Abstract
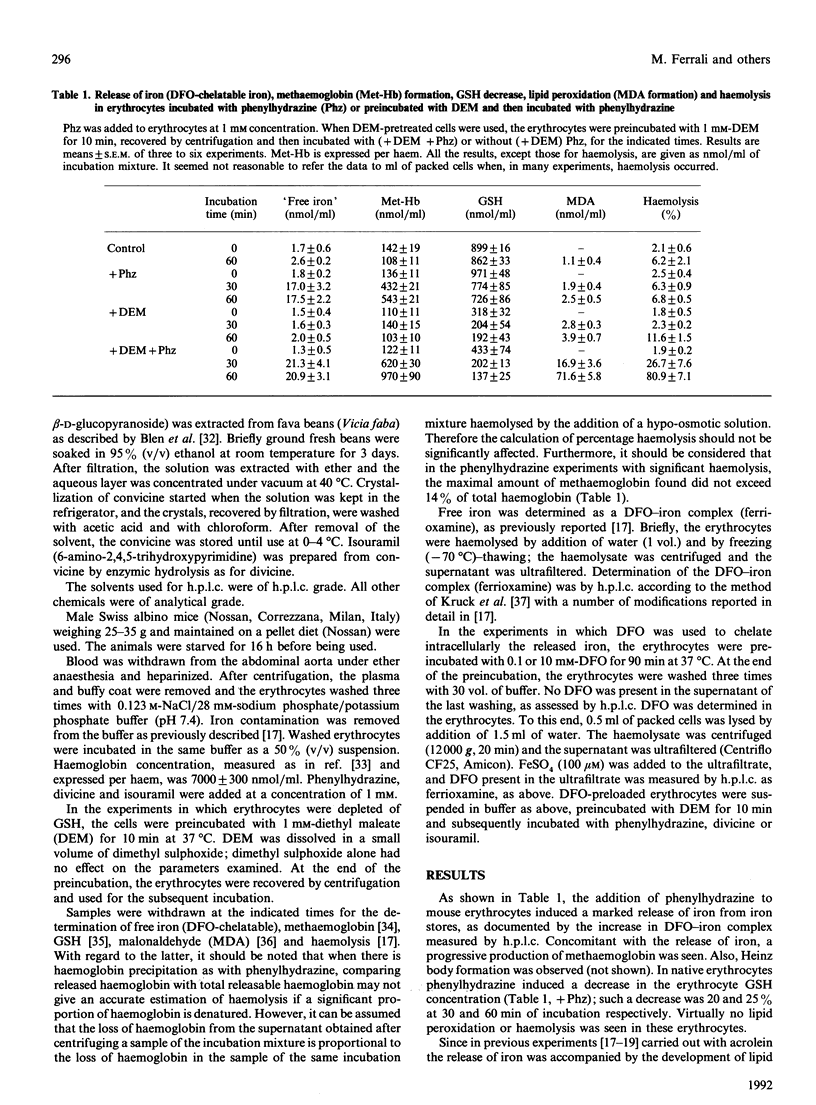
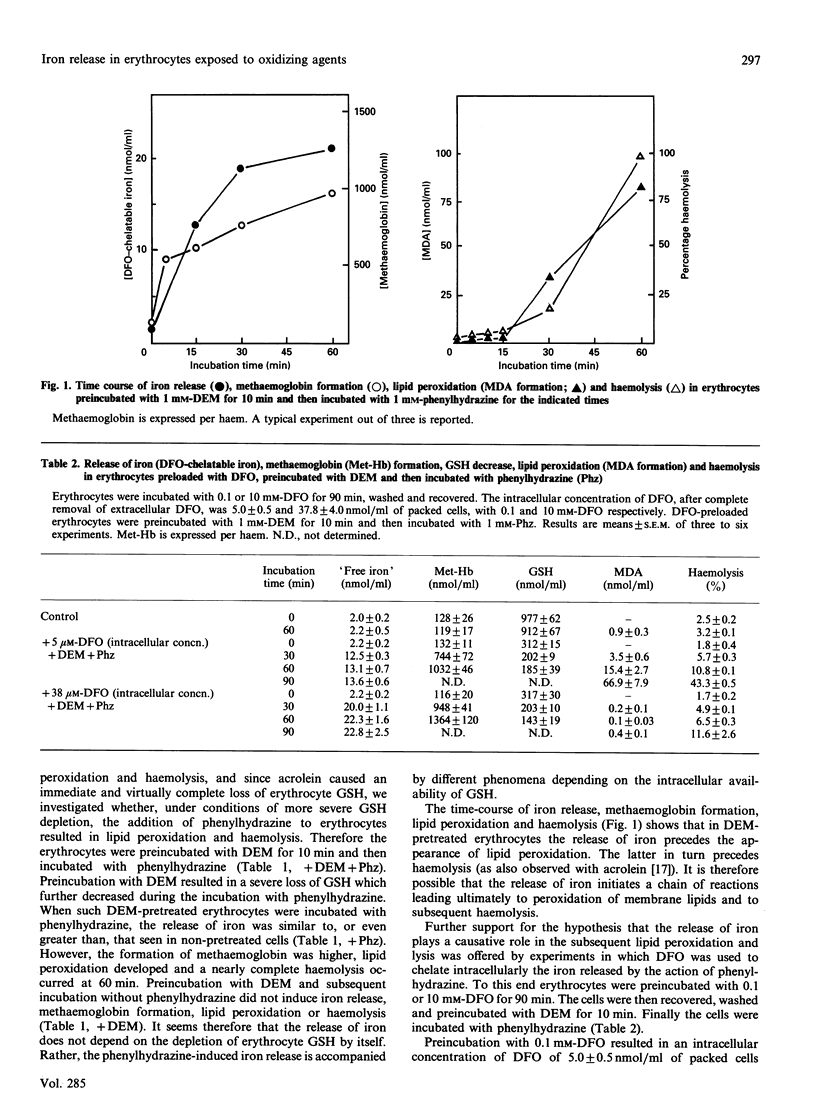
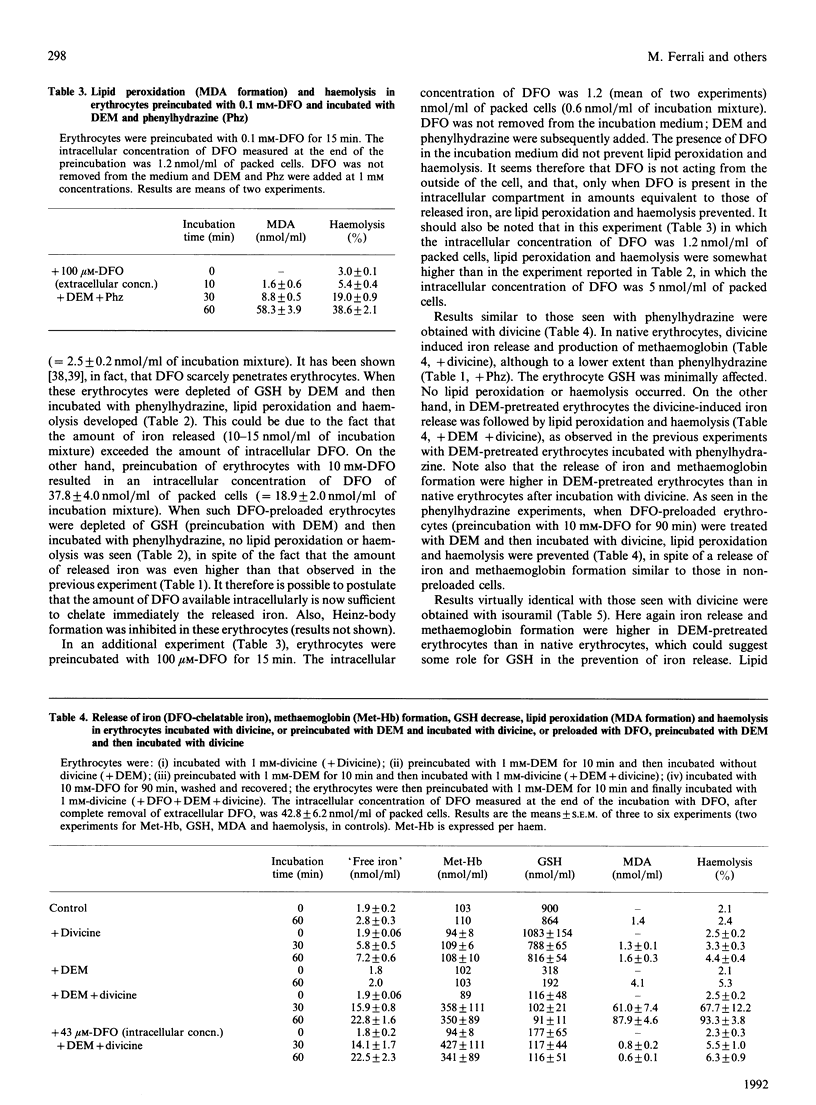
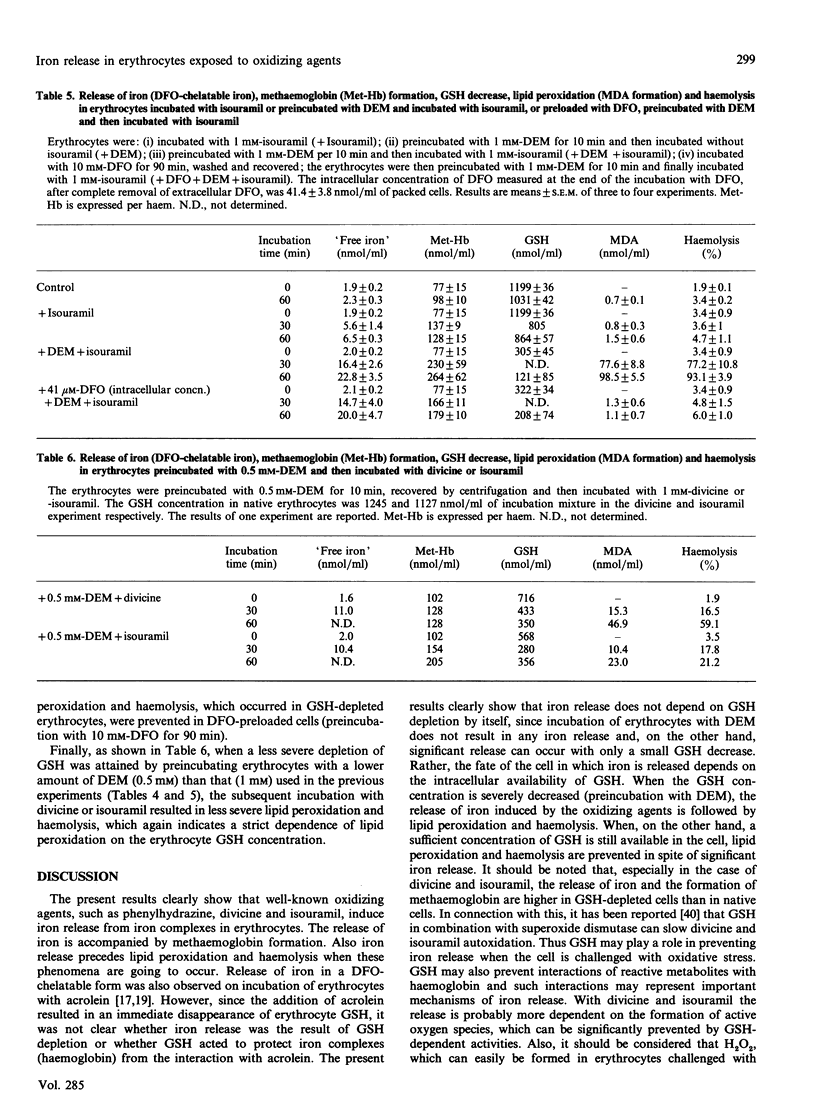
Mouse erythrocytes were incubated with oxidizing agents, phenylhydrazine, divicine and isouramil. With all the oxidants a rapid release of iron in a desferrioxamine (DFO)-chelatable form was seen and it was accompanied by methaemoglobin formation. If the erythrocytes were depleted of GSH by a short preincubation with diethyl maleate, the release of iron was accompanied by lipid peroxidation and, subsequently, haemolysis. GSH depletion by itself did not induce iron release, methaemoglobin formation, lipid peroxidation or haemolysis. Rather, the fate of the cell in which iron is released depended on the intracellular availability of GSH. In addition, iron release was higher in depleted cells than in native ones, suggesting a role for GSH in preventing iron release when oxidative stress is imposed by the oxidants. Iron release preceded lipid peroxidation. The latter was prevented when the erythrocytes were preloaded with DFO in such a way (preincubation with 10 mM-DFO) that the intracellular concentration was equivalent to that of the released iron, but not when the intracellular DFO was lower (preincubation with 0.1 mM-DFO). Extracellular DFO did not affect lipid peroxidation and haemolysis, suggesting again that the observed events occur intracellularly (intracellular chelation of released iron). The relevance of iron release from iron complexes in the mechanisms of cellular damage induced by oxidative stress is discussed.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albano E., Tomasi A., Mannuzzu L., Arese P. Detection of a free radical intermediate from divicine of Vicia faba. Biochem Pharmacol. 1984 May 15;33(10):1701–1704. doi: 10.1016/0006-2952(84)90299-5. [DOI] [PubMed] [Google Scholar]
- Arese P., De Flora A. Pathophysiology of hemolysis in glucose-6-phosphate dehydrogenase deficiency. Semin Hematol. 1990 Jan;27(1):1–40. [PubMed] [Google Scholar]
- BEUTLER E., DURON O., KELLY B. M. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963 May;61:882–888. [PubMed] [Google Scholar]
- Bakkeren D. L., de Jeu-Jaspars C. M., van der Heul C., van Eijk H. G. Analysis of iron-binding components in the low molecular weight fraction of rat reticulocyte cytosol. Int J Biochem. 1985;17(8):925–930. doi: 10.1016/0020-711x(85)90177-6. [DOI] [PubMed] [Google Scholar]
- Bien S., Salemnik G., Zamir L., Rosenblum M. The structure of convicine. J Chem Soc Perkin 1. 1968;5:496–499. doi: 10.1039/j39680000496. [DOI] [PubMed] [Google Scholar]
- Comporti M., Hartman A., Di Luzio N. R. Effect of in vivo and in vitro ethanol administration on liver lipid peroxidation. Lab Invest. 1967 Apr;16(4):616–624. [PubMed] [Google Scholar]
- Crichton R. R., Charloteaux-Wauters M. Iron transport and storage. Eur J Biochem. 1987 May 4;164(3):485–506. doi: 10.1111/j.1432-1033.1987.tb11155.x. [DOI] [PubMed] [Google Scholar]
- DiGuiseppi J., Fridovich I. The toxicology of molecular oxygen. Crit Rev Toxicol. 1984;12(4):315–342. doi: 10.3109/10408448409044213. [DOI] [PubMed] [Google Scholar]
- Ferrali M., Ciccoli L., Comporti M. Allyl alcohol-induced hemolysis and its relation to iron release and lipid peroxidation. Biochem Pharmacol. 1989 Jun 1;38(11):1819–1825. doi: 10.1016/0006-2952(89)90417-6. [DOI] [PubMed] [Google Scholar]
- Ferrali M., Ciccoli L., Signorini C., Comporti M. Iron release and erythrocyte damage in allyl alcohol intoxication in mice. Biochem Pharmacol. 1990 Oct 1;40(7):1485–1490. doi: 10.1016/0006-2952(90)90444-p. [DOI] [PubMed] [Google Scholar]
- Goldberg B., Stern A., Peisach J. The mechanism of superoxide anion generation by the interaction of phenylhydrazine with hemoglobin. J Biol Chem. 1976 May 25;251(10):3045–3051. [PubMed] [Google Scholar]
- Goldberg B., Stern A. The generation of O2-by the interaction of the hemolytic agent, phenylhydrazine, with human hemoglobin. J Biol Chem. 1975 Mar 25;250(6):2401–2403. [PubMed] [Google Scholar]
- Goldberg B., Stern A. The mechanism of oxidative hemolysis produced by phenylhydrazine. Mol Pharmacol. 1977 Sep;13(5):832–839. [PubMed] [Google Scholar]
- Goldstein B. D., Rozen M. G., Kunis R. L. Role of red cell membrane lipid peroxidation in hemolysis due to phenylhydrazine. Biochem Pharmacol. 1980 May 15;29(10):1355–1359. doi: 10.1016/0006-2952(80)90430-x. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M. Age pigments and free radicals: fluorescent lipid complexes formed by iron- and copper-containing proteins. Biochim Biophys Acta. 1985 Apr 25;834(2):144–148. doi: 10.1016/0005-2760(85)90149-3. [DOI] [PubMed] [Google Scholar]
- Gutteridge J. M., Halliwell B., Treffry A., Harrison P. M., Blake D. Effect of ferritin-containing fractions with different iron loading on lipid peroxidation. Biochem J. 1983 Feb 1;209(2):557–560. doi: 10.1042/bj2090557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutteridge J. M. Iron promoters of the Fenton reaction and lipid peroxidation can be released from haemoglobin by peroxides. FEBS Lett. 1986 Jun 9;201(2):291–295. doi: 10.1016/0014-5793(86)80626-3. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch Biochem Biophys. 1986 May 1;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984 Apr 1;219(1):1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J. M. The importance of free radicals and catalytic metal ions in human diseases. Mol Aspects Med. 1985;8(2):89–193. doi: 10.1016/0098-2997(85)90001-9. [DOI] [PubMed] [Google Scholar]
- Halliwell B. Use of desferrioxamine as a 'probe' for iron-dependent formation of hydroxyl radicals. Evidence for a direct reaction between desferal and the superoxide radical. Biochem Pharmacol. 1985 Jan 15;34(2):229–233. doi: 10.1016/0006-2952(85)90129-7. [DOI] [PubMed] [Google Scholar]
- Healing G., Gower J., Fuller B., Green C. Intracellular iron redistribution. An important determinant of reperfusion damage to rabbit kidneys. Biochem Pharmacol. 1990 Apr 1;39(7):1239–1245. doi: 10.1016/0006-2952(90)90269-q. [DOI] [PubMed] [Google Scholar]
- Hill H. A., Thornalley P. J. Phenyl radical production during the oxidation of phenylhydrazine and in phenylphydrazine-induced haemolysis. FEBS Lett. 1981 Mar 23;125(2):235–238. doi: 10.1016/0014-5793(81)80727-2. [DOI] [PubMed] [Google Scholar]
- Jacobs A. Low molecular weight intracellular iron transport compounds. Blood. 1977 Sep;50(3):433–439. [PubMed] [Google Scholar]
- Kruck T. P., Kalow W., McLachlan D. R. Determination of desferoxamine and a major metabolite by high-performance liquid chromatography. Application to the treatment of aluminium-related disorders. J Chromatogr. 1985 May 31;341(1):123–130. doi: 10.1016/s0378-4347(00)84016-5. [DOI] [PubMed] [Google Scholar]
- Lloyd J. B., Cable H., Rice-Evans C. Evidence that desferrioxamine cannot enter cells by passive diffusion. Biochem Pharmacol. 1991 May 1;41(9):1361–1363. doi: 10.1016/0006-2952(91)90109-i. [DOI] [PubMed] [Google Scholar]
- Low P. S., Waugh S. M., Zinke K., Drenckhahn D. The role of hemoglobin denaturation and band 3 clustering in red blood cell aging. Science. 1985 Feb 1;227(4686):531–533. doi: 10.1126/science.2578228. [DOI] [PubMed] [Google Scholar]
- Maples K. R., Jordan S. J., Mason R. P. In vivo rat hemoglobin thiyl free radical formation following phenylhydrazine administration. Mol Pharmacol. 1988 Mar;33(3):344–350. [PubMed] [Google Scholar]
- Miller J. P., Perkins D. J. Model experiments for the study of iron transfer from transferrin to ferritin. Eur J Biochem. 1969 Aug;10(1):146–151. doi: 10.1111/j.1432-1033.1969.tb00666.x. [DOI] [PubMed] [Google Scholar]
- Minotti G. Adriamycin-dependent release of iron from microsomal membranes. Arch Biochem Biophys. 1989 Jan;268(1):398–403. doi: 10.1016/0003-9861(89)90601-2. [DOI] [PubMed] [Google Scholar]
- Minotti G., Aust S. D. The role of iron in oxygen radical mediated lipid peroxidation. Chem Biol Interact. 1989;71(1):1–19. doi: 10.1016/0009-2797(89)90087-2. [DOI] [PubMed] [Google Scholar]
- Minotti G., Di Gennaro M. Microsomal iron-dependent NADPH oxidation: evidence for the involvement of membrane-bound nonheme iron in NADPH oxidation by rat heart microsomes. Arch Biochem Biophys. 1990 Nov 1;282(2):270–274. doi: 10.1016/0003-9861(90)90116-g. [DOI] [PubMed] [Google Scholar]
- Minotti G. tert-butyl hydroperoxide-dependent microsomal release of iron and lipid peroxidation. I. Evidence for the reductive release of nonheme, nonferritin iron. Arch Biochem Biophys. 1989 Aug 15;273(1):137–143. doi: 10.1016/0003-9861(89)90171-9. [DOI] [PubMed] [Google Scholar]
- Mulligan M., Althaus B., Linder M. C. Non-ferritin, non-heme iron pools in rat tissues. Int J Biochem. 1986;18(9):791–798. doi: 10.1016/0020-711x(86)90055-8. [DOI] [PubMed] [Google Scholar]
- O'Connell M. J., Ward R. J., Baum H., Peters T. J. The role of iron in ferritin- and haemosiderin-mediated lipid peroxidation in liposomes. Biochem J. 1985 Jul 1;229(1):135–139. doi: 10.1042/bj2290135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puppo A., Halliwell B. Formation of hydroxyl radicals from hydrogen peroxide in the presence of iron. Is haemoglobin a biological Fenton reagent? Biochem J. 1988 Jan 1;249(1):185–190. doi: 10.1042/bj2490185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice-Evans C., Baysal E., Singh S., Jones S. A., Jones J. G. The interactions of desferrioxamine and hydroxypyridone compounds with haemoglobin and erythrocytes. FEBS Lett. 1989 Oct 9;256(1-2):17–20. doi: 10.1016/0014-5793(89)81709-0. [DOI] [PubMed] [Google Scholar]
- Rouach H., Houze P., Orfanelli M. T., Gentil M., Bourdon R., Nordmann R. Effect of acute ethanol administration on the subcellular distribution of iron in rat liver and cerebellum. Biochem Pharmacol. 1990 Mar 15;39(6):1095–1100. doi: 10.1016/0006-2952(90)90289-w. [DOI] [PubMed] [Google Scholar]
- Saito S., Itano H. A. beta-meso-Phenylbiliverdin IX alpha and N-phenylprotoporphyrin IX, products of the reaction of phenylhydrazine with oxyhemoproteins. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5508–5512. doi: 10.1073/pnas.78.9.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samokyszyn V. M., Miller D. M., Reif D. W., Aust S. D. Inhibition of superoxide and ferritin-dependent lipid peroxidation by ceruloplasmin. J Biol Chem. 1989 Jan 5;264(1):21–26. [PubMed] [Google Scholar]
- Stern A. Drug-induced oxidative denaturation in red blood cells. Semin Hematol. 1989 Oct;26(4):301–306. [PubMed] [Google Scholar]
- Thomas C. E., Aust S. D. Rat liver microsomal NADPH-dependent release of iron from ferritin and lipid peroxidation. J Free Radic Biol Med. 1985;1(4):293–300. doi: 10.1016/0748-5514(85)90134-5. [DOI] [PubMed] [Google Scholar]
- Thomas C. E., Aust S. D. Reductive release of iron from ferritin by cation free radicals of paraquat and other bipyridyls. J Biol Chem. 1986 Oct 5;261(28):13064–13070. [PubMed] [Google Scholar]
- Thomas C. E., Aust S. D. Release of iron from ferritin by cardiotoxic anthracycline antibiotics. Arch Biochem Biophys. 1986 Aug 1;248(2):684–689. doi: 10.1016/0003-9861(86)90523-0. [DOI] [PubMed] [Google Scholar]
- Thomas C. E., Morehouse L. A., Aust S. D. Ferritin and superoxide-dependent lipid peroxidation. J Biol Chem. 1985 Mar 25;260(6):3275–3280. [PubMed] [Google Scholar]
- Videla L. A., Cáceres T., Lissi E. A. Antioxidant capacity of desferrioxamine and ferrioxamine in the chemically-initiated lipid peroxidation of rat erythrocyte ghost membranes. Biochem Int. 1988 May;16(5):799–807. [PubMed] [Google Scholar]
- Vile G. F., Winterbourn C. C. Adriamycin-dependent peroxidation of rat liver and heart microsomes catalysed by iron chelates and ferritin. Maximum peroxidation at low oxygen partial pressures. Biochem Pharmacol. 1988 Aug 1;37(15):2893–2897. doi: 10.1016/0006-2952(88)90273-0. [DOI] [PubMed] [Google Scholar]
- Willson R. L. Vitamin, selenium, zinc and copper interactions in free radical protection against ill-placed iron. Proc Nutr Soc. 1987 Feb;46(1):27–34. doi: 10.1079/pns19870005. [DOI] [PubMed] [Google Scholar]
- Winterbourn C. C. Inhibition of autoxidation of divicine and isouramil by the combination of superoxide dismutase and reduced glutathione. Arch Biochem Biophys. 1989 Jun;271(2):447–455. doi: 10.1016/0003-9861(89)90295-6. [DOI] [PubMed] [Google Scholar]


