Abstract
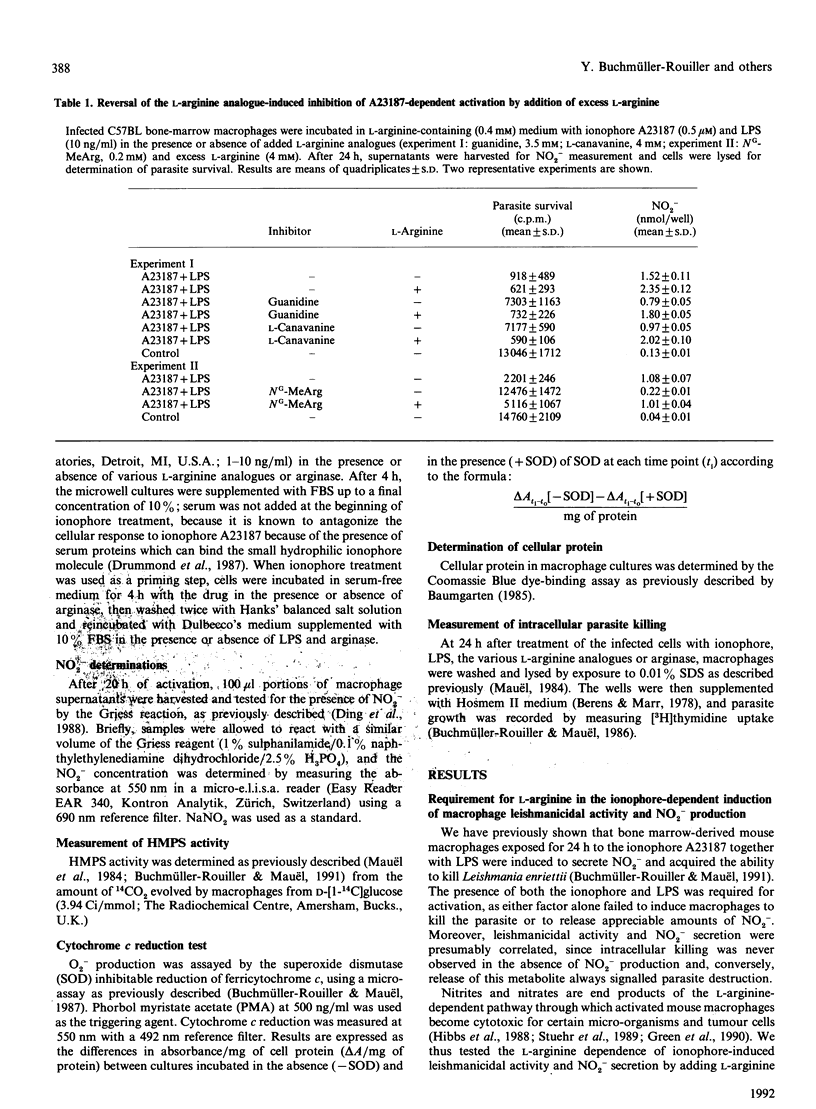
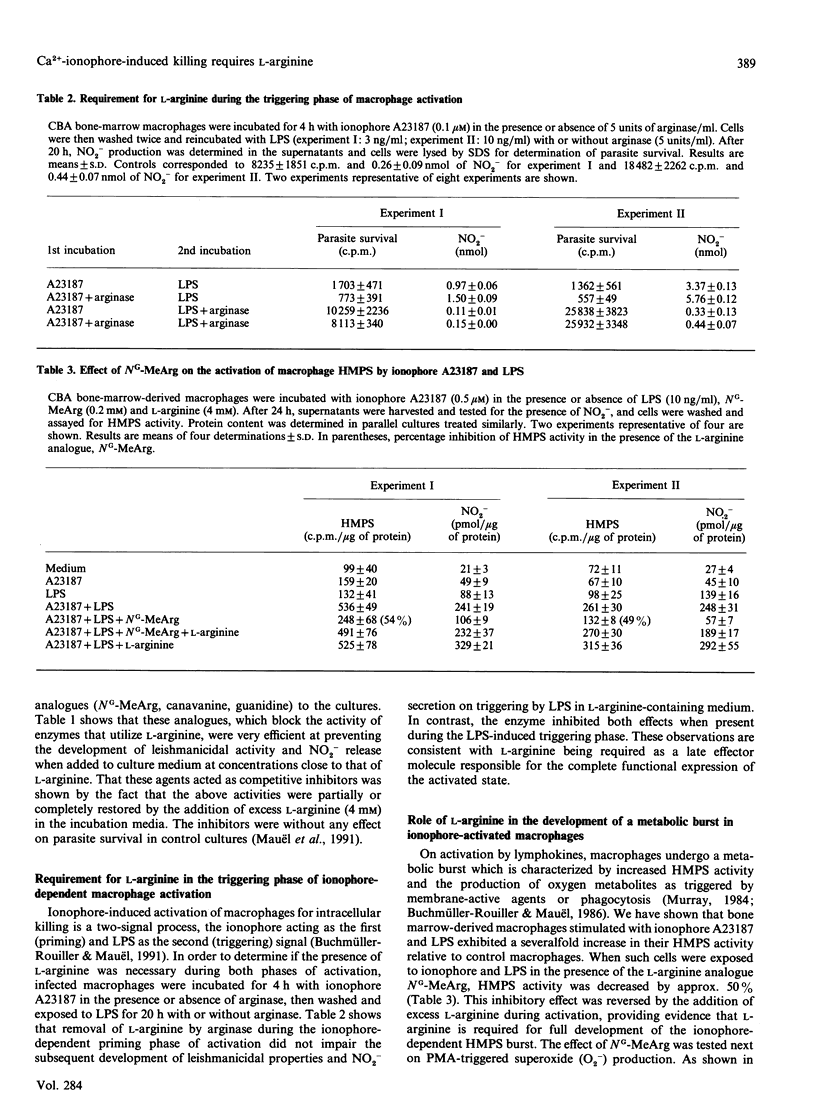
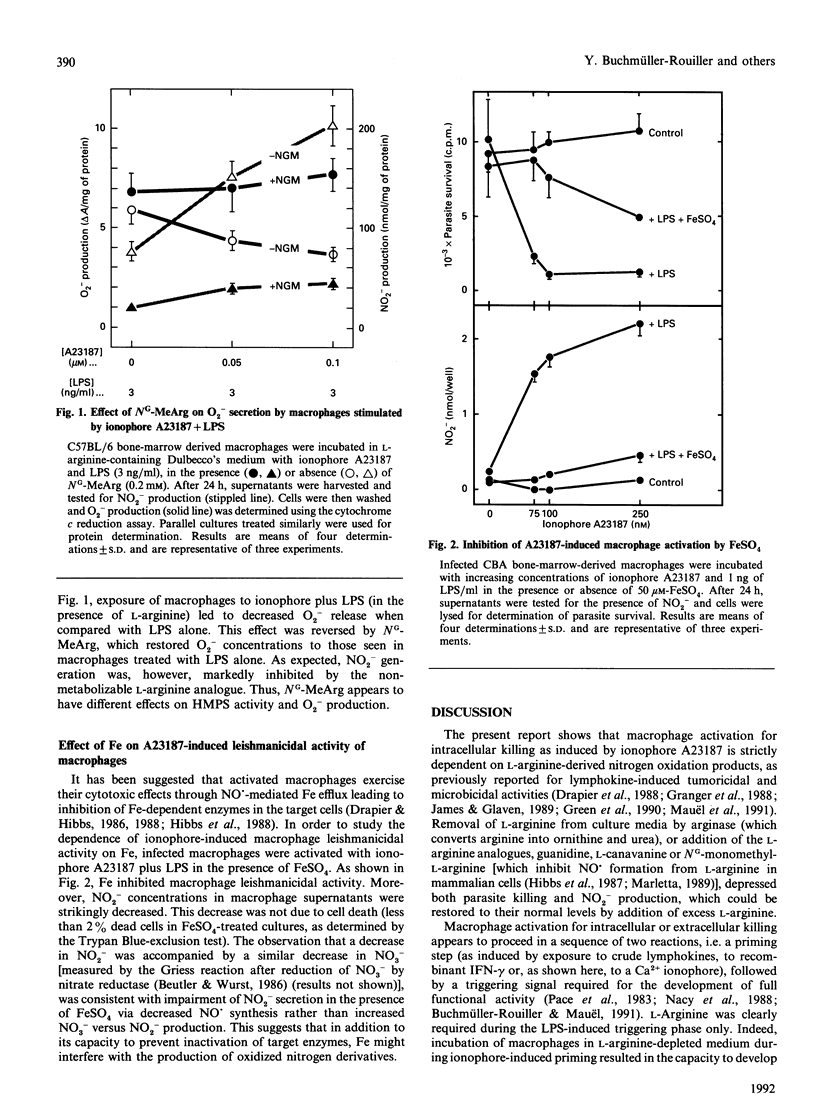
Mouse macrophages activated by interferon-gamma kill intracellular Leishmania by a process that depends on the generation of L-arginine-derived nitrogen oxidation products. Interferon-induced intracellular killing can be mimicked by exposure of macrophages to the Ca2+ ionophore A23187 in the presence of lipopolysaccharide. The mechanisms of this effect were therefore investigated. Destruction of the parasite was accompanied by accumulation of nitrite in the macrophage culture fluids. Leishmanicidal activity and nitrite production in cultures stimulated with ionophore A23187 and lipopolysaccharide were abrogated when cells were activated in medium containing arginase or the L-arginine analogues L-canavanine, guanidine or NG-monomethyl-L-arginine. L-Arginine was required during the lipopolysaccharide-induced triggering phase only. Indeed, macrophage priming with ionophore A23187 in L-arginine-depleted medium led to full microbicidal activity and nitrite generation provided that L-arginine was present during subsequent triggering by lipopolysaccharide. Addition of NG-monomethyl-L-arginine to ionophore-activated macrophages increased O2- production on phorbol myristate stimulation, while inhibiting glucose oxidation through the hexose monophosphate shunt pathway. Leishmanicidal activity and nitrite production were also inhibited when ionophore-treated cultures were incubated with excess iron, implying a role for iron as a defence mechanism against the toxicity of nitrogen derivatives. These results indicate that the ionophore-induced leishmanicidal activity occurs through a process similar to that evoked by interferon-gamma, i.e. the production of L-arginine-derived nitrogen oxidation products.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albina J. E., Mills C. D., Henry W. L., Jr, Caldwell M. D. Regulation of macrophage physiology by L-arginine: role of the oxidative L-arginine deiminase pathway. J Immunol. 1989 Dec 1;143(11):3641–3646. [PubMed] [Google Scholar]
- Badwey J. A., Karnovsky M. L. Active oxygen species and the functions of phagocytic leukocytes. Annu Rev Biochem. 1980;49:695–726. doi: 10.1146/annurev.bi.49.070180.003403. [DOI] [PubMed] [Google Scholar]
- Baumgarten H. A simple microplate assay for the determination of cellular protein. J Immunol Methods. 1985 Sep 3;82(1):25–37. doi: 10.1016/0022-1759(85)90221-2. [DOI] [PubMed] [Google Scholar]
- Beckman J. S., Beckman T. W., Chen J., Marshall P. A., Freeman B. A. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berens R. L., Marr J. J. An easily prepared defined medium for cultivation of Leishmania donovani promastigotes. J Parasitol. 1978 Feb;64(1):160–160. [PubMed] [Google Scholar]
- Bredt D. S., Snyder S. H. Isolation of nitric oxide synthetase, a calmodulin-requiring enzyme. Proc Natl Acad Sci U S A. 1990 Jan;87(2):682–685. doi: 10.1073/pnas.87.2.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmüller-Rouiller Y., Mauël J. Correlation between enhanced oxidative metabolism and leishmanicidal activity in activated macrophages from healer and nonhealer mouse strains. J Immunol. 1986 May 15;136(10):3884–3890. [PubMed] [Google Scholar]
- Buchmüller-Rouiller Y., Mauël J. Impairment of the oxidative metabolism of mouse peritoneal macrophages by intracellular Leishmania spp. Infect Immun. 1987 Mar;55(3):587–593. doi: 10.1128/iai.55.3.587-593.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmüller-Rouiller Y., Mauël J. Macrophage activation for intracellular killing as induced by calcium ionophore. Correlation with biologic and biochemical events. J Immunol. 1991 Jan 1;146(1):217–223. [PubMed] [Google Scholar]
- Buchmüller Y., Mauel J. Studies on the mechanisms of macrophage activation. II. Parasite destruction in macrophages activated by supernates from concanavalin A-stimulated lymphocytes. J Exp Med. 1979 Aug 1;150(2):359–370. doi: 10.1084/jem.150.2.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrd T. F., Horwitz M. A. Interferon gamma-activated human monocytes downregulate transferrin receptors and inhibit the intracellular multiplication of Legionella pneumophila by limiting the availability of iron. J Clin Invest. 1989 May;83(5):1457–1465. doi: 10.1172/JCI114038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988 Apr 15;140(8):2829–2838. [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Murine cytotoxic activated macrophages inhibit aconitase in tumor cells. Inhibition involves the iron-sulfur prosthetic group and is reversible. J Clin Invest. 1986 Sep;78(3):790–797. doi: 10.1172/JCI112642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapier J. C., Wietzerbin J., Hibbs J. B., Jr Interferon-gamma and tumor necrosis factor induce the L-arginine-dependent cytotoxic effector mechanism in murine macrophages. Eur J Immunol. 1988 Oct;18(10):1587–1592. doi: 10.1002/eji.1830181018. [DOI] [PubMed] [Google Scholar]
- Drummond I. A., Lee A. S., Resendez E., Jr, Steinhardt R. A. Depletion of intracellular calcium stores by calcium ionophore A23187 induces the genes for glucose-regulated proteins in hamster fibroblasts. J Biol Chem. 1987 Sep 15;262(26):12801–12805. [PubMed] [Google Scholar]
- Gemsa D., Seitz M., Kramer W., Grimm W., Till G., Resch K. Ionophore A23187 raises cyclic AMP levels in macrophages by stimulating prostaglandin E formation. Exp Cell Res. 1979 Jan;118(1):55–62. doi: 10.1016/0014-4827(79)90583-4. [DOI] [PubMed] [Google Scholar]
- Granger D. L., Hibbs J. B., Jr, Perfect J. R., Durack D. T. Specific amino acid (L-arginine) requirement for the microbiostatic activity of murine macrophages. J Clin Invest. 1988 Apr;81(4):1129–1136. doi: 10.1172/JCI113427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green S. J., Meltzer M. S., Hibbs J. B., Jr, Nacy C. A. Activated macrophages destroy intracellular Leishmania major amastigotes by an L-arginine-dependent killing mechanism. J Immunol. 1990 Jan 1;144(1):278–283. [PubMed] [Google Scholar]
- Hauschildt S., Lückhoff A., Mülsch A., Kohler J., Bessler W., Busse R. Induction and activity of NO synthase in bone-marrow-derived macrophages are independent of Ca2+. Biochem J. 1990 Sep 1;270(2):351–356. doi: 10.1042/bj2700351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Iron depletion: possible cause of tumor cell cytotoxicity induced by activated macrophages. Biochem Biophys Res Commun. 1984 Sep 17;123(2):716–723. doi: 10.1016/0006-291x(84)90288-2. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- James S. L., Glaven J. Macrophage cytotoxicity against schistosomula of Schistosoma mansoni involves arginine-dependent production of reactive nitrogen intermediates. J Immunol. 1989 Dec 15;143(12):4208–4212. [PubMed] [Google Scholar]
- Kelso A., Glasebrook A. L., Kanagawa O., Brunner K. T. Production of macrophage-activating factor by T lymphocyte clones and correlation with other lymphokine activities. J Immunol. 1982 Aug;129(2):550–556. [PubMed] [Google Scholar]
- Knowles R. G., Palacios M., Palmer R. M., Moncada S. Formation of nitric oxide from L-arginine in the central nervous system: a transduction mechanism for stimulation of the soluble guanylate cyclase. Proc Natl Acad Sci U S A. 1989 Jul;86(13):5159–5162. doi: 10.1073/pnas.86.13.5159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew F. Y., Millott S., Parkinson C., Palmer R. M., Moncada S. Macrophage killing of Leishmania parasite in vivo is mediated by nitric oxide from L-arginine. J Immunol. 1990 Jun 15;144(12):4794–4797. [PubMed] [Google Scholar]
- Marletta M. A. Nitric oxide: biosynthesis and biological significance. Trends Biochem Sci. 1989 Dec;14(12):488–492. doi: 10.1016/0968-0004(89)90181-3. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Mauel J. Intracellular parasite killing induced by electron carriers. I. Effect of electron carriers on intracellular Leishmania spp. in macrophages from different genetic backgrounds. Mol Biochem Parasitol. 1984 Sep;13(1):83–96. doi: 10.1016/0166-6851(84)90103-8. [DOI] [PubMed] [Google Scholar]
- Mauel J., Schnyder J., Baggiolini M. Intracellular parasite killing induced by electron carriers. II. Correlation between parasite killing and the induction of oxidative events in macrophages. Mol Biochem Parasitol. 1984 Sep;13(1):97–110. doi: 10.1016/0166-6851(84)90104-x. [DOI] [PubMed] [Google Scholar]
- Mauël J., Ransijn A., Buchmüller-Rouiller Y. Killing of Leishmania parasites in activated murine macrophages is based on an L-arginine-dependent process that produces nitrogen derivatives. J Leukoc Biol. 1991 Jan;49(1):73–82. doi: 10.1002/jlb.49.1.73. [DOI] [PubMed] [Google Scholar]
- Mauël J., Ransijn A., Buchmüller-Rouiller Y. Lead inhibits intracellular killing of Leishmania parasites and extracellular cytolysis of target cells by macrophages exposed to macrophage activating factor. J Leukoc Biol. 1989 May;45(5):401–409. doi: 10.1002/jlb.45.5.401. [DOI] [PubMed] [Google Scholar]
- Murray H. W. Macrophage activation: enhanced oxidative and antiprotozoal activity. Contemp Top Immunobiol. 1984;13:97–115. doi: 10.1007/978-1-4757-1445-6_5. [DOI] [PubMed] [Google Scholar]
- Pace J. L., Russell S. W., Torres B. A., Johnson H. M., Gray P. W. Recombinant mouse gamma interferon induces the priming step in macrophage activation for tumor cell killing. J Immunol. 1983 May;130(5):2011–2013. [PubMed] [Google Scholar]
- Palacios M., Knowles R. G., Palmer R. M., Moncada S. Nitric oxide from L-arginine stimulates the soluble guanylate cyclase in adrenal glands. Biochem Biophys Res Commun. 1989 Dec 15;165(2):802–809. doi: 10.1016/s0006-291x(89)80037-3. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Kwon N. S., Gross S. S., Thiel B. A., Levi R., Nathan C. F. Synthesis of nitrogen oxides from L-arginine by macrophage cytosol: requirement for inducible and constitutive components. Biochem Biophys Res Commun. 1989 Jun 15;161(2):420–426. doi: 10.1016/0006-291x(89)92615-6. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Nathan C. F. Nitric oxide. A macrophage product responsible for cytostasis and respiratory inhibition in tumor target cells. J Exp Med. 1989 May 1;169(5):1543–1555. doi: 10.1084/jem.169.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]


