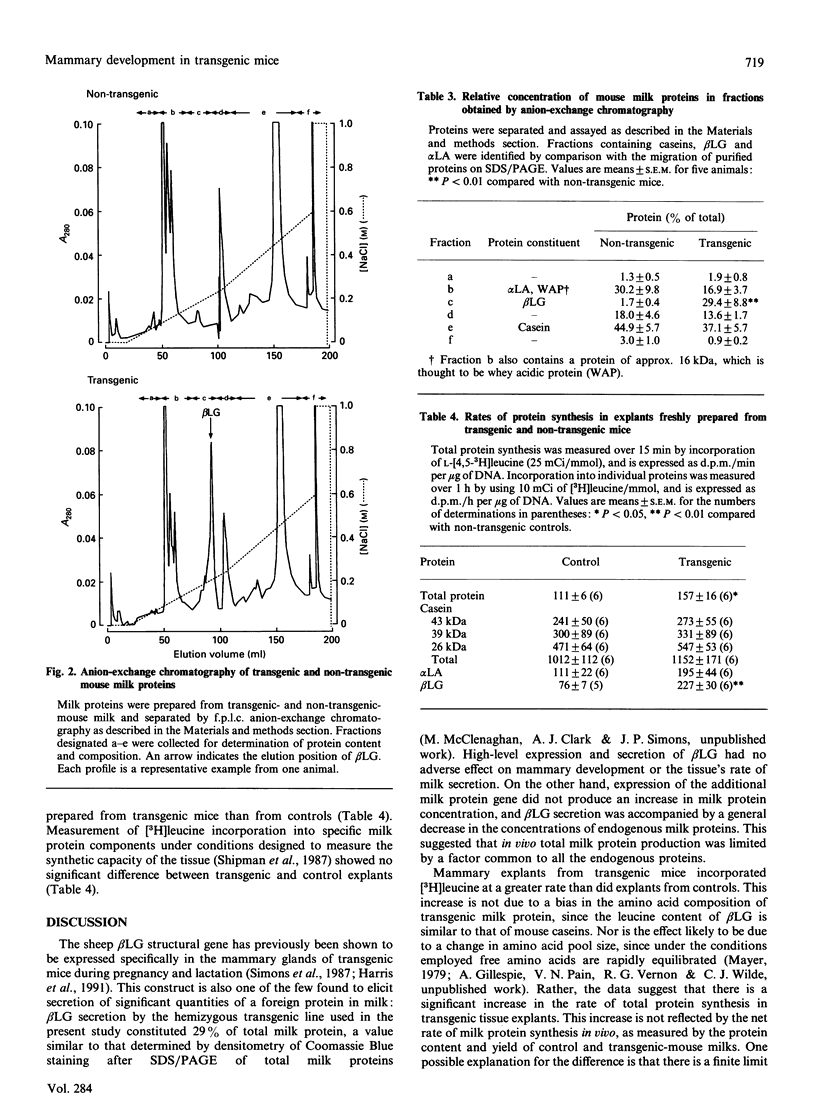
Mammary development and milk secretion were studied in transgenic mice which exhibited mammary tissue-specific expression of the sheep beta-lactoglobulin gene, and secreted significant quantities of the foreign protein in milk. Mammary development was unaffected by transgenesis. Tissue DNA content and the activities of several key enzyme markers of cell differentiation were similar in transgenic mice and non-transgenic controls. Milk yield, whether estimated by pup weight gain or measured by a 3H2O-dilution method, was unchanged by foreign gene expression. Gross milk composition, including milk protein concentration, was also similar in transgenic and non-transgenic animals, even though beta-lactoglobulin accounted for 29% of total milk protein. Therefore the foreign gene product was synthesized at the expense of endogenous milk proteins. However, transgenic mammary tissue in vitro exhibited a significantly higher rate of total protein synthesis than did control tissue. This suggested that a factor limiting milk protein synthesis or secretion in transgenic mice in vivo may have been removed by short-term explant culture of mammary tissue. The results emphasize that the use of transgenesis for manipulating milk composition may depend not only on high-level mammary-specific expression of the foreign gene, but also on the biosynthetic capacity of the mammary gland itself.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archibald A. L., McClenaghan M., Hornsey V., Simons J. P., Clark A. J. High-level expression of biologically active human alpha 1-antitrypsin in the milk of transgenic mice. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5178–5182. doi: 10.1073/pnas.87.13.5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Clark A. J., Ali S., Archibald A. L., Bessos H., Brown P., Harris S., McClenaghan M., Prowse C., Simons J. P., Whitelaw C. B. The molecular manipulation of milk composition. Genome. 1989;31(2):950–955. doi: 10.1139/g89-166. [DOI] [PubMed] [Google Scholar]
- Devinoy E., Houdebine L. M., Delouis C. Role of prolactin and glucocorticoids in the expression of casein genes in rabbit mammary gland organ culture. Quantification of casein mRNA. Biochim Biophys Acta. 1978 Feb 16;517(2):360–366. doi: 10.1016/0005-2787(78)90202-2. [DOI] [PubMed] [Google Scholar]
- Harris S., McClenaghan M., Simons J. P., Ali S., Clark A. J. Developmental regulation of the sheep beta-lactoglobulin gene in the mammary gland of transgenic mice. Dev Genet. 1991;12(4):299–307. doi: 10.1002/dvg.1020120407. [DOI] [PubMed] [Google Scholar]
- Hennighausen L. The mammary gland as a bioreactor: production of foreign proteins in milk. Protein Expr Purif. 1990 Sep;1(1):3–8. doi: 10.1016/1046-5928(90)90037-y. [DOI] [PubMed] [Google Scholar]
- Houdebine L. M. Effects of prolactin and progesterone on expression of casein genes. Titration of casein mRNA by hybridization with complementary DNA. Eur J Biochem. 1976 Sep;68(1):219–225. doi: 10.1111/j.1432-1033.1976.tb10781.x. [DOI] [PubMed] [Google Scholar]
- Knight C. H., Maltz E., Docherty A. H. Milk yield and composition in mice: effects of litter size and lactation number. Comp Biochem Physiol A Comp Physiol. 1986;84(1):127–133. doi: 10.1016/0300-9629(86)90054-x. [DOI] [PubMed] [Google Scholar]
- Kuhn N. J. The lactose and neuraminlactose content of rat milk and mammary tissue. Biochem J. 1972 Nov;130(1):177–180. doi: 10.1042/bj1300177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee K. F., DeMayo F. J., Atiee S. H., Rosen J. M. Tissue-specific expression of the rat beta-casein gene in transgenic mice. Nucleic Acids Res. 1988 Feb 11;16(3):1027–1041. doi: 10.1093/nar/16.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer R. J. Hormonal factors in lipogenesis in mammary gland. Vitam Horm. 1978;36:101–163. doi: 10.1016/s0083-6729(08)60983-8. [DOI] [PubMed] [Google Scholar]
- Pittius C. W., Hennighausen L., Lee E., Westphal H., Nicols E., Vitale J., Gordon K. A milk protein gene promoter directs the expression of human tissue plasminogen activator cDNA to the mammary gland in transgenic mice. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5874–5878. doi: 10.1073/pnas.85.16.5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razooki Hasan H., White D. A., Mayer R. J. Extensive destruction of newly synthesized casein in mammary explants in organ culture. Biochem J. 1982 Jan 15;202(1):133–138. doi: 10.1042/bj2020133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipman L. J., Docherty A. H., Knight C. H., Wilde C. J. Metabolic adaptations in mouse mammary gland during a normal lactation cycle and in extended lactation. Q J Exp Physiol. 1987 Jul;72(3):303–311. doi: 10.1113/expphysiol.1987.sp003076. [DOI] [PubMed] [Google Scholar]
- Simons J. P., McClenaghan M., Clark A. J. Alteration of the quality of milk by expression of sheep beta-lactoglobulin in transgenic mice. Nature. 1987 Aug 6;328(6130):530–532. doi: 10.1038/328530a0. [DOI] [PubMed] [Google Scholar]
- Wall R. J., Pursel V. G., Shamay A., McKnight R. A., Pittius C. W., Hennighausen L. High-level synthesis of a heterologous milk protein in the mammary glands of transgenic swine. Proc Natl Acad Sci U S A. 1991 Mar 1;88(5):1696–1700. doi: 10.1073/pnas.88.5.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitelaw C. B., Archibald A. L., Harris S., McClenaghan M., Simons J. P., Clark A. J. Targeting expression to the mammary gland: intronic sequences can enhance the efficiency of gene expression in transgenic mice. Transgenic Res. 1991 Dec;1(1):3–13. doi: 10.1007/BF02512991. [DOI] [PubMed] [Google Scholar]
- Wilde C. J., Addey C. V., Knight C. H. Regulation of intracellular casein degradation by secreted milk proteins. Biochim Biophys Acta. 1989 Sep 15;992(3):315–319. doi: 10.1016/0304-4165(89)90090-1. [DOI] [PubMed] [Google Scholar]
- Wilde C. J., Hasan H. R., Mayer R. J. Comparison of collagen gels and mammary extracellular matrix as substrata for study of terminal differentiation in rabbit mammary epithelial cells. Exp Cell Res. 1984 Apr;151(2):519–532. doi: 10.1016/0014-4827(84)90400-2. [DOI] [PubMed] [Google Scholar]
- Wilde C. J., Henderson A. J., Knight C. H. Metabolic adaptations in goat mammary tissue during pregnancy and lactation. J Reprod Fertil. 1986 Jan;76(1):289–298. doi: 10.1530/jrf.0.0760289. [DOI] [PubMed] [Google Scholar]
- Wilde C. J., Kerr M. A., Calvert D. T. Intracellular degradation of newly synthesized casein in perfused rat mammary gland. Exp Physiol. 1991 Jul;76(4):533–537. doi: 10.1113/expphysiol.1991.sp003519. [DOI] [PubMed] [Google Scholar]
- Wilde C. J., Knight C. H. Degradation of newly-synthesised casein in mammary explants from pregnant and lactating goats. Comp Biochem Physiol B. 1986;84(2):197–201. doi: 10.1016/0305-0491(86)90205-1. [DOI] [PubMed] [Google Scholar]



