Abstract
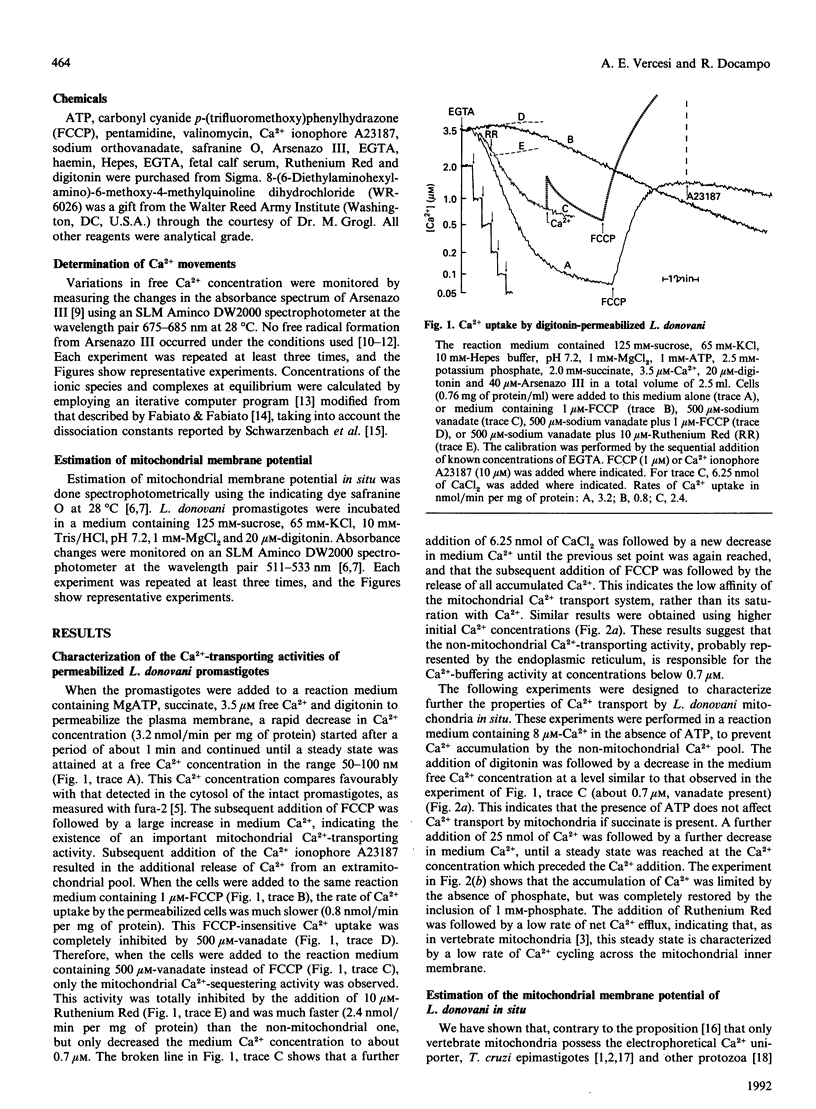
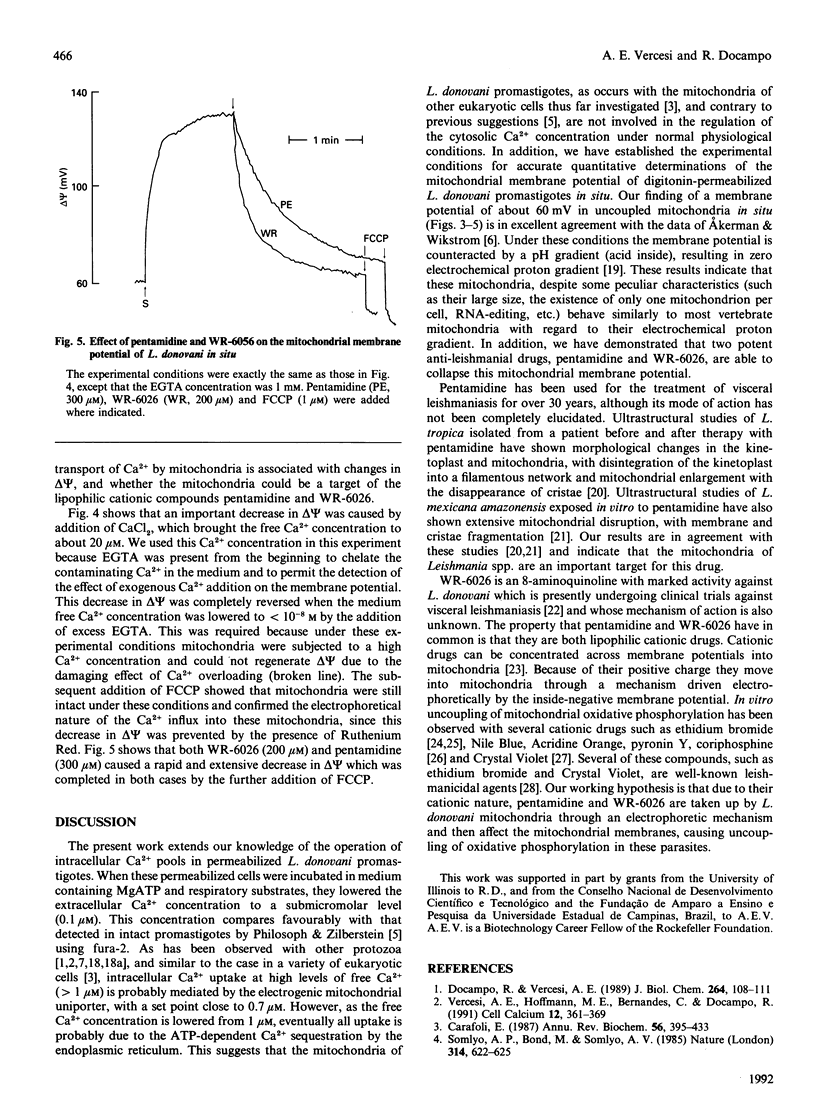
The use of low concentrations of digitonin allowed the quantitative determination of the mitochondrial membrane potential of Leishmania donovani promastigotes in situ using safranine O. L. donovani mitochondria were able to build up and retain a membrane potential of a value comparable with that of mammalian mitochondria. The response of promastigotes mitochondrial membrane potential to phosphate, carbonyl cyanide p-(trifluoromethoxy)phenylhydrazone (FCCP), valinomycin and Ca2+ indicates that these mitochondria behave similarly to vertebrate mitochondria with regard to the properties of their electrochemical proton gradient. When L. donovani promastigotes were permeabilized with digitonin in a reaction medium containing MgATP, succinate and 3.5 microM free Ca2+, they lowered the medium Ca2+ concentration to the submicromolar level (0.05-0.1 microM). The presence of 1 microM-FCCP decreased by about 75% the initial rate of Ca2+ sequestration by these permeabilized cells. This FCCP-insensitive Ca2+ uptake, probably by the endoplasmic reticulum, was completely inhibited by 500 microM-vanadate. On the other hand, when vanadate instead of FCCP was present, the initial rate of Ca2+ accumulation was decreased by about 25% and the Ca2+ set point was increased to 0.7 microM. The succinate-dependence and FCCP-and Ruthenium Red-sensitivity of the Ca2+ uptake detected in the presence of vanadate indicate that this uptake is probably by the mitochondria. This interpretation was further supported by the Ruthenium Red-sensitive decrease in the mitochondrial membrane potential caused by Ca2+ addition. The anti-leishmanial cationic drugs pentamidine and WR-6026 also induced a rapid collapse of the mitochondrial inner membrane potential of L. donovani promastigotes.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akerman K. E., Wikström M. K. Safranine as a probe of the mitochondrial membrane potential. FEBS Lett. 1976 Oct 1;68(2):191–197. doi: 10.1016/0014-5793(76)80434-6. [DOI] [PubMed] [Google Scholar]
- Benaim G., Bermudez R., Urbina J. A. Ca2+ transport in isolated mitochondrial vesicles from Leishmania braziliensis promastigotes. Mol Biochem Parasitol. 1990 Feb;39(1):61–68. doi: 10.1016/0166-6851(90)90008-a. [DOI] [PubMed] [Google Scholar]
- Carafoli E. Intracellular calcium homeostasis. Annu Rev Biochem. 1987;56:395–433. doi: 10.1146/annurev.bi.56.070187.002143. [DOI] [PubMed] [Google Scholar]
- Chen L. B. Mitochondrial membrane potential in living cells. Annu Rev Cell Biol. 1988;4:155–181. doi: 10.1146/annurev.cb.04.110188.001103. [DOI] [PubMed] [Google Scholar]
- Croft S. L., Brazil R. P. Effect of pentamidine isethionate on the ultrastructure and morphology of Leishmania mexicana amazonensis in vitro. Ann Trop Med Parasitol. 1982 Feb;76(1):37–43. doi: 10.1080/00034983.1982.11687502. [DOI] [PubMed] [Google Scholar]
- Docampo R., Moreno S. N., Mason R. P. Generation of free radical metabolites and superoxide anion by the calcium indicators arsenazo III, antipyrylazo III, and murexide in rat liver microsomes. J Biol Chem. 1983 Dec 25;258(24):14920–14925. [PubMed] [Google Scholar]
- Docampo R., Vercesi A. E. Ca2+ transport by coupled Trypanosoma cruzi mitochondria in situ. J Biol Chem. 1989 Jan 5;264(1):108–111. [PubMed] [Google Scholar]
- Docampo R., Vercesi A. E. Characteristics of Ca2+ transport by Trypanosoma cruzi mitochondria in situ. Arch Biochem Biophys. 1989 Jul;272(1):122–129. doi: 10.1016/0003-9861(89)90202-6. [DOI] [PubMed] [Google Scholar]
- Fabiato A., Fabiato F. Calculator programs for computing the composition of the solutions containing multiple metals and ligands used for experiments in skinned muscle cells. J Physiol (Paris) 1979;75(5):463–505. [PubMed] [Google Scholar]
- Grimwood B. G., Wagner R. P. Direct action of ethidium bromide upon mitochondrial oxidative phosphorylation and morphology. Arch Biochem Biophys. 1976 Sep;176(1):43–52. doi: 10.1016/0003-9861(76)90139-9. [DOI] [PubMed] [Google Scholar]
- Hentzer B., Kobayasi T. The ultrastructural changes of Leishmania tropica after treatment with pentamidine. Ann Trop Med Parasitol. 1977 Jun;71(2):157–166. doi: 10.1080/00034983.1977.11687174. [DOI] [PubMed] [Google Scholar]
- Inesi G., Kurzmack M., Coan C., Lewis D. E. Cooperative calcium binding and ATPase activation in sarcoplasmic reticulum vesicles. J Biol Chem. 1980 Apr 10;255(7):3025–3031. [PubMed] [Google Scholar]
- Mai M. S., Allison W. S. Inhibition of an oligomycin-sensitive ATPase by cationic dyes, some of which are atypical uncouplers of intact mitochondria. Arch Biochem Biophys. 1983 Mar;221(2):467–476. doi: 10.1016/0003-9861(83)90165-0. [DOI] [PubMed] [Google Scholar]
- Miko M., Chance B. Ethidium bromide as an uncoupler of oxidative phosphorylation. FEBS Lett. 1975 Jul 1;54(3):347–352. doi: 10.1016/0014-5793(75)80937-9. [DOI] [PubMed] [Google Scholar]
- Moreno S. N., Gadelha F. R., Docampo R. Crystal violet as an uncoupler of oxidative phosphorylation in rat liver mitochondria. J Biol Chem. 1988 Sep 5;263(25):12493–12499. [PubMed] [Google Scholar]
- Moreno S. N., Mason R. P., Docampo R. Ca2+ and Mg2+-enhanced reduction of arsenazo III to its anion free radical metabolite and generation of superoxide anion by an outer mitochondrial membrane azoreductase. J Biol Chem. 1984 Dec 10;259(23):14609–14616. [PubMed] [Google Scholar]
- Moreno S. N., Mason R. P., Docampo R. Reduction of the metallochromic indicators arsenazo III and antipyrylazo III to their free radical metabolites by cytoplasmic enzymes. FEBS Lett. 1985 Jan 28;180(2):229–233. doi: 10.1016/0014-5793(85)81076-0. [DOI] [PubMed] [Google Scholar]
- Nicholls D. G. The influence of respiration and ATP hydrolysis on the proton-electrochemical gradient across the inner membrane of rat-liver mitochondria as determined by ion distribution. Eur J Biochem. 1974 Dec 16;50(1):305–315. doi: 10.1111/j.1432-1033.1974.tb03899.x. [DOI] [PubMed] [Google Scholar]
- Philosoph H., Zilberstein D. Regulation of intracellular calcium in promastigotes of the human protozoan parasite Leishmania donovani. J Biol Chem. 1989 Jun 25;264(18):10420–10424. [PubMed] [Google Scholar]
- Rabinovitch M., Dedet J. P., Ryter A., Robineaux R., Topper G., Brunet E. Destruction of Leishmania mexicana amazonensis amastigotes within macrophages in culture by phenazine methosulfate and other electron carriers. J Exp Med. 1982 Feb 1;155(2):415–431. doi: 10.1084/jem.155.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scarpa A. Measurements of cation transport with metallochromic indicators. Methods Enzymol. 1979;56:301–338. doi: 10.1016/0076-6879(79)56030-3. [DOI] [PubMed] [Google Scholar]
- Somlyo A. P., Bond M., Somlyo A. V. Calcium content of mitochondria and endoplasmic reticulum in liver frozen rapidly in vivo. Nature. 1985 Apr 18;314(6012):622–625. doi: 10.1038/314622a0. [DOI] [PubMed] [Google Scholar]
- Vercesi A. E., Bernardes C. F., Hoffmann M. E., Gadelha F. R., Docampo R. Digitonin permeabilization does not affect mitochondrial function and allows the determination of the mitochondrial membrane potential of Trypanosoma cruzi in situ. J Biol Chem. 1991 Aug 5;266(22):14431–14434. [PubMed] [Google Scholar]
- Vercesi A. E., Hoffmann M. E., Bernardes C. F., Docampo R. Regulation of intracellular calcium homeostasis in Trypanosoma cruzi. Effects of calmidazolium and trifluoperazine. Cell Calcium. 1991 May;12(5):361–369. doi: 10.1016/0143-4160(91)90052-g. [DOI] [PubMed] [Google Scholar]
- Vercesi A. E., Macedo D. V., Lima S. A., Gadelha F. R., Docampo R. Ca2+ transport in digitonin-permeabilized trypanosomatids. Mol Biochem Parasitol. 1990 Aug;42(1):119–124. doi: 10.1016/0166-6851(90)90119-7. [DOI] [PubMed] [Google Scholar]


