Abstract
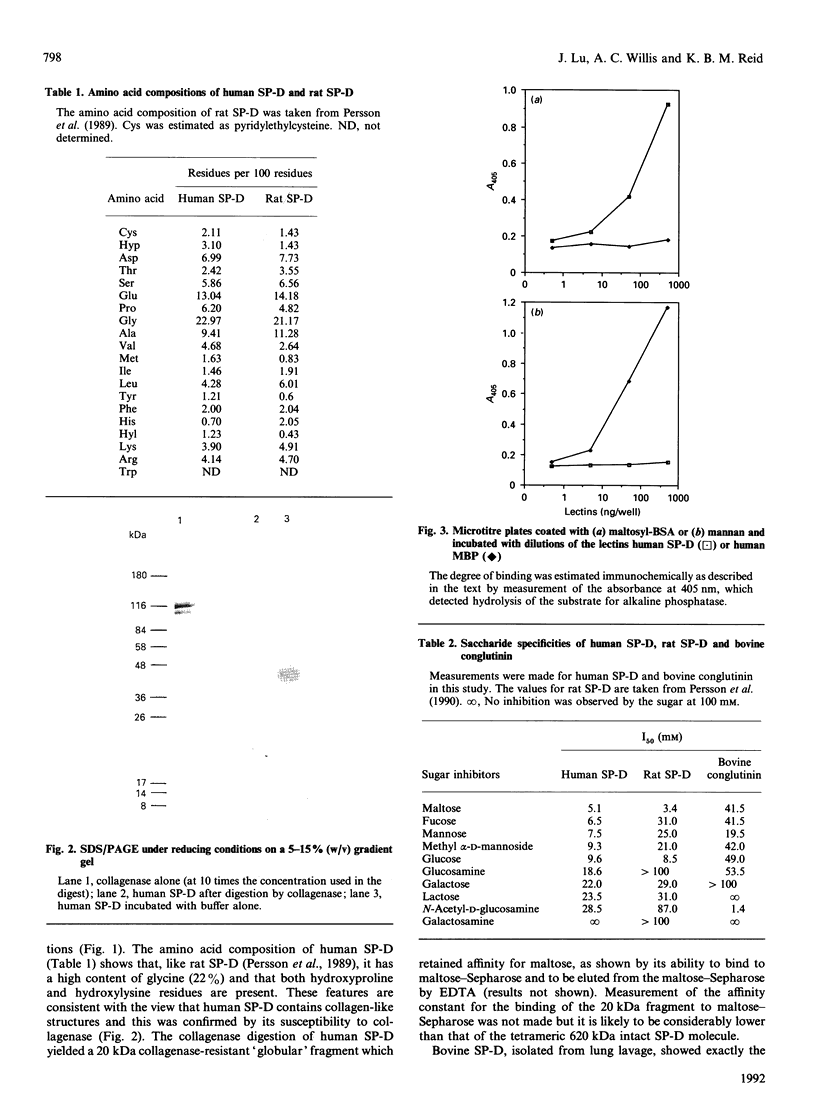
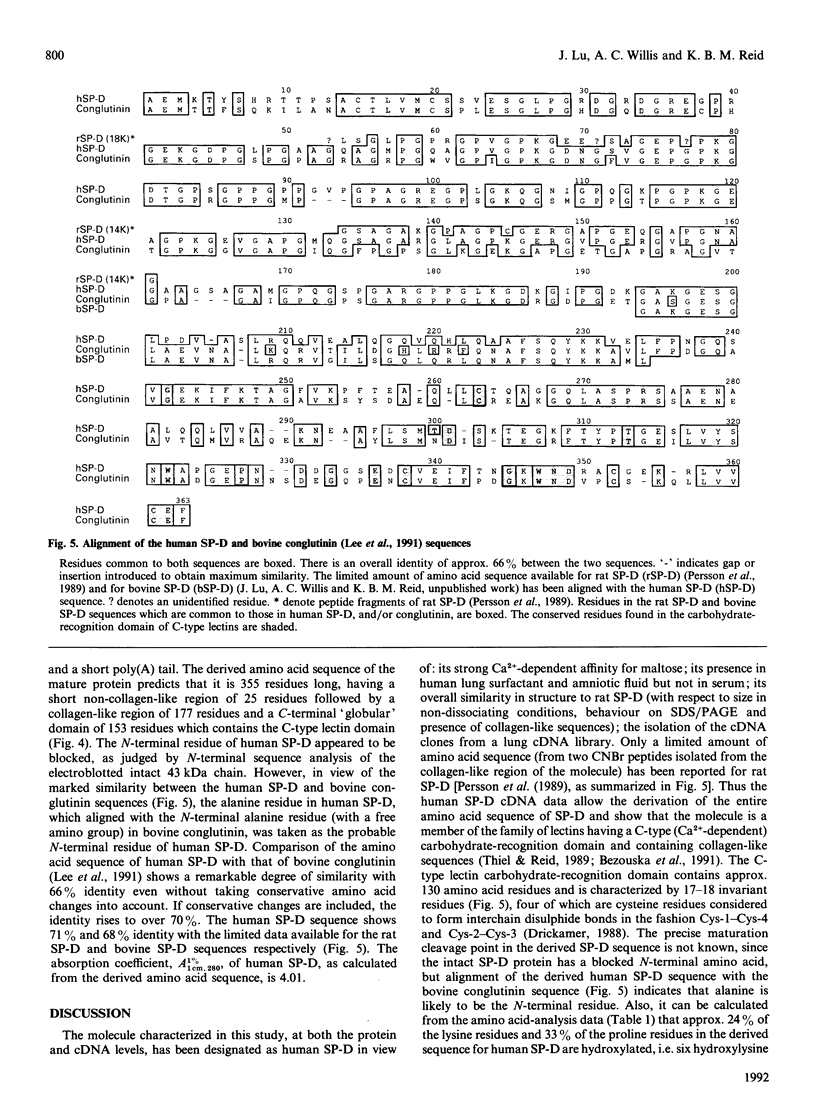
Human pulmonary surfactant protein D (SP-D) was identified in lung lavage by its similarity to rat SP-D in both its molecular mass and its Ca(2+)-dependent-binding affinity for maltose [Persson, Chang & Crouch (1990) J. Biol. Chem. 265, 5755-5760]. For structural studies, human SP-D was isolated from amniotic fluid by affinity chromatography on maltose-Sepharose followed by f.p.l.c. on Superose 6, which showed it to have a molecular mass of approx. 620 kDa in non-dissociating conditions. On SDS/PAGE the human SP-D behaved as a single band of 150 kDa or 43 kDa in non-reducing or reducing conditions respectively. The presence of a high concentration of glycine (22%), hydroxyproline and hydroxylysine in the amino acid composition of human SP-D indicated that it contained collagen-like structure. Collagenase digestion yielded a 20 kDa collagenase-resistant globular fragment which retained affinity for maltose. Use of maltosyl-BSA as a neoglycoprotein ligand in a solid-phase binding assay showed that human SP-D has a similar carbohydrate-binding specificity to rat SP-D, but a clearly distinct specificity from that of other lectins, such as conglutinin, for a range of simple saccharides. Amino acid sequence analysis established the presence of collagen-like Gly-Xaa-Yaa triplets in human SP-D and also provided sequence data from the globular region of the molecule which was used in the synthesis of oligonucleotide probes. Screening of a human lung cDNA library with the oligonucleotide probes, and also with rabbit anti-(human SP-D), allowed the isolation of two cDNA clones which overlap to give the full coding sequence of human SP-D. The derived amino acid sequence indicates that the mature human SP-D polypeptide chain is 355 residues long, having a short non-collagen-like N-terminal section of 25 residues, followed by a collagen-like region of 177 residues and a C-terminal C-type lectin domain of 153 residues. Comparison of the human SP-D and bovine serum conglutinin amino acid sequences indicated that they showed 66% identity despite their marked differences in carbohydrate specificity.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baritussio A. G., Magoon M. W., Goerke J., Clements J. A. Precursor-product relationship between rabbit type II cell lamellar bodies and alveolar surface-active material. Surfactant turnover time. Biochim Biophys Acta. 1981 Dec 23;666(3):382–393. doi: 10.1016/0005-2760(81)90297-6. [DOI] [PubMed] [Google Scholar]
- Bezouska K., Crichlow G. V., Rose J. M., Taylor M. E., Drickamer K. Evolutionary conservation of intron position in a subfamily of genes encoding carbohydrate-recognition domains. J Biol Chem. 1991 Jun 25;266(18):11604–11609. [PubMed] [Google Scholar]
- Childs R. A., Drickamer K., Kawasaki T., Thiel S., Mizuochi T., Feizi T. Neoglycolipids as probes of oligosaccharide recognition by recombinant and natural mannose-binding proteins of the rat and man. Biochem J. 1989 Aug 15;262(1):131–138. doi: 10.1042/bj2620131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford C., Brown N. R., Willis A. C. Investigation of the structural basis of the interaction of calpain II with phospholipid and with carbohydrate. Biochem J. 1990 Jan 15;265(2):575–579. doi: 10.1042/bj2650575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curstedt T., Jörnvall H., Robertson B., Bergman T., Berggren P. Two hydrophobic low-molecular-mass protein fractions of pulmonary surfactant. Characterization and biophysical activity. Eur J Biochem. 1987 Oct 15;168(2):255–262. doi: 10.1111/j.1432-1033.1987.tb13414.x. [DOI] [PubMed] [Google Scholar]
- Davis A. E., 3rd, Lachmann P. J. Bovine conglutinin is a collagen-like protein. Biochemistry. 1984 May 8;23(10):2139–2144. doi: 10.1021/bi00305a006. [DOI] [PubMed] [Google Scholar]
- Dobbs L. G., Wright J. R., Hawgood S., Gonzalez R., Venstrom K., Nellenbogen J. Pulmonary surfactant and its components inhibit secretion of phosphatidylcholine from cultured rat alveolar type II cells. Proc Natl Acad Sci U S A. 1987 Feb;84(4):1010–1014. doi: 10.1073/pnas.84.4.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drickamer K. Two distinct classes of carbohydrate-recognition domains in animal lectins. J Biol Chem. 1988 Jul 15;263(20):9557–9560. [PubMed] [Google Scholar]
- Fisher J. H., Shannon J. M., Hofmann T., Mason R. J. Nucleotide and deduced amino acid sequence of the hydrophobic surfactant protein SP-C from rat: expression in alveolar type II cells and homology with SP-C from other species. Biochim Biophys Acta. 1989 May 1;995(3):225–230. doi: 10.1016/0167-4838(89)90040-x. [DOI] [PubMed] [Google Scholar]
- Floros J., Steinbrink R., Jacobs K., Phelps D., Kriz R., Recny M., Sultzman L., Jones S., Taeusch H. W., Frank H. A. Isolation and characterization of cDNA clones for the 35-kDa pulmonary surfactant-associated protein. J Biol Chem. 1986 Jul 5;261(19):9029–9033. [PubMed] [Google Scholar]
- Glasser S. W., Korfhagen T. R., Weaver T., Pilot-Matias T., Fox J. L., Whitsett J. A. cDNA and deduced amino acid sequence of human pulmonary surfactant-associated proteolipid SPL(Phe). Proc Natl Acad Sci U S A. 1987 Jun;84(12):4007–4011. doi: 10.1073/pnas.84.12.4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haagsman H. P., Hawgood S., Sargeant T., Buckley D., White R. T., Drickamer K., Benson B. J. The major lung surfactant protein, SP 28-36, is a calcium-dependent, carbohydrate-binding protein. J Biol Chem. 1987 Oct 15;262(29):13877–13880. [PubMed] [Google Scholar]
- Hawgood S., Benson B. J., Schilling J., Damm D., Clements J. A., White R. T. Nucleotide and amino acid sequences of pulmonary surfactant protein SP 18 and evidence for cooperation between SP 18 and SP 28-36 in surfactant lipid adsorption. Proc Natl Acad Sci U S A. 1987 Jan;84(1):66–70. doi: 10.1073/pnas.84.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinz H. P. Biological functions of C1q expressed by conformational changes. Behring Inst Mitt. 1989 Jul;(84):20–31. [PubMed] [Google Scholar]
- King R. J., Klass D. J., Gikas E. G., Clements J. A. Isolation of apoproteins from canine surface active material. Am J Physiol. 1973 Apr;224(4):788–795. doi: 10.1152/ajplegacy.1973.224.4.788. [DOI] [PubMed] [Google Scholar]
- King R. J., Phillips M. C., Horowitz P. M., Dang S. C. Interaction between the 35 kDa apolipoprotein of pulmonary surfactant and saturated phosphatidylcholines. Effects of temperature. Biochim Biophys Acta. 1986 Oct 24;879(1):1–13. doi: 10.1016/0005-2760(86)90259-6. [DOI] [PubMed] [Google Scholar]
- King R. J., Ruch J., Gikas E. G., Platzker A. C., Creasy R. K. Appearance of paoproteins of pulmonary surfactant in human amniotic fluid. J Appl Physiol. 1975 Nov;39(5):735–741. doi: 10.1152/jappl.1975.39.5.735. [DOI] [PubMed] [Google Scholar]
- Kuroki Y., Mason R. J., Voelker D. R. Pulmonary surfactant apoprotein A structure and modulation of surfactant secretion by rat alveolar type II cells. J Biol Chem. 1988 Mar 5;263(7):3388–3394. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee Y. M., Leiby K. R., Allar J., Paris K., Lerch B., Okarma T. B. Primary structure of bovine conglutinin, a member of the C-type animal lectin family. J Biol Chem. 1991 Feb 15;266(5):2715–2723. [PubMed] [Google Scholar]
- Loveless R. W., Feizi T., Childs R. A., Mizuochi T., Stoll M. S., Oldroyd R. G., Lachmann P. J. Bovine serum conglutinin is a lectin which binds non-reducing terminal N-acetylglucosamine, mannose and fucose residues. Biochem J. 1989 Feb 15;258(1):109–113. doi: 10.1042/bj2580109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MACKLIN C. C. The pulmonary alveolar mucoid film and the pneumonocytes. Lancet. 1954 May 29;266(6822):1099–1104. doi: 10.1016/s0140-6736(54)92154-6. [DOI] [PubMed] [Google Scholar]
- Malhotra R., Thiel S., Reid K. B., Sim R. B. Human leukocyte C1q receptor binds other soluble proteins with collagen domains. J Exp Med. 1990 Sep 1;172(3):955–959. doi: 10.1084/jem.172.3.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogasawara Y., Kuroki Y., Shiratori M., Shimizu H., Miyamura K., Akino T. Ontogeny of surfactant apoprotein D, SP-D, in the rat lung. Biochim Biophys Acta. 1991 Jun 3;1083(3):252–256. doi: 10.1016/0005-2760(91)90079-w. [DOI] [PubMed] [Google Scholar]
- Persson A., Chang D., Crouch E. Surfactant protein D is a divalent cation-dependent carbohydrate-binding protein. J Biol Chem. 1990 Apr 5;265(10):5755–5760. [PubMed] [Google Scholar]
- Persson A., Chang D., Rust K., Moxley M., Longmore W., Crouch E. Purification and biochemical characterization of CP4 (SP-D), a collagenous surfactant-associated protein. Biochemistry. 1989 Jul 25;28(15):6361–6367. doi: 10.1021/bi00441a031. [DOI] [PubMed] [Google Scholar]
- Persson A., Rust K., Chang D., Moxley M., Longmore W., Crouch E. CP4: a pneumocyte-derived collagenous surfactant-associated protein. Evidence for heterogeneity of collagenous surfactant proteins. Biochemistry. 1988 Nov 15;27(23):8576–8584. doi: 10.1021/bi00423a011. [DOI] [PubMed] [Google Scholar]
- Phelps D. S., Floros J. Localization of surfactant protein synthesis in human lung by in situ hybridization. Am Rev Respir Dis. 1988 Apr;137(4):939–942. doi: 10.1164/ajrccm/137.4.939. [DOI] [PubMed] [Google Scholar]
- Rice W. R., Ross G. F., Singleton F. M., Dingle S., Whitsett J. A. Surfactant-associated protein inhibits phospholipid secretion from type II cells. J Appl Physiol (1985) 1987 Aug;63(2):692–698. doi: 10.1152/jappl.1987.63.2.692. [DOI] [PubMed] [Google Scholar]
- Rust K., Grosso L., Zhang V., Chang D., Persson A., Longmore W., Cai G. Z., Crouch E. Human surfactant protein D: SP-D contains a C-type lectin carbohydrate recognition domain. Arch Biochem Biophys. 1991 Oct;290(1):116–126. doi: 10.1016/0003-9861(91)90597-c. [DOI] [PubMed] [Google Scholar]
- Ryan R. M., Morris R. E., Rice W. R., Ciraolo G., Whitsett J. A. Binding and uptake of pulmonary surfactant protein (SP-A) by pulmonary type II epithelial cells. J Histochem Cytochem. 1989 Apr;37(4):429–440. doi: 10.1177/37.4.2926121. [DOI] [PubMed] [Google Scholar]
- Schwartz B. A., Gray G. R. Proteins containing reductively aminated disaccharides. Synthesis and chemical characterization. Arch Biochem Biophys. 1977 Jun;181(2):542–549. doi: 10.1016/0003-9861(77)90261-2. [DOI] [PubMed] [Google Scholar]
- Shimizu H., Fisher J. H., Papst P., Benson B., Lau K., Mason R. J., Voelker D. R. Primary structure of rat pulmonary surfactant protein D. cDNA and deduced amino acid sequence. J Biol Chem. 1992 Jan 25;267(3):1853–1857. [PubMed] [Google Scholar]
- Suzuki Y., Curstedt T., Grossmann G., Kobayashi T., Nilsson R., Nohara K., Robertson B. The role of the low-molecular weight (less than or equal to 15,000 daltons) apoproteins of pulmonary surfactant. Eur J Respir Dis. 1986 Nov;69(5):336–345. [PubMed] [Google Scholar]
- Suzuki Y., Fujita Y., Kogishi K. Reconstitution of tubular myelin from synthetic lipids and proteins associated with pig pulmonary surfactant. Am Rev Respir Dis. 1989 Jul;140(1):75–81. doi: 10.1164/ajrccm/140.1.75. [DOI] [PubMed] [Google Scholar]
- Takahashi A., Fujiwara T. Proteolipid in bovine lung surfactant: its role in surfactant function. Biochem Biophys Res Commun. 1986 Mar 13;135(2):527–532. doi: 10.1016/0006-291x(86)90026-4. [DOI] [PubMed] [Google Scholar]
- Tenner A. J., Robinson S. L., Borchelt J., Wright J. R. Human pulmonary surfactant protein (SP-A), a protein structurally homologous to C1q, can enhance FcR- and CR1-mediated phagocytosis. J Biol Chem. 1989 Aug 15;264(23):13923–13928. [PubMed] [Google Scholar]
- Thiel S., Reid K. B. Structures and functions associated with the group of mammalian lectins containing collagen-like sequences. FEBS Lett. 1989 Jun 19;250(1):78–84. doi: 10.1016/0014-5793(89)80689-1. [DOI] [PubMed] [Google Scholar]
- Voss T., Eistetter H., Schäfer K. P., Engel J. Macromolecular organization of natural and recombinant lung surfactant protein SP 28-36. Structural homology with the complement factor C1q. J Mol Biol. 1988 May 5;201(1):219–227. doi: 10.1016/0022-2836(88)90448-2. [DOI] [PubMed] [Google Scholar]
- Walker S. R., Williams M. C., Benson B. Immunocytochemical localization of the major surfactant apoproteins in type II cells, Clara cells, and alveolar macrophages of rat lung. J Histochem Cytochem. 1986 Sep;34(9):1137–1148. doi: 10.1177/34.9.2426341. [DOI] [PubMed] [Google Scholar]
- Warr R. G., Hawgood S., Buckley D. I., Crisp T. M., Schilling J., Benson B. J., Ballard P. L., Clements J. A., White R. T. Low molecular weight human pulmonary surfactant protein (SP5): isolation, characterization, and cDNA and amino acid sequences. Proc Natl Acad Sci U S A. 1987 Nov;84(22):7915–7919. doi: 10.1073/pnas.84.22.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White R. T., Damm D., Miller J., Spratt K., Schilling J., Hawgood S., Benson B., Cordell B. Isolation and characterization of the human pulmonary surfactant apoprotein gene. 1985 Sep 26-Oct 2Nature. 317(6035):361–363. doi: 10.1038/317361a0. [DOI] [PubMed] [Google Scholar]
- Young N. M., Leon M. A. The carbohydrate specificity of conglutinin and its homology to proteins in the hepatic lectin family. Biochem Biophys Res Commun. 1987 Mar 13;143(2):645–651. doi: 10.1016/0006-291x(87)91402-1. [DOI] [PubMed] [Google Scholar]
- Young S. L., Kremers S. A., Apple J. S., Crapo J. D., Brumley G. W. Rat lung surfactant kinetics biochemical and morphometric correlation. J Appl Physiol Respir Environ Exerc Physiol. 1981 Aug;51(2):248–253. doi: 10.1152/jappl.1981.51.2.248. [DOI] [PubMed] [Google Scholar]




