Abstract
Summary
Background:
Irritable bowel syndrome (IBS) is a heterogeneous disorder of gut-brain interaction (DGBI) maintained by interacting biological, psychological, and social processes. Interestingly, there are two contrasting yet evidence-based treatment approaches for reducing IBS symptoms: exclusion diets such as those low in fermentable oligo-, di-, mono-saccharides and polyols (FODMAPs) and exposure-based cognitive-behavioural therapy (CBT). Exclusion diets recommend patients avoid foods thought to be symptom-inducing, whereas exposure-based CBT encourages patients to expose themselves to foods.
Aims:
In this review, we address the paradox of conceptually opposite exclusion diets and exposure-based CBT for IBS.
Methods:
In this conceptual review, we describe the rationale, practical implementation, evidence base, and strengths and weaknesses for each treatment. An up-to-date literature search concerning the low FODMAP diet and CBT was conducted, and a secondary analysis of a previously conducted trial was performed to illustrate a key point in our review.
Results:
The low FODMAP diet has demonstrated efficacy, but problems with adherence, nutritional compromise, and heightened gastrointestinal-specific anxiety raise caution. Exposure-based CBT has demonstrated efficacy with substantial evidence for gastrointestinal-specific anxiety as a key mechanism of action. Mediation analysis also showed that increased FODMAP intake mediated decreased symptom severity in exposure-based CBT. However, there is minimal evidence supporting which treatment “works best for whom” and how these approaches could be best integrated.
Conclusions:
Even though exclusion diets and exposure-based CBT are conceptually opposite, they have proven efficacy. Clinicians should familiarise themselves with both treatments, and further research is needed on predictors, mechanisms, and moderators of treatment outcome.
Keywords: Irritable bowel syndrome, low FODMAP diet, exclusion diets, exposure-based therapy, cognitive-behavioural therapy
Introduction
Irritable bowel syndrome (IBS) is a prevalent disorder of gut-brain interaction, formerly called functional gastrointestinal disorders, defined by recurrent abdominal pain associated with defaecation or change in bowel habits.1 Its aetiology and pathophysiology remain incompletely understood, but there is consensus that IBS symptoms result from complex interactions between biological, psychological, and social processes, for which the microbiota-gut-brain axis constitutes the mechanistic basis.2 Mechanisms shown to be involved in IBS are manifold, including but not limited to altered microbiota composition and function, low-grade inflammation, nutrient intolerances, visceral hypersensitivity, altered motility, and dysfunction of the neural and neuroendocrine stress response systems. In addition, psychological processes including (symptom-specific) anxiety and heightened attention towards gastrointestinal sensations (i.e., hypervigilance) and social events (i.e., observational learning)3 may influence these biological processes. IBS is a heterogeneous condition, and the relative contribution of different pathophysiological mechanisms may vary widely between patients. In other words, different mechanisms along the microbiota-gut-brain axis may result in very similar phenotypical presentations at the symptom level.
This pathophysiological heterogeneity renders IBS treatment difficult. Various treatments including pre-, pro-, and antibiotics,4 neuromodulators,5 exclusion diets6 and brain-gut behavioural therapies5,7 all have proven some effectiveness, but numbers needed to treat are relatively high (ranging from 3 to 9), indicating that a substantial number of patients do not benefit from these treatments. Curiously, two conceptually opposite treatments are both efficacious in IBS (Table 1). On the one hand, exclusion diets recommend that patients avoid foods that are thought to be symptom-inducing. On the other hand, exposure-based cognitive behavioural therapy (CBT) encourages patients to expose themselves to these foods with the aim of improving gastrointestinal symptom severity via a reduction in gastrointestinal-specific fear and anxiety. In this perspective paper, we analyse this paradox and its implications for the status of IBS as a disorder and its treatment. Before doing so, we first discuss both treatments in detail.
TABLE 1.
Similarities and differences between the approaches of exclusion-based diets versus exposure-based therapy for irritable bowel syndrome
| Exclusion diets (low FODMAP diet) | Exposure therapy (CBT) | ||
|---|---|---|---|
| Similarities | Patient-practitioner relationship | Time spent between patient-practitioner in assessment, implementation and review appointments (minimum 2 hrs). | Time spent between patient-practitioner in assessment, implementation and monitoring (minimum 2 hrs). |
| Individualised | Dietetic advice tailors to the individual’s habitual diet, symptom severity and understanding of absorptive capacities and tolerance levels. | Exposure exercises are tailored to the individual to challenge the behaviours that are most debilitating and most likely to maintain the fear of IBS symptoms. | |
| Differences | Mechanisms | Recommends patients to exclude (avoid) foods that are thought to be symptom-inducing through a reduction in osmotic activity and fermentation effects. | Recommends patients to expose themselves to foods with the aim of reducing gastrointestinal symptoms purportedly through a reduction in gastrointestinal- specific fear and anxiety. |
| Favourable patient characteristics | Good knowledge of food, and confidence in selecting and managing appropriate food choices in various environments. | Buy-in to the brain-gut regulation model (i.e. biopsychosocial illness beliefs), presence of avoidance behaviours, willingness and motivation to try a non-pharmacologic and non-dietary intervention. | |
| Unfavourable patient characteristics | Long-term avoidance of foods that trigger symptoms and potential reluctance to reintroduce foods that may not even be implicated in symptoms: the presence of avoidant/restrictive food intake disorder. | The belief that CBT implies that IBS symptoms are “not real”, lack of trust in or motivation for CBT. and somatic/medical illness beliefs. |
Methods
A literature search concerning the low FODMAP diet and CBT was performed. The search was conducted using PubMed with keywords including ‘low FODMAP’ or ‘exclusion diet’, ‘cognitive behaviour therapy’ or ‘exposure therapy’, and ‘irritable bowel syndrome’ or ‘gastrointestinal symptoms’. Keywords were restricted to “Title/Abstract” and additional references were identified through bibliographies of retrieved articles and author suggestions. A secondary analysis of a previously conducted trial8 was also conducted to illustrate a key point in our review, demonstrating how food avoidance data changed between two intervention groups of exposure therapy or stress management, and mediated symptom improvement. Further detail for this analysis is provided in the CBT section.
1. Exclusion diets for IBS
What are exclusion diets?
In exclusion diets, patients are advised to exclude (hence, avoid) food items or nutrients that are thought to induce symptoms. This makes sense theoretically in that avoiding food substances with properties that have been shown to augment water delivery and accumulate colonic gas, can induce symptoms in IBS.9 There is also good empirical scientific evidence showing that exclusion diets reduce symptoms. Exclusion diets for the management of IBS have been previously developed following the principle that the fewer foods allowed in the diet, the greater the chance of success.10 Historically, the use of the exclusion diet is a regime that aims to identify food intolerance via steps of strict exclusion followed by a structured reintroduction program.10 Different types of exclusion diets have been studied and used as dietary therapy for IBS including high-fibre, gluten-free, lactose-reduced, low fat and low fermentable diets.11
Exclusion diets have been growing in popularity in both the scientific and popular literature. Given that most patients relate their IBS symptoms to foods they consume, exclusion diets have high credibility and patient acceptability. Indeed, humans are programmed to avoid foods perceived to cause harm12 and, dietary exclusion is a commonly adopted self-management strategy in 70–89% of individuals with IBS,13 where the more severe the symptoms reported, the higher the number of foods excluded.14 In addition, the majority of patients report trialling dietary therapies and more than half will simultaneously use dietary manipulations.15
The low FODMAP diet as the prototypical exclusion diet for IBS
Introduction of the FODMAP concept and rationale for the exclusion diet.
Among the many dietary strategies investigated for IBS management, a diet low in fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAPs) is the most commonly recommended by healthcare providers11 and there is strong evidence for its effectiveness. The FODMAP concept aims to reduce the intake of several short-chain carbohydrates commonly found in the diet, leading to reduced small intestinal water content and reduced microbial fermentation in the colon, thereby limiting symptom induction in people with IBS.9 Achieving symptom reduction with reduced intake of high FODMAP foods is then indicative for further exclusion of those foods.
Rationale for the low FODMAP Diet in IBS.
In the low FODMAP diet, the dietary exclusion focuses on key ingredients that are poorly absorbed and that have osmotic or fermentation effects purported to induce symptoms of IBS. Mechanistic studies demonstrate FODMAPs increase small intestinal water content in ileostomy outputs16 and increase colonic gas shown via breath hydrogen levels,17 leading to increased gas, distention of the colon and causing flatulence, bloating and discomfort in IBS compared to healthy subjects.17 Induction of symptoms by FODMAPs is specific to IBS due to the heightened visceral hypersensitivity rather than increased intestinal gas production or water content, shown via bowel magnetic resonance imaging in IBS patients compared to healthy subjects.18 Symptom onset occurs within 4 hours of FODMAP intake17,18 and symptom improvement occurs within days of starting a low FODMAP diet.19
Practical implementation of the three phases of the FODMAP exclusion diet.
The practical implementation of the low FODMAP diet follows a three-phase dietary intervention involving (i) an exclusion (or “elimination”) phase, (ii) re-introduction phase and, (iii) a challenge (or “personalization”) period.20 The initial exclusion phase involves the restriction of FODMAPs for a recommended duration of four weeks. During this period, the goal in practice is to ascertain if FODMAP exclusion leads to any symptomatic relief (where response is defined by patients self-reporting symptom improvement), indicating whether to proceed with the FODMAP approach or not. The second phase focuses on re-introduction of dietary FODMAPs. Challenge foods are consumed in increasing amounts over a 3-day period whilst continuing with an overall low FODMAP intake. Challenges aim to identify individual FODMAP triggers and a personal threshold to FODMAP containing foods. Lastly, after re-introduction (which can last 6–10 weeks), the personalisation phase begins with the goal of consuming a varied diet inclusive of FODMAPs, but below the threshold to which symptoms arise.20,21 All of these steps are performed with dietary counselling, under the guidance of a gastrointestinal experienced dietitian.
Evidence for efficacy of the FODMAP exclusion diet.
There is evidence for efficacy of the low FODMAP diet based on several meta-analyses, showing it can significantly improve global IBS symptoms, abdominal pain, bloating and flatulence.6,22–25 The most recent meta-analysis found a low FODMAP diet was more efficacious than a habitual diet for reducing global symptoms (RR of 0.67; 95% CI 0.48 to 0.91), and was superior to alternative interventions for abdominal bloating or distension (RR of 0.72; 95% CI 0.55 to 0.94) and abdominal pain severity (RR of 0.51; 95% CI 0.30 to 0.87) using data from thirteen RCTs.26 The alternative interventions included the British Dietetic Association (BDA) or the National Institute for Health and Care Excellence (NICE) guidelines for people with IBS as well as inactive control interventions such as sham dietary advice. The quality of evidence was considered low due to risk of bias from lack of double blinding and suboptimal adverse event reporting.26 According to the GRADE criteria, evidence for efficacy of the low FODMAP diet has been considered moderate.27 This is due to the inherent limitations of trial design including blinding challenges and conduct of dietary intervention trials for disorders of gut-brain interaction in general28 that are further compounded in FODMAP research specifically due to the nature of its whole diet delivery. Factors attributed as important to the efficacy across these RCTs include dietitian-delivered intervention and using relatively standardised FDA recommended endpoints.26 The RCTs implementing the low FODMAP diet have been mostly short-term and conducted in secondary or tertiary care, have examined the exclusion phase only, or excluded subgroups of IBS, particularly IBS-C.
Moderate evidence for efficacy versus active control conditions.
Assessing the efficacy of the low FODMAP diet is highly dependent on the control intervention, where the evidence shows a clear advantage over high FODMAP diets29 and habitual or usual diets,19,30–32 but less conclusive results when assessing the trials that use more appropriate active control diets individually, such as those based on dietary recommendations from the NICE33 and the BDA.34–37 Interestingly, the most recent meta-analysis, that includes these studies shows the low FODMAP diet to be significantly more efficacious.26 Overall, the evidence supports recommending a low FODMAP diet in IBS26 and data demonstrates the low FODMAP diet to be effective in 50–80% of individuals with IBS.9 This has led to the diet being included in the NICE Guidelines for IBS management in primary care in the UK and recommended as second line advice by the BDA Guidelines.38
Reduction in FODMAP intake correlates with symptom improvement.
Improvement at the individual symptom level (e.g., abdominal pain, bloating, flatulence and diarrhoea) has been found to be weakly associated with adherence to the low FODMAP diet (r=−0.26).39 This was further emphasized in more recent association analyses from a Swedish RCT in 66 patients with IBS, that showed participants who adhered better to a 4-week low FODMAP diet (consumed lower FODMAP intake) had a larger symptom response (r=−0.30).40 Together, these findings highlight that adherence may predict symptom improvement, albeit only weakly.
Limitations and pitfalls of the low FODMAP exclusion diet
Instructing patients to exclude a broad range of purportedly symptom-provoking (but often otherwise healthy) foods can be challenging for them in several ways. Below, we detail three key limitations/pitfalls of the low FODMAP exclusion diet—adherence, nutritional compromise, and in some cases, heightened fear and anxiety around gastrointestinal symptoms leading to greater, generalized, and potentially problematic food avoidance. Due to these three pitfalls, a subset of patients may develop a level of food avoidance/restriction that crosses the threshold into avoidant/restrictive food intake disorder due to resulting medical and/or functioning impairments.41 In fact, recent data suggests that history of trying an exclusion diet like the low FODMAP diet is associated with a three times greater likelihood of having avoidant/restrictive food intake disorder symptoms in a broad range of DGBI.42
Challenges to adherence.
Although the literature consistently shows adherence to the low FODMAP diet to be good,40 patients often describe difficulty in following the diet, particularly when eating away from home due to finding the diet to be restrictive, expensive, time-consuming, or difficult topurchase.43 Factors associated with efficacy and adherence include dietitian consultation and education materials.39 Patient self-initiation of dietary management is common and while gastroenterology providers may feel comfortable prescribing the low FODMAP diet,11 patients may have difficulty with implementation without close guidance (e.g., without a dietitian).44
Over one-third of patients follow the first exclusion phase for longer than recommended and only 40% move onto the later phases.15 It has been suggested that the re-introduction is difficult due to the patient’s fear of symptom recurrence. Overall, there is limited data on the re-introduction and personalisation phases of the diet outside of prospective and retrospective real-world cohorts. Similarly, high quality studies regarding the long-term effects of the low FODMAP diet are lacking. Given 80% of patients report still excluding particular FODMAP-rich foods (median of 24 months following dietitian-led education) for longer term maintenance of symptom control,45 there is potential concern for detrimental effects on their microbiota profiles.9 However, Bifidobacteria abundance may normalise after 12 months of FODMAP personalisation,46 demonstrating importance of the re-introduction and personalisation phases.
Nutritional compromise.
Due to the restrictive nature of the elimination phase of the low FODMAP diet, some patients may be at risk of nutritional inadequacies, particularly in non-dietitian led dietary implementation and compounding the IBS individuals who already fail to meet national nutrient recommendations.47 Previous studies have reported lower carbohydrate, lower energy and lower calcium intakes in individuals following a low FODMAP diet compared with habitual diets, however these nutrients as well as overall nutrient intake and measures of diet diversity were not significantly impacted when delivered by a specialist dietitian.47 Overall scores for diet quality have been shown to be lower after 4-weeks of following low FODMAP advice,47 and a case-series study showed over half of patients reported using concurrent dietary strategies (‘diet-stacking’),15 highlighting concerns for an over-restrictive diet and further compromising nutritional adequacy.
Reduced food-related quality of life.
Use of exclusion diets has been associated with reduced food-related quality of life in IBS48 and high dietary avoidance has been associated with both poorer IBS-related quality of life and poorer nutrient intake.49 In fact, greater dietary restriction with quality of life impairments may be indicative of disordered eating in line with avoidant/restrictive food intake disorder.50
Gastrointestinal-Specific Anxiety and Risk for Avoidant/Restrictive Food Intake Disorder.
Food avoidance/dietary restriction in some patients may heighten food-related fears and paradoxically perpetuate IBS symptom maintenance via increased anxiety around IBS symptoms (i.e., gastrointestinal-specific anxiety). The low FODMAP exclusion diet explicitly instructs patients to avoid foods to attempt absence of gastrointestinal symptoms, and there is evidence that the opportunity to avoid increases fear levels when avoidance is not possible.51 Although the elimination phase of the low FODMAP diet is intended to be short-term, patients may struggle to re-introduce foods post-elimination because of increased threat value of certain “forbidden” foods. Indeed, real-world evaluation (via case-series) have shown compliance is poor in the re-introduction phase and even less in the personalization phase, especially without dietitian guidance.15 For example, while re-introducing FODMAP foods, patients may pay heightened attention to gastrointestinal symptoms and as soon as they notice a symptom, interpret the food they consumed to have caused the symptom. While this reaction can be normal in some cases where patients identify a handful of foods to avoid in the long-term, some patients may become conditioned to ascribe any symptom change to foods, even generalized beyond high FODMAP foods. The conditioned fear learning process that can occur aligns with the Fear-Avoidance model of pain, through which avoidance of a stimulus (e.g., food) for the purposes of avoiding an aversive outcome (pain or other symptoms) heightens fear of that stimulus.52 That is, avoidance of foods perceived to be associated with GI symptoms, increases fear of certain foods.
As a result of this conditioned fear learning, there is growing awareness and concern that the low FODMAP diet may put a subset of patients at risk for developing food avoidance/dietary restriction at the level of avoidant/restrictive food intake disorder.41,50,53–57 Avoidant/restrictive food intake disorder is classified in the Diagnostic and Statistical Manual of Mental Disorders, fifth edition (DSM-5) as a feeding and eating disorder characterized by restricted food volume and/or variety that leads to medical (e.g., nutritional deficiency, weight loss) and/or psychosocial impairments (e.g., social eating avoidance).58 Food avoidance/restriction in avoidant/restrictive food intake disorder is not motivated by body image disturbance seen in other restrictive feeding/eating disorders (e.g., as in anorexia nervosa); instead, patients with avoidant/restrictive food intake disorder describe motivations of one or multiple prototypical presentations—sensory sensitivity (e.g., taste, smell, texture), lack of interest in eating/low appetite (e.g., forgetting to eat, high satiety), and fear of aversive consequences (e.g., of diarrhoea, pain, constipation).58,59 Cross sectional data shows that between 13–40% of paediatric and adult patients with disorders of gut-brain interaction have avoidant/restrictive food intake disorder symptoms,60–63 with avoidant/restrictive food intake disorder in close to 50% of those with IBS.64 Across studies, the most frequent food avoidant/restrictive presentation has been fear of aversive consequences, specifically fear of gastrointestinal symptoms.60,62–64
Although further longitudinal research is needed to identify predictors of the development of avoidant/restrictive food intake disorder, the presence of avoidant/restrictive symptoms among patients with IBS is of crucial importance for treatment decision-making. Specifically, the treatment of avoidant/restrictive food intake disorder is diametrically opposed to that of the low FODMAP diet. Food avoidance/restriction is treated with CBT during which purposeful and repeated exposure to food volume and variety challenges conditioned learning around food.65 In the case of the avoidant/restrictive food intake disorder fear of aversive consequences presentation, the mechanism of change through which exposure purportedly works is via a violation of expected negative outcomes such as gastrointestinal symptoms.65 While exclusion diets recommend patients avoid foods that are thought to be symptom-inducing, exposure-based CBT advises patients to expose themselves to these foods. Exposure-based CBT is in fact an evidence-based treatment for IBS, outside the context of avoidant/restrictive food intake disorder, and there is growing research indicating decreases in gastrointestinal-specific anxiety is the core mechanism of change.
2. Exposure-based cognitive behavioural therapy for IBS
What is exposure-based CBT?
Cognitive behavioural therapies (CBTs) include a myriad of techniques that target interactions between cognitions, emotions, and behaviours. Not all CBTs include the same techniques (i.e., the “ingredients”) because the ingredients that aim for symptom change can be different. Exposure-based CBT focuses on behavioural techniques that promote exposure to stimuli that are feared and/or avoided in order to create an expectancy violation, usually followed by more approach behaviour and fear reduction. A recommended application of exposure-based CBT is with specific phobias (e.g., spider phobia), and exposure-based techniques are viewed as the treatment of choice for anxiety disorders.66
Exposure-based CBT for IBS
CBTs for IBS have included different CBT techniques with varying emphasis. CBTs that purport general stress as the targeted IBS maintenance mechanism have included relaxation training (e.g., diaphragmatic breathing)67 and traditional cognitive techniques (e.g., cognitive restructuring).68 However, heightened stress is not always a predictor of IBS symptoms69 and there is limited evidence that targeting stress improves IBS symptoms (for a more extensive discussion, see Ljótsson et. al).69 Additionally, stress-targeted CBTs for IBS do not explicitly address food avoidance. Instead, CBTs that purport gastrointestinal-specific anxiety as the targeted IBS symptom maintenance mechanism use exposure-based techniques and have demonstrated that decrease in gastrointestinal-specific anxiety mediates treatment effects on symptom.69–71 Exposure-based CBT for IBS has patients conduct planned experiments to approach a food-related stimulus expected to provoke IBS symptoms. Below, we describe gastrointestinal-specific anxiety’s role in the maintenance of IBS and outline the rationale and evidence for exposure-based CBT in IBS.
Gastrointestinal-specific anxiety as a maintenance mechanism in IBS.
Gastrointestinal-specific anxiety, defined as food-related concerns, fear of symptoms and excessive behavioural avoidance,72 has been identified as the primary variable mediating the relations between risk factors (e.g., general trait anxiety) and IBS symptoms severity.73 Moreover, gastrointestinal-specific anxiety has been shown to be associated with increased IBS symptoms in cross-sectional studies74,75 and in a longitudinal study with a 5-year follow-up.76 Mayer et al. suggested that aversive experiences in IBS can over time produce a learned association between visceral pain and food-related stimuli, resulting in fear of those stimuli.77 Negative expectancies increase the likelihood of IBS symptoms. In addition, the psychophysiological arousal associated with fear also induces altered motility, which can further exacerbate IBS symptoms.77,78 A natural response to threatening stimuli is avoidance. However, avoidance precludes learning that the stimuli are actually not dangerous.79 To illustrate, a patient with IBS experiences abdominal pain after drinking milk, which leads to an association between milk and pain, resulting in the avoidance of drinking milk. If they would try that food again, the learned fear response can lead to both altered gastrointestinal motility and increased visceral sensations, meaning that the patient experiences pain again, but not only because of the effect of the food itself on the digestive system. As a result, the patient starts to avoid this food, which perpetuates the fear of the food, and the fear may even generalize to other foods. In the long term, avoidance may in itself lead to heightened fear of gastrointestinal sensations, thereby perpetuating gut-brain dysregulation in IBS.
Rationale for exposure-based CBT in IBS.
Exposure-based CBT involves approaching previously avoided stimuli in order to create an expectancy violation, namely that the catastrophic outcome does not occur. Such a surprise experience generates a new association ‘food – no pain’ that competes with the existing one, and can inhibit it.66 In exposure-based CBT for IBS, repeated exposure to stimuli that are perceived to provoke symptoms reduces: (1) avoidance of the stimuli, (2) fear of those stimuli, and (3) worry and concerns about the symptoms. Decreases in these mechanisms in turn produce a decrease in IBS symptoms.
Practical implementation of exposure therapy for IBS.
Exposure-based CBT techniques have patients expose themselves to stimuli believed to provoke symptoms. With some stimuli, the goal is for patients to either experience that the likelihood of symptoms actually happening is low or to learn that even if symptoms occur, they are able to function despite having symptoms. This latter learning experience is arguably the most realistic, as experiencing some degree of gastrointestinal distress is to be expected when initially re-introducing some foods that had been removed from the patient’s diet. Exposure-based CBT techniques for IBS have been included in various protocols80 and in various formats including guided self-help with therapist email support,8,81–84 mobile self-help,85 and in-person formats.66,86 While several studies have included exposure as one of several components of the treatment, one of the authors (BL) has led a series of studies of a 10-week CBT delivered over the internet that primarily focuses on exposure for IBS in adults.8,82–84 Below, we briefly describe the protocol.
The protocol focuses on behavioural exposure, but first uses brief training to promote awareness of feared stimuli and one’s cognitive and behavioural reactions to these stimuli. Awareness training is also intended to promote engagement in behavioural exposure through acceptance of symptoms and cognitive/emotional reactions to symptoms. Before the behavioural exposure starts, patients create a list of avoidance behaviours to facilitate planning exposure exercises. Exposure exercises are tailored to address the behavioural pattern of each individual patient. Common examples of exposure exercises are extended time between toilet visits, going by public transport, physical exercise, and provoking symptoms before attending a dinner or social event. The exposure exercises will for most patients include eating certain foods that are perceived by them to provoke symptoms. Patients may even expose themselves to foods that they will not necessarily want to have in their diet in the longer term, to facilitate decreasing fear and anxiety around IBS symptoms.
Evidence for exposure-based CBT in improving IBS outcomes.
Exposure-based CBT for IBS has demonstrated efficacy in improving IBS symptoms and associated quality of life, but it is still a growing area of research. A meta-analysis on the comparative effectiveness concluded that CBT had the largest effect on improving IBS-related quality of life, noting that the inclusion of exposure-based techniques may be responsible for this finding.87 In addition, the above internet-delivered protocol in one study had the largest effect size of 41 behavioural health treatment studies for improving symptom severity.87,88 The exposure-based protocol has shown large effects compared to waitlist control in improving IBS symptoms (Cohen’s d=0.75–1.21) and IBS-related quality of life (d=0.82–0.93).82,83 The protocol has also shown small effects in improving IBS symptoms (d=0.38) and IBS-related quality of life (d=0.26) compared to stress management.84 A dismantling study demonstrated that the exposure component was necessary to achieve large treatments effects (incremental effect d=0.47).8 Further analyses that took treatment adherence status into account showed a much larger difference between those who received the exposure component and those who did not (incremental d=0.81), further supporting the claim that increased repeated exposure improves IBS symptom severity.89 Although other meta-analyses have reported inconclusive effects of CBT on IBS symptoms,5,90,91 they did not compare the effects of different types of CBTs for IBS (e.g., those with versus those without exposure-based techniques). It should be noted that while we do not yet have evidence comparing internet-delivered to provider-delivered exposure CBT in IBS, there is increasing evidence in other conditions (e.g., anxiety) that internet-delivered CBT overall shows similar effects.92
Evidence for exposure-based CBT mechanisms of action.
What are the mechanisms through which CBT works?93 In exposure-based CBT for IBS, the ingredient of behavioural exposure concerns the decrease of gastrointestinal-specific anxiety, via which IBS symptoms decrease. Three studies have shown that change in gastrointestinal-specific anxiety indeed mediated treatment outcomes.69–71 Specifically for the internet-delivered exposure-based CBT protocol described above, decreases in both gastrointestinal-specific anxiety69,71 and avoidance behaviors71 mediated the difference in change in IBS severity in those who received exposure-based CBT compared to control groups, whereas reduced stress did not mediate treatment effects.69 Furthermore, highlighting the focus of the exposure therapy on avoidance and control behaviour, a moderation analysis showed that the exposure-based CBT is more effective in terms of symptom improvement for individuals who have greater avoidance at the beginning of treatment.94
The effect of exposure-based CBT on FODMAP avoidance.
Specifically for the present article, we performed a secondary analysis on food avoidance based on data collected in the online RCT that compared exposure-based CBT therapy to stress management.84 In that trial, 98 patients received exposure therapy that encouraged them to try all types of food that they thought could trigger IBS symptoms, and 97 patients received stress management that included advice on identifying symptom triggering food and reducing or avoiding intake of that food. Both these interventions were delivered online and included written therapist support on a regular basis. Before and after treatment, patients completed a questionnaire where they indicated how often they avoided specific foods because of their IBS (on a 0=never avoid to 3=always avoid scale). Twenty of the food items were identified by the first author (JB) as high FODMAP foods and were included in the analysis. We first investigated if food avoidance changed in the two intervention groups (exposure vs. stress management). In the second analysis we investigated if the post-treatment food avoidance status mediated the difference between the groups in post-treatment symptom severity, measured by the GSRS-IBS.95,96 In the mediation analysis, we calculated the ab product, i.e., the mediated effect, with a bootstrapped 95% confidence interval (CI; based on 5000 replications) as proposed by Preacher and Hayes (2004).97 The ab/c proportion was also calculated, i.e., how much of the effect of the exposure treatment (vs stress management) on IBS symptom severity was accounted by the difference in food avoidance between the intervention groups.98
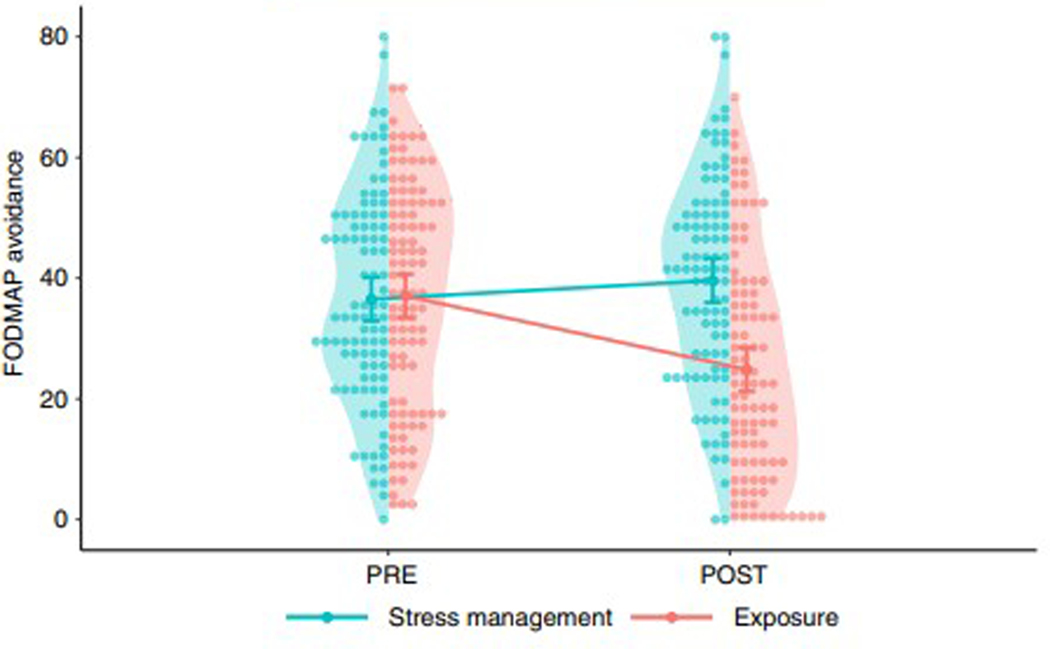
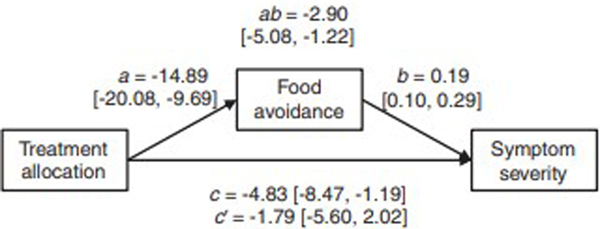
Post-treatment FODMAP avoidance data was available for 85 (87%) of 98 patients in the exposure group and 81 (84%) of 97 patients in the stress management groups. Linear mixed models identified a significant difference (p<.001) in how food avoidance changed in the two groups during treatment (Figure 1). In line with the treatment rationales, the exposure group decreased their FODMAP avoidance (p<.001), while an increase was observed in the stress management group (p=.013). In the mediation analysis, the mediated effect was ab=−2.90, bootstrapped 95% CI [−5.08, −1.22], i.e., the ab product was statistically significant, which indicates that the effect of treatment on symptom severity was mediated by change in food avoidance. The direct effect of treatment allocation was c=−4.83 points on the GSRS-IBS, meaning that −2.90/−4.83 = 60% of the difference in symptom severity between exposure treatment and stress management was accounted for by the difference in food avoidance. The remaining effect after controlling for the mediator (usually denoted c’, c-prime) was −1.79, 95% CI [−5.60, 2.02], i.e., the remaining effect of treatment allocation not explained by reduced FODMAP avoidance was not significant. Figure 2 shows a path diagram of the mediation analysis.
FIGURE 1.
Pre- and post-treatment values of FODMAP avoidance in the online randomised controlled trial of exposure therapy vs. stress management for IBS. The exposure therapy arm (n = 98) involved completing exposures to foods and situations that patients feared provoked IBS symptoms. The stress management arm (n = 97) involved advice on identifying symptoms triggering food and reducing or avoiding intake of that food. The shaded areas show the distribution of values, and the dots show individual scores. Lines show average scores with 95% confidence intervals.
FIGURE 2.
Path diagram of mediation analysis for post- treatment FODMAP avoidance data comparing exposure therapy to stress management. Estimates are given with 95% confidence intervals in brackets. A-path = effect of treatment allocation on FODMAP avoidance. B-path = effect of FODMAP avoidance on symptom severity. Ab-path = Total mediated (indirect) effect. C-path = direct treatment effect (not controlling for FODMAP avoidance) on symptom severity. C’-path = remaining treatment effect on symptom severity when controlling for the mediator (FODMAP avoidance).
These results indicate that the paradox that two opposite treatments work for the same disorder is not only conceptual, but also exists at the level of mediating mechanisms. That is, the increase in FODMAP intake mediated the decrease in IBS symptoms after exposure-based CBT, while the decrease in FODMAP intake (supposedly) mediated the decrease in symptoms after the low FODMAP diet. Specifically, a decrease in FODMAP avoidance partly explains why exposure-based CBT led to larger improvement in IBS symptoms versus stress management. This indicates that decreases in FODMAP avoidance (i.e., increased dietary exposure to FODMAPs) is a key mechanism that exposure-based CBT engages to produce IBS symptom improvement. Thus, in exposure therapy, increasing high FODMAP foods actually improves IBS symptoms.
Limitations and pitfalls of exposure-based CBT for IBS
There are also limitations to the current application of exposure-based CBT for IBS. First, not all patients with IBS report gastrointestinal-specific anxiety and associated avoidance behaviour—for these patients, behavioural therapy that focuses on improving symptom management or a dietary approach could be more appropriate. We also do not know of those with IBS who meet criteria for ARFID, for whom exposure-based CBT for IBS is sufficient, or for whom more specialized CBT focused on ARFID is needed (e.g., for patients who need to gain weight). Second, not all patients may find exposure-based CBT acceptable, as exposure may lead to temporary increase in symptom load and both therapists and patients need to be prepared for this. If this effect is unexpected or unwanted, it might lead to early termination of a treatment that could potentially be beneficial. Third, we do not yet know which “ingredients” are needed in exposure-based CBT; for example, some protocols have combined exposure with other CBT techniques (e.g., cognitive restructuring, awareness training), which may not be necessary or may not be necessary for all patients.
3. The paradox of conceptually opposite yet efficacious treatments – what does it tell us about IBS and its treatment?
IBS as a pathophysiologically heterogeneous syndrome
One way to reconcile the paradox is to acknowledge IBS (in its current symptom-based definition) as a pathophysiologically heterogeneous syndrome. Please note that we use pathophysiology in the broad sense here, including psycho(physio)logical mechanisms, in line with IBS conceptualization as a disorder of gut-brain interaction. This may indeed explain why conceptually opposite treatments have proven efficacy in the same disorder: they may both work “on average” (i.e. at the group level in clinical trials) because they work for pathophysiologically different subgroups of patients. The relatively high NNTs for these treatments (and IBS treatments in general) are in line with such interpretation in that every study will contain subgroups of patients with an underlying pathophysiology that is not being targeted by the treatment under study, for whom the treatment consequently will not work. Indeed, patients are usually recruited based on symptom-based diagnostic criteria only, rather than pathophysiological mechanisms. That said, it is also conceivable that patient samples in different treatment studies differ because of selection bias. For example, a dietary trial may attract more patients for whom nutritional factors and (perceived) intolerances may play a more prominent role. Conversely, an (exposure-based) CBT study may primarily recruit patients for whom (symptom-specific) anxiety is a major pathophysiological factor, even though both trials recruit based on Rome IV diagnostic criteria. This is highly relevant in explaining the proven efficacy of the conceptually different treatments especially since patient preferences and beliefs about the nature of their disorder and, hence, about the rationale and effectiveness of the treatment for it shape expectations about treatment success, which is an important predictor of its actual success especially when outcomes are symptoms as is the case for IBS.99,100 Consequently, trying to establish superiority of one treatment over the other for IBS as a whole may not be the highest priority in head-to-head trials, but focusing on differential predictors, whether clinical, psychological or physiological, of treatment success may be more important to tailor treatment optimally to each individual patient.101 This is especially the case as treatment selection is currently often done on a trial-and-error basis, or in a rigid serial fashion according to guidelines (see below), to the frustration of patients and clinicians alike.
What works for whom?
Unfortunately, research on such predictors, in other words on the question “what works for whom”, remains relatively scarce in IBS, and should therefore figure prominently on the future research agenda on IBS treatments. Although faecal microbiota profiles102 and methane and fatty acid metabolism pathways103 of patients with IBS may be predictive of low FODMAP diet efficacy, there is minimal research into clinical predictors of responsiveness. As mentioned above, it has recently been shown that exposure-based CBT works better for subject with higher levels of avoidance prior to treatment.94 However, more research into this important question is clearly needed. For example, while we show that decreased food avoidance mediates exposure-based CBT outcomes, the presence of avoidant/restrictive food intake disorder has not yet been examined as a predictor (i.e. moderator) of treatment outcomes for exposure-based CBT. It is conceivable that in addition to treatment-specific predictors of success (e.g., higher degree of avoidance behaviour for exposure-based CBT, lower levels of trait anxiety and anxiety sensitivity for more cognitively oriented CBT versus education),104 general predictors of success across treatments may also exist. Patient beliefs and expectations may constitute such an important general predictor, which would imply that taking them into account when allocating patients to a certain treatment could improve success rates, in addition to specific interventions to boost patients’ treatment expectations regardless of which treatment they undergo.105,100
Aligned with precision medicine efforts, growing research is investigating profiles of patient presentations to inform treatment recommendations. For example, a recent investigation identified seven profiles for IBS integrating both IBS symptom severity and psychological burden.106,107 Future research should investigate how such profiles may differentially predict outcomes in dietary and behavioural treatments.
Outcome studies adopting a single-case experimental design may be helpful in testing the efficacy of treatments in single persons considering their idiosyncratic characteristics. These are designs in which a single entity (the individual) is observed repeatedly during a certain period under different levels of at least one manipulated variable (the treatment). Given their flexibility, their low cost and their focus on outcomes on the level of the individual, single-case experimental designs have become increasingly popular in the behavioural and medical sciences.108,109 Single-case experimental designs are preferable to more conventional group-based RCT’s, when the magnitude of the effect within an individual is more relevant than the average effect across a group of individuals.110,111 Additionally, meta-analytic procedures can be used for the aggregation of replicated single-case experiments, which provides decision-making opportunities at both the individual and the population level.112 Our suggestions for a future research agenda are outlined in Table 2.
TABLE 2.
Future research agenda for exclusion diets and exposure-based therapy for irritable bowel syndrome
| Research questions to be answered | How this might be achieved |
|---|---|
| What is the efficacy of the two treatments in single individuals? | Single-case experimental design where an individual is repeatedly observed whilst undertaking different treatments. |
| What are predictors (i.e. moderators) of treatment response? | Clinical trials assessing likely common (e.g. [outcome] expectancy and beliefs) as well as treatment- specific (e.g. microbiota for low FODMAP diet, severity of Gl-specific avoidance behaviours) predictors of response. |
| Can predictors of response be used to improve the choice of treatment and therefore response? | Randomised controlled trial 2×2 design where patients are allocated to different treatments most/ least suited. |
| What are likely mechanisms of treatment effect? | Clinical trials measuring mediators (e.g. FODMAP intake as a common mediator. Gl-specific anxiety as a differential mediator) and outcomes at regular intervals during the treatment phase. |
| Does treatment augmentation and/or integration improve response? | Clinical trials evaluating incorporation of low FODMAP diet and CBT (e.g. incorporating exposure- based therapy education and principles more directly into dietary management practices for exclusion diets). |
Clinical implications
Since both treatments discussed in this paper, even though they are conceptually opposite (Table 1), have proven efficacy, we believe clinicians working with IBS patients, regardless of their specialty/background, should know both treatments with their pros and cons. This should also allow them to combine elements from both treatments where needed. For example, knowledge of the rationale and theoretical foundation of exposure-based CBT highlights the importance of the reintroduction and personalization phases of the low FODMAP diet to prevent the development of ARFID or other overly restrictive eating behaviours as well as nutritional deficiencies. Individuals reporting high gastrointestinal-specific anxiety/avoidance and/or weight loss due to gastrointestinal symptoms may be considered for exposure-based CBT over the low FODMAP diet, or for modified dietary exclusion approaches. Moreover, knowledge of exposure-based techniques could be used by the dietician to help patients overcome excessive fear of re-emerging symptoms during reintroduction. Conversely, knowledge of the exclusion diet literature may help the CBT therapist when meeting resistance towards exposure-based CBT, or when confronted with exposure to certain foods leading to uncontrollable symptoms early in treatment.
However, integrating elements of both treatments and tailoring treatment to the individual patient is not without challenges. Accessibility of both dietitians and psychologists specialised in this matter will not always be available, but we believe it is key, not only to be able to offer both treatments to patients and/or integrate them, but also to perform dietetic and psychological assessment prior to assigning a patient to one of the treatments. In practice, dietary interventions are typically more easily accessible from gastroenterology clinics, and are also positioned earlier in typical treatment guidelines compared to brain-gut behavioural therapy, which often causes them to be attempted first, without taking individual patient characteristics into account. We believe a more individually tailored treatment approach is warranted, especially as the problems with access to brain-gut behavioural therapies can be largely overcome by implementing internet-based approaches, which have proven efficacy as evident from our review.
Conclusion
The paradox of two conceptually opposite yet efficacious treatments for IBS, the FODMAP exclusion diet (promoting avoidance) and exposure-based CBT (reducing avoidance) highlight the pathophysiologically heterogeneous nature of IBS as a symptom-based disorder as well as a potential role of non-specific factors such as treatment beliefs and expectations in determining treatment efficacy. This paradox can hence be resolved by performing research on differential predictors for response to each of these treatments, (e.g. by applying single-case experimental designs), which should lead to a better individual tailoring of treatment options for IBS patients. Clinicians should be aware of both treatment options including their pros and cons, as integrating elements from both treatments may lead to better patient care by avoiding some of the pitfalls and limitations of each individual treatment modality.
Acknowledgement
JV is supported by the Asthenes long-term structural funding – Methusalem grant (#METH/15/011) by the Flemish Government, Belgium, and a grant from the Dutch Research Council NWO (Grant# 406.20.GO.032). HBM is supported by the National Institutes of Health (K23 DK131334 and U24NS113850–02).
Footnotes
Conflict of interest
BL owns and licenses an irritable bowel syndrome CBT manual for commercial purposes.
References
- 1.Lacy BE, Mearin F, Chang L, et al. Bowel Disorders. Gastroenterology. 2016;150(6):1393–1407.e1395. [DOI] [PubMed] [Google Scholar]
- 2.Van Oudenhove L, Levy RL, Crowell MD, et al. Biopsychosocial Aspects of Functional Gastrointestinal Disorders: How Central and Environmental Processes Contribute to the Development and Expression of Functional Gastrointestinal Disorders. Gastroenterology. 2016;150(6):1355–1367.e1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karos K, Williams ACC, Meulders A, Vlaeyen JWS. Pain as a threat to the social self: a motivational account. Pain. 2018;159(9):1690–1695. [DOI] [PubMed] [Google Scholar]
- 4.Ford AC, Harris LA, Lacy BE, Quigley EMM, Moayyedi P. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther. 2018;48(10):1044–1060. [DOI] [PubMed] [Google Scholar]
- 5.Ford AC, Lacy BE, Harris LA, Quigley EMM, Moayyedi P. Effect of Antidepressants and Psychological Therapies in Irritable Bowel Syndrome: An Updated Systematic Review and Meta-Analysis. Am J Gastroenterol. 2019;114(1):21–39. [DOI] [PubMed] [Google Scholar]
- 6.Dionne J, Ford AC, Yuan Y, et al. A Systematic Review and Meta-Analysis Evaluating the Efficacy of a Gluten-Free Diet and a Low FODMAPs Diet in Treating Symptoms of Irritable Bowel Syndrome. Am J Gastroenterol. 2018;113(9):1290–1300. [DOI] [PubMed] [Google Scholar]
- 7.Keefer L, Ballou SK, Drossman DA, Ringstrom G, Elsenbruch S, Ljótsson B. A Rome Working Team Report on Brain-Gut Behavior Therapies for Disorders of Gut-Brain Interaction. Gastroenterology. 2022;162(1):300–315. [DOI] [PubMed] [Google Scholar]
- 8.Ljótsson B, Hesser H, Andersson E, et al. Provoking symptoms to relieve symptoms: a randomized controlled dismantling study of exposure therapy in irritable bowel syndrome. Behaviour research and therapy. 2014;55:27–39. [DOI] [PubMed] [Google Scholar]
- 9.Staudacher HM, Whelan K. The low FODMAP diet: recent advances in understanding its mechanisms and efficacy in IBS. Gut. 2017;66(8):1517–1527. [DOI] [PubMed] [Google Scholar]
- 10.Parker T, Naylor S, Riordan A, Hunter J. Management of patients with food intolerance in irritable bowel syndrome: the development and use of an exclusion diet. Journal of Human Nutrition and Dietetics. 1995;8(3):159–166. [Google Scholar]
- 11.Lenhart A, Ferch C, Shaw M, Chey WD. Use of Dietary Management in Irritable Bowel Syndrome: Results of a Survey of Over 1500 United States Gastroenterologists. Journal of neurogastroenterology and motility. 2018;24(3):437–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garcia J, Lasiter PS, Bermudez-Rattoni F, Deems DA. A general theory of aversion learning. Annals of the New York Academy of Sciences. 1985;443:8–21. [DOI] [PubMed] [Google Scholar]
- 13.Werlang ME, Palmer WC, Lacy BE. Irritable bowel syndrome and dietary interventions. Gastroenterology & hepatology. 2019;15(1):16. [PMC free article] [PubMed] [Google Scholar]
- 14.Bohn L, Storsrud S, Tornblom H, Bengtsson U, Simren M. Self-Reported Food-Related Gastrointestinal Symptoms in IBS Are Common and Associated With More Severe Symptoms and Reduced Quality of Life. Am J Gastroenterol. 2013;108(5):634–641. [DOI] [PubMed] [Google Scholar]
- 15.Tuck CJ, Reed DE, Muir JG, Vanner SJ. Implementation of the low FODMAP diet in functional gastrointestinal symptoms: A real-world experience. Neurogastroenterology & Motility. 2020;32(1):e13730. [DOI] [PubMed] [Google Scholar]
- 16.Barrett JS, Gearry RB, Muir JG, et al. Dietary poorly absorbed, short-chain carbohydrates increase delivery of water and fermentable substrates to the proximal colon. Aliment Pharmacol Ther. 2010;31(8):874–882. [DOI] [PubMed] [Google Scholar]
- 17.Ong DK, Mitchell SB, Barrett JS, et al. Manipulation of dietary short chain carbohydrates alters the pattern of gas production and genesis of symptoms in irritable bowel syndrome. J Gastroenterol Hepatol. 2010;25(8):1366–1373. [DOI] [PubMed] [Google Scholar]
- 18.Major G, Pritchard S, Murray K, et al. Colon Hypersensitivity to Distension, Rather Than Excessive Gas Production, Produces Carbohydrate-related Symptoms in Individuals with Irritable Bowel Syndrome. Gastroenterology. 2017;152(1):124–133. [DOI] [PubMed] [Google Scholar]
- 19.Halmos EP, Power VA, Shepherd SJ, Gibson PR, Muir JG. A Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome. Gastroenterology. 2014;146(1):67–75.e65. [DOI] [PubMed] [Google Scholar]
- 20.Whelan K, Martin LD, Staudacher HM, Lomer MCE. The low FODMAP diet in the management of irritable bowel syndrome: an evidence-based review of FODMAP restriction, reintroduction and personalisation in clinical practice. Journal of Human Nutrition and Dietetics. 2018;31(2):239. [DOI] [PubMed] [Google Scholar]
- 21.Tuck C, Barrett J. Re-challenging FODMAPs: the low FODMAP diet phase two. Journal of gastroenterology and hepatology. 2017;32:11–15. [DOI] [PubMed] [Google Scholar]
- 22.Marsh A, Eslick E, Eslick G. Does a diet low in FODMAPs reduce symptoms associated with functional gastrointestinal disorders? A comprehensive systematic review and meta-analysis. European Journal of Nutrition. 2016;55(3):897–906. [DOI] [PubMed] [Google Scholar]
- 23.Altobelli E, Del Negro V, Angeletti PM, Latella G. Low-FODMAP diet improves irritable bowel syndrome symptoms: a meta-analysis. Nutrients. 2017;9(9):940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schumann D, Klose P, Lauche R, Dobos G, Langhorst J, Cramer H. Low fermentable, oligo-, di-, mono-saccharides and polyol diet in the treatment of irritable bowel syndrome: A systematic review and meta-analysis. Nutrition. 2018;45:24–31. [DOI] [PubMed] [Google Scholar]
- 25.Varjú P, Farkas N, Hegyi P, et al. Low fermentable oligosaccharides, disaccharides, monosaccharides and polyols (FODMAP) diet improves symptoms in adults suffering from irritable bowel syndrome (IBS) compared to standard IBS diet: A meta-analysis of clinical studies. PloS One. 2017;12(8):e0182942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Black CJ, Staudacher HM, Ford AC. Efficacy of a low FODMAP diet in irritable bowel syndrome: systematic review and network meta-analysis. Gut. 2021:gutjnl-2021–325214. [DOI] [PubMed] [Google Scholar]
- 27.Wang J, Yang P, Zhang L, Hou X. A Low-FODMAP Diet Improves the Global Symptoms and Bowel Habits of Adult IBS Patients: A Systematic Review and Meta-Analysis. Front Nutr. 2021;8:683191–683191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yao CK, Gibson PR, Shepherd SJ. Design of clinical trials evaluating dietary interventions in patients with functional gastrointestinal disorders. Am J Gastroenterol. 2013;108(5):748–758. [DOI] [PubMed] [Google Scholar]
- 29.McIntosh K, Reed DE, Schneider T, et al. FODMAPs alter symptoms and the metabolome of patients with IBS: a randomised controlled trial. Gut. 2017;66(7):1241–1251. [DOI] [PubMed] [Google Scholar]
- 30.Staudacher HM, Lomer MC, Anderson JL, et al. Fermentable Carbohydrate Restriction Reduces Luminal Bifidobacteria and Gastrointestinal Symptoms in Patients with Irritable Bowel Syndrome. J Nutr. 2012;142:1510–1518. [DOI] [PubMed] [Google Scholar]
- 31.Pedersen N, Ankersen DV, Felding M, et al. Low-FODMAP diet reduces irritable bowel symptoms in patients with inflammatory bowel disease. World journal of gastroenterology. 2017;23(18):3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harvie RM, Chisholm AW, Bisanz JE, et al. Long-term irritable bowel syndrome symptom control with reintroduction of selected FODMAPs. World journal of gastroenterology. 2017;23(25):4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eswaran SL, Chey WD, Han-Markey T, Ball S, Jackson K. A randomized controlled trial comparing the low FODMAP diet vs. modified NICE guidelines in US adults with IBS-D. The American Journal of Gastroenterology. 2016;111(12):1824–1832. [DOI] [PubMed] [Google Scholar]
- 34.Böhn L, Störsrud S, Liljebo T, et al. Diet low in FODMAPs reduces symptoms of irritable bowel syndrome as well as traditional dietary advice: a randomized controlled trial. Gastroenterology. 2015;149(6):1399–1407. e1392. [DOI] [PubMed] [Google Scholar]
- 35.Zahedi MJ, Behrouz V, Azimi M. Low fermentable oligo-di-mono-saccharides and polyols diet versus general dietary advice in patients with diarrhea-predominant irritable bowel syndrome: A randomized controlled trial. Journal of gastroenterology and hepatology. 2018;33(6):1192–1199. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Feng L, Wang X, et al. Low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols diet compared with traditional dietary advice for diarrhea-predominant irritable bowel syndrome: a parallel-group, randomized controlled trial with analysis of clinical and microbiological factors associated with patient outcomes. The American Journal of Clinical Nutrition. 2021;113(6):1531–1545. [DOI] [PubMed] [Google Scholar]
- 37.Goyal O, Batta S, Nohria S, et al. Low fermentable oligosaccharide, disaccharide, monosaccharide, and polyol diet in patients with diarrhea-predominant irritable bowel syndrome: A prospective, randomized trial. Journal of Gastroenterology and Hepatology. 2021;36(8):2107–2115. [DOI] [PubMed] [Google Scholar]
- 38.McKenzie Y, Bowyer R, Leach H, et al. British Dietetic Association systematic review and evidence-based practice guidelines for the dietary management of irritable bowel syndrome in adults (2016 update). Journal of Human Nutrition and Dietetics. 2016;29(5):549–575. [DOI] [PubMed] [Google Scholar]
- 39.de Roest RH, Dobbs BR, Chapman BA, et al. The low FODMAP diet improves gastrointestinal symptoms in patients with irritable bowel syndrome: a prospective study. International Journal of Clinical Practice. 2013;67(9):895–903. [DOI] [PubMed] [Google Scholar]
- 40.Clevers E, Tran M, Van Oudenhove L, et al. Adherence to diet low in fermentable carbohydrates and traditional diet for irritable bowel syndrome. Nutrition. 2020;73:110719. [DOI] [PubMed] [Google Scholar]
- 41.Chey WD, Hashash JG, Manning L, Chang L. AGA Clinical Practice Update on the Role of Diet in Irritable Bowel Syndrome: Expert Review. Gastroenterology. 2022;162(6):1737–1745.e1735. [DOI] [PubMed] [Google Scholar]
- 42.Atkins M, Zar-Kessler C, Madva EN, et al. Prevalence of exclusion diets and relationship with avoidant/restrictive food intake disorder in adult neurogastroenterology patients. Digestive Disease Week; May, 2022; San Diego, USA. [Google Scholar]
- 43.Pedersen N, Andersen NN, Végh Z, et al. Ehealth: low FODMAP diet vs Lactobacillus rhamnosus GG in irritable bowel syndrome. World Journal of Gastroenterology. 2014;20(43):16215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trott N, Aziz I, Rej A, Surendran Sanders D. How Patients with IBS Use Low FODMAP Dietary Information Provided by General Practitioners and Gastroenterologists: A Qualitative Study. Nutrients. 2019;11(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Weynants A, Goossens L, Genetello M, De Looze D, Van Winckel M. The long-term effect and adherence of a low fermentable oligosaccharides disaccharides monosaccharides and polyols (FODMAP) diet in patients with irritable bowel syndrome. Journal of Human Nutrition and Dietetics. 2020;33(2):159–169. [DOI] [PubMed] [Google Scholar]
- 46.Staudacher HM, Rossi M, Kaminski T, et al. Long-term personalized low FODMAP diet improves symptoms and maintains luminal Bifidobacteria abundance in irritable bowel syndrome. Neurogastroenterology & Motility. 2021:e14241. [DOI] [PubMed] [Google Scholar]
- 47.Staudacher HM, Ralph FS, Irving PM, Whelan K, Lomer MC. Nutrient intake, diet quality, and diet diversity in irritable bowel syndrome and the impact of the low FODMAP diet. Journal of the Academy of Nutrition and Dietetics. 2020;120(4):535–547. [DOI] [PubMed] [Google Scholar]
- 48.Guadagnoli L, Mutlu EA, Doerfler B, Ibrahim A, Brenner D, Taft TH. Food-related quality of life in patients with inflammatory bowel disease and irritable bowel syndrome. Quality of Life Research. 2019;28(8):2195–2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Melchior C, Algera J, Colomier E, Törnblom H, Simrén M, Störsrud S. Food Avoidance and Restriction in Irritable Bowel Syndrome: Relevance for Symptoms, Quality of Life and Nutrient Intake. Clinical Gastroenterology and Hepatology. 2021. [DOI] [PubMed] [Google Scholar]
- 50.Murray HB, Staller K. When food moves from friend to foe: Why avoidant/restrictive food intake matters in irritable bowel syndrome. Clin Gastroenterol Hepatol. 2021. [DOI] [PubMed] [Google Scholar]
- 51.van Vliet CM, Meulders A, Vancleef LMG, Vlaeyen JWS. The Perceived Opportunity to Avoid Pain Paradoxically Increases Pain-Related Fear Through Increased Threat Appraisals. Ann Behav Med. 2021;55(3):216–227. [DOI] [PubMed] [Google Scholar]
- 52.Vlaeyen JW, Crombez G, Linton SJ. The fear-avoidance model of pain. Pain. 2016;157(8):1588–1589. [DOI] [PubMed] [Google Scholar]
- 53.Harer KN, Eswaran SL. Irritable Bowel Syndrome: Food as a Friend or Foe? Gastroenterology Clinics. 2021;50(1):183–199. [DOI] [PubMed] [Google Scholar]
- 54.Scarlata K, Catsos P, Smith J. From a dietitian’s perspective, diets for irritable bowel syndrome are not one size fits all. Clinical Gastroenterology and Hepatology. 2020;18(3):543–545. [DOI] [PubMed] [Google Scholar]
- 55.Chey WD, Keefer L, Whelan K, Gibson PR. Behavioral and diet therapies in integrated care for patients with irritable bowel syndrome. Gastroenterology. 2020. [DOI] [PubMed] [Google Scholar]
- 56.Werlang ME, Sim LA, Lebow JR, Lacy BE. Assessing for eating disorders: A primer for gastroenterologists. Official journal of the American College of Gastroenterology| ACG. 2021;116(1):68–76. [DOI] [PubMed] [Google Scholar]
- 57.McGowan A, Harer KN. Irritable Bowel Syndrome and Eating Disorders: A Burgeoning Concern in Gastrointestinal Clinics. Gastroenterology Clinics of North America. 2021;50(3):595–610. [DOI] [PubMed] [Google Scholar]
- 58.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th ed: American Psychiatric Association; Washington, DC; 2013. [Google Scholar]
- 59.Thomas JJ, Lawson EA, Micali N, Misra M, Deckersbach T, Eddy KT. Avoidant/Restrictive Food Intake Disorder: a Three-Dimensional Model of Neurobiology with Implications for Etiology and Treatment. Current Psychiatry Reports. 2017;19(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Murray HB, Bailey AP, Keshishian AC, et al. Prevalence and characteristics of avoidant/restrictive food intake disorder in adult neurogastroenterology patients. Clinical Gastroenterology and Hepatology. 2020;18(9):1995–2002. e1991. [DOI] [PubMed] [Google Scholar]
- 61.Burton Murray H, Jehangir A, Silvernale CJ, Kuo B, Parkman HP. Avoidant/restrictive food intake disorder symptoms are frequent in patients presenting for symptoms of gastroparesis. Neurogastroenterol Motil. 2020;32(12):e13931. [DOI] [PubMed] [Google Scholar]
- 62.Harer KN, Jagielski CH, Riehl ME, Chey WD. 272–Avoidant/restrictive food intake disorder among adult gastroenterology behavioral health patients: demographic and clinical characteristics. Gastroenterology. 2019;156(6):S–53. [Google Scholar]
- 63.Burton Murray H, Rao F, Baker C, al. e. Prevalence and Characteristics of Avoidant/Restrictive Food Intake Disorder in Pediatric Neurogastroenterology Patients. J Pediatr Gastroenterol Nutr. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Burton Murray H, Riddle M, Rao F, et al. Eating disorder symptoms, including avoidant/restrictive food intake disorder, in patients with disorders of gut-brain interaction. Neurogastroenterol Motil. in press. [DOI] [PubMed] [Google Scholar]
- 65.Thomas JJ, Eddy KT. Cognitive-behavioral therapy for avoidant/restrictive food intake disorder: children, adolescents, and adults. Cambridge University Press; 2018. [Google Scholar]
- 66.Craske MG, Treanor M, Conway CC, Zbozinek T, Vervliet B. Maximizing exposure therapy: an inhibitory learning approach. Behaviour research and therapy. 2014;58:10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Everitt H, Moss-Morris R, Sibelli A, et al. Management of irritable bowel syndrome in primary care: the results of an exploratory randomised controlled trial of mebeverine, methylcellulose, placebo and a self-management website. BMC Gastroenterol. 2013;13:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Moss-Morris R, McAlpine L, Didsbury LP, Spence MJ. A randomized controlled trial of a cognitive behavioural therapy-based self-management intervention for irritable bowel syndrome in primary care. Psychological Medicine. 2010;40(1):85–94. [DOI] [PubMed] [Google Scholar]
- 69.Ljótsson B, Hesser H, Andersson E, et al. Mechanisms of change in an exposure-based treatment for irritable bowel syndrome. J Consult Clin Psychol. 2013;81(6):1113–1126. [DOI] [PubMed] [Google Scholar]
- 70.Wolitzky-Taylor K, Craske MG, Labus JS, Mayer EA, Naliboff BD. Visceral sensitivity as a mediator of outcome in the treatment of irritable bowel syndrome. Behaviour research and therapy. 2012;50(10):647–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hesser H, Hedman-Lagerlöf E, Andersson E, Lindfors P, Ljótsson B. How does exposure therapy work? A comparison between generic and gastrointestinal anxiety-specific mediators in a dismantling study of exposure therapy for irritable bowel syndrome. J Consult Clin Psychol. 2018;86(3):254–267. [DOI] [PubMed] [Google Scholar]
- 72.Labus JS, Bolus R, Chang L, et al. The Visceral Sensitivity Index: development and validation of a gastrointestinal symptom-specific anxiety scale. Aliment Pharmacol Ther. 2004;20(1):89–97. [DOI] [PubMed] [Google Scholar]
- 73.Labus JS, Mayer EA, Chang L, Bolus R, Naliboff BD. The central role of gastrointestinal-specific anxiety in irritable bowel syndrome: further validation of the visceral sensitivity index. Psychosom Med. 2007;69(1):89–98. [DOI] [PubMed] [Google Scholar]
- 74.Reme SE, Darnley S, Kennedy T, Chalder T. The development of the irritable bowel syndrome-behavioral responses questionnaire. J Psychosom Res. 2010;69(3):319–325. [DOI] [PubMed] [Google Scholar]
- 75.Jerndal P, Ringström G, Agerforz P, et al. Gastrointestinal-specific anxiety: an important factor for severity of GI symptoms and quality of life in IBS. Neurogastroenterol Motil. 2010;22(6):646–e179. [DOI] [PubMed] [Google Scholar]
- 76.Clevers E, Tack J, Törnblom H, et al. Development of Irritable Bowel Syndrome Features Over a 5-year Period. Clin Gastroenterol Hepatol. 2018;16(8):1244–1251.e1241. [DOI] [PubMed] [Google Scholar]
- 77.Mayer EA, Craske M, Naliboff BD. Depression, anxiety, and the gastrointestinal system. J Clin Psychiatry. 2001;62 Suppl 8:28–36; discussion 37. [PubMed] [Google Scholar]
- 78.Kano M, Muratsubaki T, Van Oudenhove L, et al. Altered brain and gut responses to corticotropin-releasing hormone (CRH) in patients with irritable bowel syndrome. Sci Rep. 2017;7(1):12425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.LeDoux JE, Moscarello J, Sears R, Campese V. The birth, death and resurrection of avoidance: a reconceptualization of a troubled paradigm. Molecular psychiatry. 2017;22(1):24–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shah K, Ramos-Garcia M, Bhavsar J, Lehrer P. Mind-body treatments of irritable bowel syndrome symptoms: An updated meta-analysis. Behaviour research and therapy. 2020;128:103462. [DOI] [PubMed] [Google Scholar]
- 81.Hunt MG, Moshier S, Milonova M. Brief cognitive-behavioral internet therapy for irritable bowel syndrome. Behaviour research and therapy. 2009;47(9):797–802. [DOI] [PubMed] [Google Scholar]
- 82.Ljótsson B, Andersson G, Andersson E, et al. Acceptability, effectiveness, and cost-effectiveness of internet-based exposure treatment for irritable bowel syndrome in a clinical sample: a randomized controlled trial. BMC Gastroenterol. 2011;11:110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ljótsson B, Falk L, Vesterlund AW, et al. Internet-delivered exposure and mindfulness based therapy for irritable bowel syndrome--a randomized controlled trial. Behaviour research and therapy. 2010;48(6):531–539. [DOI] [PubMed] [Google Scholar]
- 84.Ljótsson B, Hedman E, Andersson E, et al. Internet-delivered exposure-based treatment vs. stress management for irritable bowel syndrome: a randomized trial. Am J Gastroenterol. 2011;106(8):1481–1491. [DOI] [PubMed] [Google Scholar]
- 85.Hunt M, Miguez S, Dukas B, Onwude O, White S. Efficacy of Zemedy, a Mobile Digital Therapeutic for the Self-management of Irritable Bowel Syndrome: Crossover Randomized Controlled Trial. JMIR Mhealth Uhealth. 2021;9(5):e26152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Craske MG, Wolitzky-Taylor KB, Labus J, et al. A cognitive-behavioral treatment for irritable bowel syndrome using interoceptive exposure to visceral sensations. Behaviour research and therapy. 2011;49(6–7):413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Laird KT, Tanner-Smith EE, Russell AC, Hollon SD, Walker LS. Comparative efficacy of psychological therapies for improving mental health and daily functioning in irritable bowel syndrome: A systematic review and meta-analysis. Clin Psychol Rev. 2017;51:142–152. [DOI] [PubMed] [Google Scholar]
- 88.Laird KT, Tanner-Smith EE, Russell AC, Hollon SD, Walker LS. Short-term and Long-term Efficacy of Psychological Therapies for Irritable Bowel Syndrome: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016;14(7):937–947.e934. [DOI] [PubMed] [Google Scholar]
- 89.Hesser H, Hedman E, Lindfors P, Andersson E, Ljótsson B. The specific effect of systematic exposure in irritable bowel syndrome: complier average causal effect analysis using growth mixture modeling. Psychol Med. 2017;47(15):2653–2662. [DOI] [PubMed] [Google Scholar]
- 90.Black CJ, Thakur ER, Houghton LA, Quigley EMM, Moayyedi P, Ford AC. Efficacy of psychological therapies for irritable bowel syndrome: systematic review and network meta-analysis. Gut. 2020;69(8):1441–1451. [DOI] [PubMed] [Google Scholar]
- 91.Hanlon I, Hewitt C, Bell K, Phillips A, Mikocka-Walus A. Systematic review with meta-analysis: online psychological interventions for mental and physical health outcomes in gastrointestinal disorders including irritable bowel syndrome and inflammatory bowel disease. Aliment Pharmacol Ther. 2018;48(3):244–259. [DOI] [PubMed] [Google Scholar]
- 92.Carlbring P, Andersson G, Cuijpers P, Riper H, Hedman-Lagerlöf E. Internet-based vs. face-to-face cognitive behavior therapy for psychiatric and somatic disorders: an updated systematic review and meta-analysis. Cognitive behaviour therapy. 2018;47(1):1–18. [DOI] [PubMed] [Google Scholar]
- 93.Kazdin AE. Mediators and mechanisms of change in psychotherapy research. Annu Rev Clin Psychol. 2007;3:1–27. [DOI] [PubMed] [Google Scholar]
- 94.Hesser H, Hedman-Lagerlöf E, Lindfors P, Andersson E, Ljótsson B. Behavioral avoidance moderates the effect of exposure therapy for irritable bowel syndrome: A secondary analysis of results from a randomized component trial. Behaviour research and therapy. 2021;141:103862. [DOI] [PubMed] [Google Scholar]
- 95.Wiklund IK, Fullerton S, Hawkey CJ, et al. An irritable bowel syndrome-specific symptom questionnaire: development and validation. Scand J Gastroenterol. 2003;38(9):947–954. [DOI] [PubMed] [Google Scholar]
- 96.Ljótsson B, Jones M, Talley NJ, Kjellström L, Agréus L, Andreasson A. Discriminant and convergent validity of the GSRS-IBS symptom severity measure for irritable bowel syndrome: A population study. United European Gastroenterol J. 2020;8(3):284–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Preacher KJ, Hayes AF. SPSS and SAS procedures for estimating indirect effects in simple mediation models. Behav Res Methods Instrum Comput. 2004;36(4):717–731. [DOI] [PubMed] [Google Scholar]
- 98.Preacher KJ, Kelley K. Effect size measures for mediation models: quantitative strategies for communicating indirect effects. Psychol Methods. 2011;16(2):93–115. [DOI] [PubMed] [Google Scholar]
- 99.Smeets RJ, Beelen S, Goossens ME, Schouten EG, Knottnerus JA, Vlaeyen JW. Treatment expectancy and credibility are associated with the outcome of both physical and cognitive-behavioral treatment in chronic low back pain. Clin J Pain. 2008;24(4):305–315. [DOI] [PubMed] [Google Scholar]
- 100.Schmidt K, Berding T, Kleine-Borgmann J, et al. The beneficial effect of positive treatment expectations on pharmacological migraine prophylaxis. Pain. 2022;163(2). [DOI] [PubMed] [Google Scholar]
- 101.Vlaeyen JW, Morley S. Cognitive-behavioral treatments for chronic pain: what works for whom? Clin J Pain. 2005;21(1):1–8. [DOI] [PubMed] [Google Scholar]
- 102.Bennet SM, Böhn L, Störsrud S, et al. Multivariate modelling of faecal bacterial profiles of patients with IBS predicts responsiveness to a diet low in FODMAPs. Gut. 2018;67(5):872–881. [DOI] [PubMed] [Google Scholar]
- 103.Eetemadi A, Tagkopoulos I. Methane and fatty acid metabolism pathways are predictive of Low-FODMAP diet efficacy for patients with irritable bowel syndrome. Clin Nutr. 2021;40(6):4414–4421. [DOI] [PubMed] [Google Scholar]
- 104.Lackner JM, Jaccard J, Firth R, et al. Factors associated with efficacy of cognitive behavior therapy vs education for patients with irritable bowel syndrome. Clinical Gastroenterology and Hepatology. 2019;17(8):1500–1508. e1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cormier S, Lavigne GL, Choinière M, Rainville P. Expectations predict chronic pain treatment outcomes. Pain. 2016;157(2):329–338. [DOI] [PubMed] [Google Scholar]
- 106.Polster A, Van Oudenhove L, Jones M, Öhman L, Törnblom H, Simrén M. Mixture model analysis identifies irritable bowel syndrome subgroups characterised by specific profiles of gastrointestinal, extraintestinal somatic and psychological symptoms. Aliment Pharmacol Ther. 2017;46(5):529–539. [DOI] [PubMed] [Google Scholar]
- 107.Black CJ, Yiannakou Y, Guthrie EA, West R, Houghton LA, Ford AC. A Novel Method to Classify and Subgroup Patients With IBS Based on Gastrointestinal Symptoms and Psychological Profiles. Am J Gastroenterol. 2021;116(2):372–381. [DOI] [PubMed] [Google Scholar]
- 108.Heyvaert M, Onghena P. Randomization tests for single-case experiments: State of the art, state of the science, and state of the application. Journal of Contextual Behavioral Science. 2014;3(1):51–64. [Google Scholar]
- 109.Vlaeyen JWS, Wicksell RK, Simons LE, et al. From Boulder to Stockholm in 70 Years: Single Case Experimental Designs in Clinical Research. The Psychological Record. 2020;70(4):659–670. [Google Scholar]
- 110.Basilisco G, Group ISoNMS, Barbara G, et al. Patient dissatisfaction with medical therapy for chronic constipation or irritable bowel syndrome with constipation: analysis of N-of-1 prospective trials in 81 patients. Alimentary pharmacology & therapeutics. 2020;51(6):629–636. [DOI] [PubMed] [Google Scholar]
- 111.Kaplan HC, Opipari-Arrigan L, Schmid CH, et al. Evaluating the comparative effectiveness of two diets in pediatric inflammatory bowel disease: a study protocol for a series of N-of-1 trials. Paper presented at: Healthcare2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Onghena P, Michiels B, Jamshidi L, Moeyaert M, Van den Noortgate W. One by one: Accumulating evidence by using meta-analytical procedures for single-case experiments. Brain Impairment. 2018;19(1):33–58. [Google Scholar]