Abstract
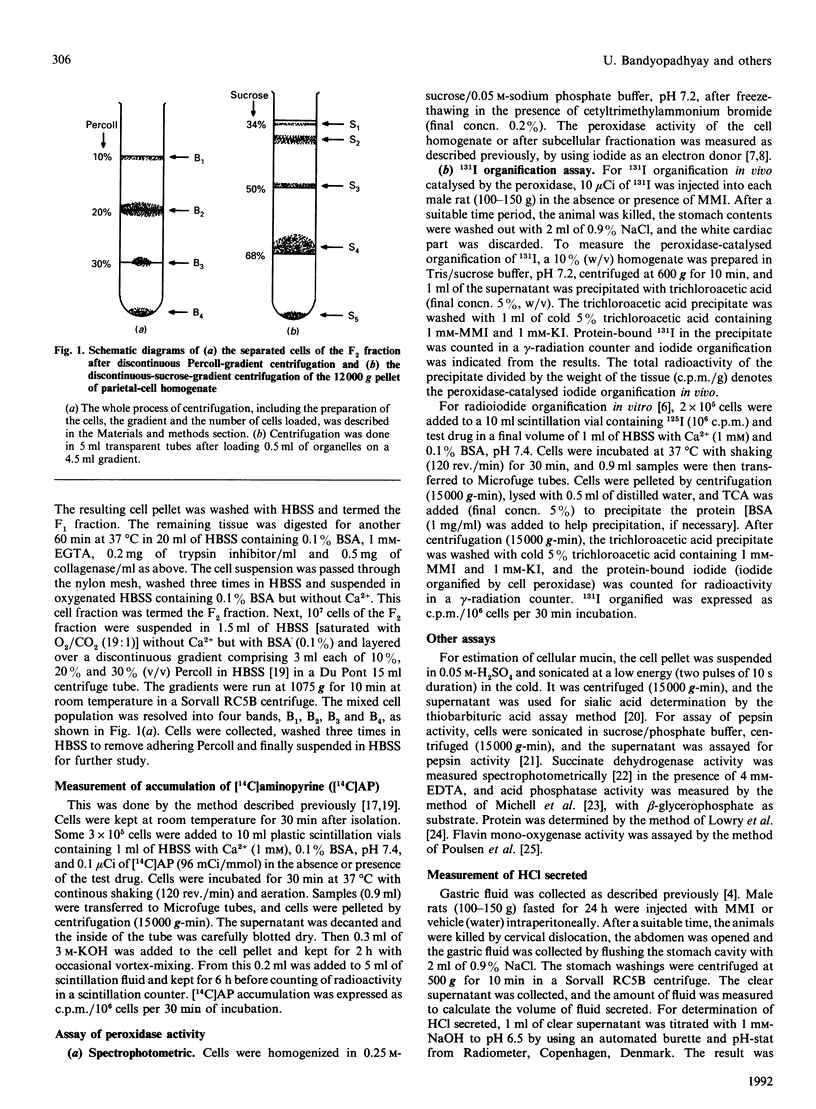
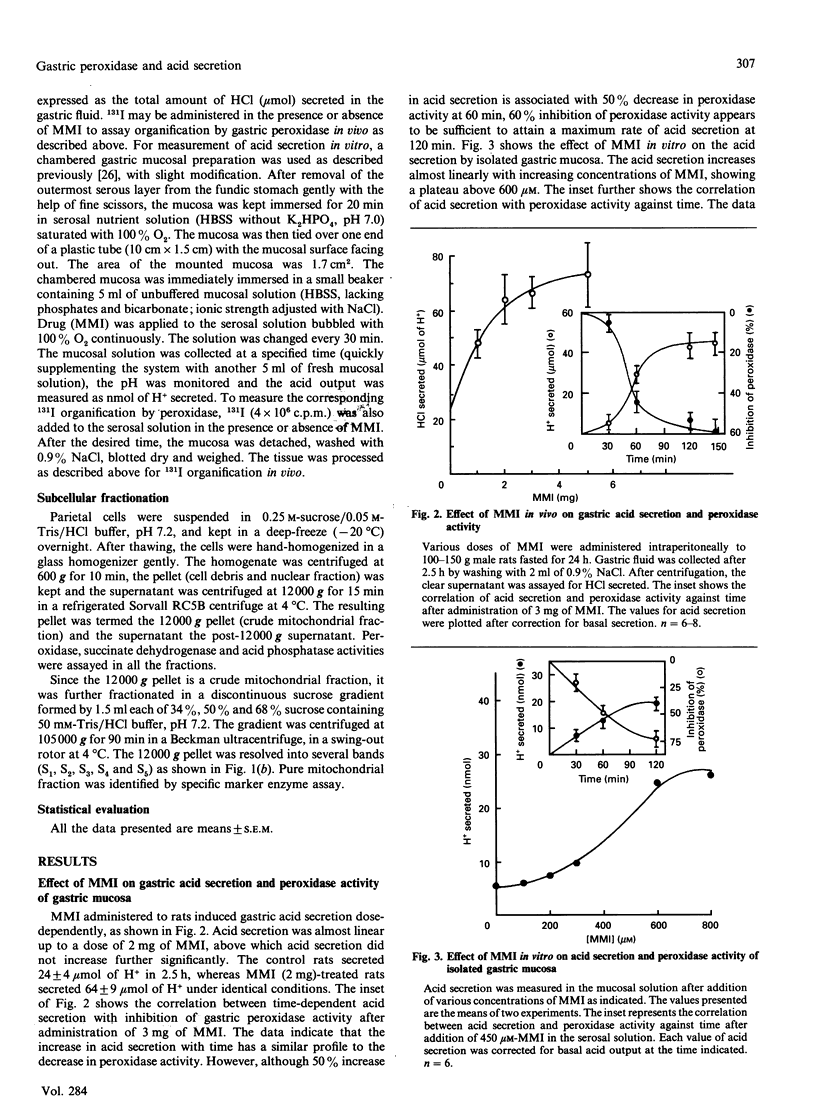
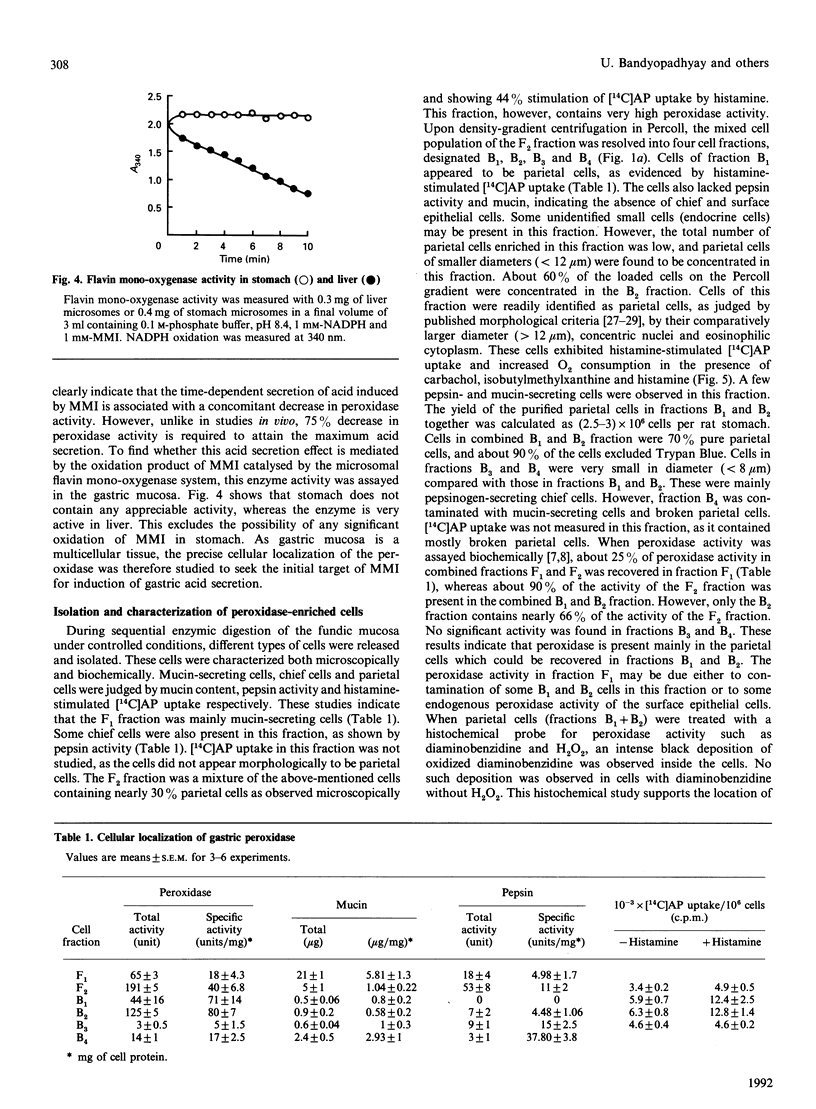
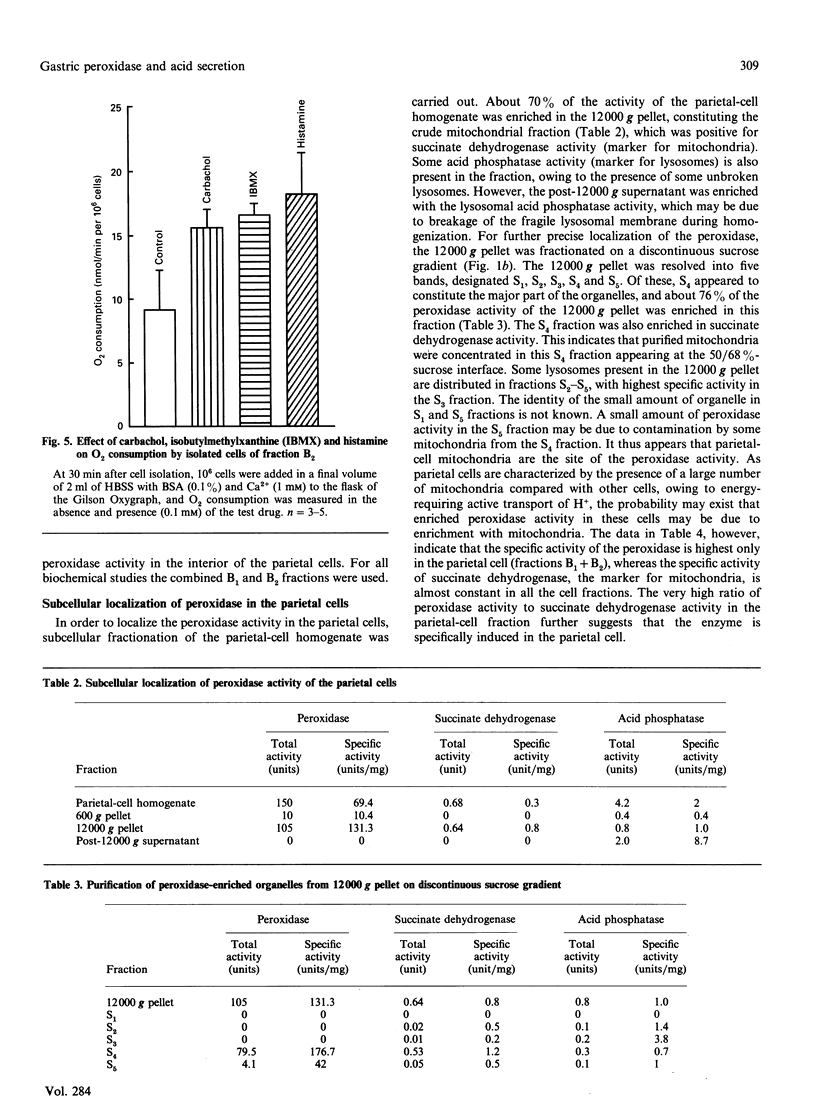
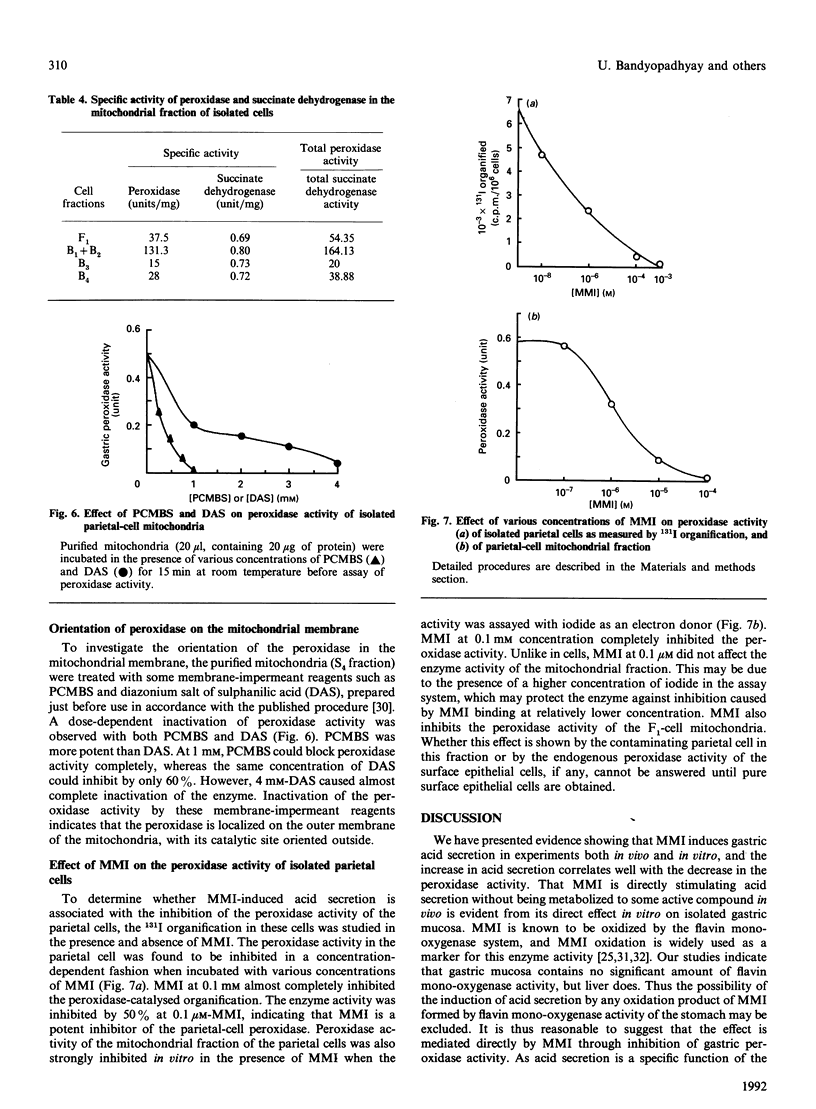
Mercaptomethylimidazole (MMI) is a potent inducer of gastric acid secretion which is associated with significant inhibition of peroxidase activity of rat gastric mucosa in vivo. A time-dependent increase in acid secretion correlates well with time-dependent decrease in the peroxidase activity. In a chamber experiment in vitro using isolated gastric mucosa, MMI stimulates acid secretion, showing an almost linear response up to 600 microM. The time-dependent increase in acid secretion is also correlated with time-dependent inhibition of the peroxidase activity. This effect is not mediated through oxidation of MMI by flavin-containing mono-oxygenase, which is absent from gastric mucosa. The peroxidase has been localized mainly in parietal cells isolated and purified from gastric mucosa by controlled digestion with collagenase followed by Percoll-density-gradient centrifugation. Peroxidase activity was further localized in the outer membrane of the purified mitochondria of the parietal cell by some membrane-impermeant reagents, indicating outward orientation of the enzyme. MMI can inhibit the peroxidase activity of both the parietal cell and its mitochondria in a concentration-dependent manner. The possible involvement of the parietal-cell peroxidase-H2O2 system in MMI-induced acid secretion may be suggested.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai I., Hirose H., Usuki C., Muramatsu M., Aihara H. Effects of indomethacin and cold-stress on gastric acid secretion and ulceration. The effects of anti-acid secretory agents in rats. Res Commun Chem Pathol Pharmacol. 1987 Sep;57(3):313–327. [PubMed] [Google Scholar]
- Armstrong C. P., Blower A. L. Non-steroidal anti-inflammatory drugs and life threatening complications of peptic ulceration. Gut. 1987 May;28(5):527–532. doi: 10.1136/gut.28.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee R. K., Bose A. K., Chakraborty T. K., De S. K., Datta A. G. Peroxidase-catalysed iodotyrosine formation in dispersed cells of mouse extrathyroidal tissues. J Endocrinol. 1985 Aug;106(2):159–165. doi: 10.1677/joe.0.1060159. [DOI] [PubMed] [Google Scholar]
- Banerjee R. K., Bose A. K., Chakravartty T. K., Datta A. G. Solubilisation & properties of mitochondrial peroxidase from mouse gastric mucosa. Indian J Biochem Biophys. 1982 Oct;19(5):324–329. [PubMed] [Google Scholar]
- Banerjee R. K. Nonsteroidal anti-inflammatory drugs inhibit gastric peroxidase activity. Biochim Biophys Acta. 1990 Jun 20;1034(3):275–280. doi: 10.1016/0304-4165(90)90050-7. [DOI] [PubMed] [Google Scholar]
- Berg H. C. Sulfanilic acid diazonium salt: a label for the outside of the human erythrocyte membrane. Biochim Biophys Acta. 1969 Jun 3;183(1):65–78. doi: 10.1016/0005-2736(69)90130-8. [DOI] [PubMed] [Google Scholar]
- Berglindh T., Helander H. F., Obrink K. J. Effects of secretagogues on oxygen consumption, aminopyrine accumulation and morphology in isolated gastric glands. Acta Physiol Scand. 1976 Aug;97(4):401–414. doi: 10.1111/j.1748-1716.1976.tb10281.x. [DOI] [PubMed] [Google Scholar]
- Bhattacharjee M., Bose A. K., Banerjee R. K. Histamine H2-receptor mediated stimulation of gastric acid secretion by mercaptomethylimidazole. Biochem Pharmacol. 1989 Mar 15;38(6):907–914. doi: 10.1016/0006-2952(89)90279-7. [DOI] [PubMed] [Google Scholar]
- DePierre J. W., Karnovsky M. L. Ecto-enzymes of the guinea pig polymorphonuclear leukocyte. I. Evidence for an ecto-adenosine monophosphatase, adenosine triphosphatase, and -p-nitrophenyl phosphates. J Biol Chem. 1974 Nov 25;249(22):7111–7120. [PubMed] [Google Scholar]
- De S. K., Banerjee R. K. Glucocorticoid effects on gastric peroxidase activity. Biochim Biophys Acta. 1984 Aug 21;800(3):233–241. doi: 10.1016/0304-4165(84)90401-x. [DOI] [PubMed] [Google Scholar]
- De S. K., Banerjee R. K. Purification, characterization and origin of rat gastric peroxidase. Eur J Biochem. 1986 Oct 15;160(2):319–325. doi: 10.1111/j.1432-1033.1986.tb09974.x. [DOI] [PubMed] [Google Scholar]
- De S. K., De M., Banerjee R. K. Localization and origin of the intestinal peroxidase--effect of adrenal glucocorticoids. J Steroid Biochem. 1986 Feb;24(2):629–635. doi: 10.1016/0022-4731(86)90130-5. [DOI] [PubMed] [Google Scholar]
- Ecknauer R., Dial E., Thompson W. J., Johnson L. R., Rosenfeld G. C. Isolated rat gastric parietal cells: cholinergic response and pharmacology. Life Sci. 1981 Feb 9;28(6):609–621. doi: 10.1016/0024-3205(81)90124-7. [DOI] [PubMed] [Google Scholar]
- Engler H., Taurog A., Dorris M. L. Preferential inhibition of thyroxine and 3,5,3'-triiodothyronine formation by propylthiouracil and methylmercaptoimidazole in thyroid peroxidase-catalyzed iodination of thyroglobulin. Endocrinology. 1982 Jan;110(1):190–197. doi: 10.1210/endo-110-1-190. [DOI] [PubMed] [Google Scholar]
- Foerder C. A., Klebanoff S. J., Shapiro B. M. Hydrogen peroxide production, chemiluminescence, and the respiratory burst of fertilization: interrelated events in early sea urchin development. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3183–3187. doi: 10.1073/pnas.75.7.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halter F., Müllener C., Kohler B., Saner U., Smith G. M. Die Wirkung von Kortikosteroiden auf die Magensäuresekretion der Ratte. Vorläufige Mitteilung. Schweiz Med Wochenschr. 1971 May 22;101(20):755–756. [PubMed] [Google Scholar]
- Helander H. F., Sundell G. W. Ultrastructure of inhibited parietal cells in the rat. Gastroenterology. 1984 Nov;87(5):1064–1071. [PubMed] [Google Scholar]
- Ito S., Munro D. R., Schofield G. C. Morphology of the isolated mouse oxyntic cell and some physiological parameters. Gastroenterology. 1977 Oct;73(4 Pt 2):887–898. [PubMed] [Google Scholar]
- Ivey K. J. Mechanisms of nonsteroidal anti-inflammatory drug-induced gastric damage. Actions of therapeutic agents. Am J Med. 1988 Feb 22;84(2A):41–48. doi: 10.1016/0002-9343(88)90253-7. [DOI] [PubMed] [Google Scholar]
- Jakoby W. B., Ziegler D. M. The enzymes of detoxication. J Biol Chem. 1990 Dec 5;265(34):20715–20718. [PubMed] [Google Scholar]
- Jensen M. S., Bainton D. F. Temporal changes in pH within the phagocytic vacuole of the polymorphonuclear neutrophilic leukocyte. J Cell Biol. 1973 Feb;56(2):379–388. doi: 10.1083/jcb.56.2.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLEBANOFF S. J. INACTIVATION OF ESTROGEN BY RAT UTERINE PREPARATIONS. Endocrinology. 1965 Feb;76:301–311. doi: 10.1210/endo-76-2-301. [DOI] [PubMed] [Google Scholar]
- Kay E., Eddy E. M., Shapiro B. M. Assembly of the fertilization membrane of the sea urchin: isolation of a divalent cation-dependent intermediate and its crosslinking in vitro. Cell. 1982 Jul;29(3):867–875. doi: 10.1016/0092-8674(82)90448-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lyttle C. R., Jellinck P. H. Metabolism of (4- 14 C)oestradiol by oestrogen-induced uterine peroxidase. Biochem J. 1972 Apr;127(3):481–487. doi: 10.1042/bj1270481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michell R. H., Karnovsky M. J., Karnovsky M. L. The distributions of some granule-associated enzymes in guinea-pig polymorphonuclear leucocytes. Biochem J. 1970 Jan;116(2):207–216. doi: 10.1042/bj1160207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison M., Allen P. Z. Lactoperoxidase: identification and isolation from Harderian and lacrimal glands. Science. 1966 Jun 17;152(3729):1626–1628. doi: 10.1126/science.152.3729.1626. [DOI] [PubMed] [Google Scholar]
- Ohtaki S., Nakagawa H., Nakamura M., Yamazaki I. Reactions of purified hog thyroid peroxidase with H2O2, tyrosine, and methylmercaptoimidazole (goitrogen) in comparison with bovine lactoperoxidase. J Biol Chem. 1982 Jan 25;257(2):761–766. [PubMed] [Google Scholar]
- Poulsen L. L., Hyslop R. M., Ziegler D. M. S-oxidation of thioureylenes catalyzed by a microsomal flavoprotein mixed-function oxidase. Biochem Pharmacol. 1974 Dec 15;23(24):3431–3440. doi: 10.1016/0006-2952(74)90346-3. [DOI] [PubMed] [Google Scholar]
- ROBERT A., NEZAMIS J. E. Ulcerogenic property of steroids. Proc Soc Exp Biol Med. 1958 Nov;99(2):443–447. doi: 10.3181/00379727-99-24378. [DOI] [PubMed] [Google Scholar]
- Reeves J. J., Stables R. Effects of indomethacin, piroxicam and selected prostanoids on gastric acid secretion by the rat isolated gastric mucosa. Br J Pharmacol. 1985 Nov;86(3):677–684. doi: 10.1111/j.1476-5381.1985.tb08945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romrell L. J., Coppe M. R., Munro D. R., Ito S. Isolation and separation of highly enriched fractions of viable mouse gastric parietal cells by velocity sedimentation. J Cell Biol. 1975 May;65(2):428–438. doi: 10.1083/jcb.65.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld G. C. Isolated parietal cells: adrenergic response and pharmacology. J Pharmacol Exp Ther. 1984 Jun;229(3):763–767. [PubMed] [Google Scholar]
- SBARRA A. J., KARNOVSKY M. L. The biochemical basis of phagocytosis. I. Metabolic changes during the ingestion of particles by polymorphonuclear leukocytes. J Biol Chem. 1959 Jun;234(6):1355–1362. [PubMed] [Google Scholar]
- SCHLAMOWITZ M., PETERSON L. U. Studies on the optimum pH for the action of pepsin on "native" and denatured bovine serum albumin and bovine hemoglobin. J Biol Chem. 1959 Dec;234:3137–3145. [PubMed] [Google Scholar]
- SINGER T. P., KEARNEY E. B. Determination of succinic dehydrogenase activity. Methods Biochem Anal. 1957;4:307–333. doi: 10.1002/9780470110201.ch9. [DOI] [PubMed] [Google Scholar]
- Slungaard A., Mahoney J. R., Jr Thiocyanate is the major substrate for eosinophil peroxidase in physiologic fluids. Implications for cytotoxicity. J Biol Chem. 1991 Mar 15;266(8):4903–4910. [PubMed] [Google Scholar]
- Tai A., Burton R. C., Warner N. L. Differential natural killer cell reactivity against T cell lymphomas by cells from normal or stimulated mice. J Immunol. 1980 Apr;124(4):1705–1711. [PubMed] [Google Scholar]
- Thompson W. J., Chang L. K., Rosenfeld G. C. Histamine regulation of adenylyl cyclase of enriched rat gastric parietal cells. Am J Physiol. 1981 Jan;240(1):G76–G84. doi: 10.1152/ajpgi.1981.240.1.G76. [DOI] [PubMed] [Google Scholar]
- Turner E., Somers C. E., Shapiro B. M. The relationship between a novel NAD(P)H oxidase activity of ovoperoxidase and the CN- -resistant respiratory burst that follows fertilization of sea urchin eggs. J Biol Chem. 1985 Oct 25;260(24):13163–13171. [PubMed] [Google Scholar]
- WALDER A. I., LUNSETH J. B. A technic for separation of the cells of the gastric mucosa. Proc Soc Exp Biol Med. 1963 Feb;112:494–496. doi: 10.3181/00379727-112-28086. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Ziegler D. M. Flavin-containing monooxygenases: enzymes adapted for multisubstrate specificity. Trends Pharmacol Sci. 1990 Aug;11(8):321–324. doi: 10.1016/0165-6147(90)90235-z. [DOI] [PubMed] [Google Scholar]


