Abstract
Common cancer complications include bone cancer pain (BCP), which was not sufficiently alleviated by traditional analgesics. More safe and effective therapy was urgent needed. Metformin relieved osteoarthritis pain, but the analgesia of Metformin in BCP was not well studied. The study aimed to explore the Metformin-mediated analgesic effect and its molecular mechanisms in BCP rats. We demonstrated that Walker 256 cell transplantation into the medullary cavity of the tibia worsened mechanical allodynia in BCP rats, increased the expression of TGFβ1 in the metastatic bone tissue, and raised the expression of TGFβRI and TRPV1 in the L4-6 dorsal root ganglion (DRG) of BCP rats. While, selectively blockade of TGFβRI by SD208 could obviously elevated the paw withdraw threshold (PWT) of BCP rats, together with decreased TRPV1 expression in L4-6 DRG. Notably, continuous Metformin treatment reduced TGFβ1, TGFβRI and TRPV1 expression, and relieved mechanical allodynia of BCP rats in a long-term effect. In conclusion, these results illustrated that Metformin ameliorated bone cancer pain, and the downregulation of TGFβ1-TGFβRI-TRPV1 might be a potential mechanism of Metformin-mediated analgesia in BCP.
Keywords: Bone cancer pain, Metformin, TGFβ1, TGFβRI, TRPV1
1. Introduction
One of the most intense and intractable chronic pains is cancer pain, whether it is induced by a primary cancer or metastatic tumor [1,2]. Numerous cancer cells, including those from the breasts, lungs, and prostate, often invade several bone tissues before producing excruciating bone cancer pain (BCP) [[3], [4], [5]]. The quality of life and functional level of patients with bone cancer are significantly impacted by BCP, which often leads to depression and suicide in cancer pain patients [6,7]. There are very few safe and effective pharmacotherapy to treat BCP [[8], [9], [10]], therefore new therapies for cancer pain patients are urgently needed.
One of the most popular hypoglycemic medications for the type 2 diabetes treatment is Metformin. In addition to diabetes, it has been demonstrated to protect against a number of other disorders, such as cancer [[11], [12], [13]], anxiety [[14], [15], [16]], depression [[17], [18], [19]], aging [20], pain [[21], [22], [23], [24], [25], [26], [27]], cognitive impairment [[28], [29], [30]], and cardiovascular disease [[31], [32], [33]]. By suppressing transient receptor potential ion channel (TRPV1) expression, Metformin lessens the mechanical allodynia in BCP rats [25]. However, the exact signaling pathway by which Metformin affects TRPV1 expression is yet unknown.
It was reported that Metformin is a novel suppressor for Transforming growth factor-β1 (TGFβ1) [[34], [35], [36]]. TGFβ is a main bone-derived growth factor, produced by bone cells in a latent structure and preserved in the bone matrix [37]. Previous studies have demonstrated that TGFβ is crucial for the development of bone metastases [38]. Excessive TGFβ promotes tumor development, invasion, and metastasis in progressive bone cancer [[39], [40], [41]]. TGFβ1 signaling has been related to the overexpression and sensitivity of TRPV1 in primary sensory neurons [42]. However, it is unclear whether metformin plays an analgesic role in bone cancer pain by inhibiting TGFβ1-TRPV1 signaling.
In the study, the BCP rats were used to examine the analgesic benefits of Metformin and mechanical impacts on the TGFβ1-TRPV1 signaling. Our findings provide a potential pathway for the analgesic action of Metformin and might bring possible approaches to the therapeutic management of BCP.
2. Materials and methods
2.1. Animals
Female Sprague-Dawley (SD) rats weighing 180–200 g were obtained from Soochow University's experimental animal facility. The animals were kept in a 12/12 light/dark cycle with unlimited access to food and drink. The Soochow University Institutional Animal Care and Use Committee provided its consent to the study's experimental procedure. All techniques were conducted in accordance with the National Research Council's recommendations for the handling and utilization of laboratory animals. There was an attempt made to minimize the number of animals utilized and their suffering.
2.2. Rat model of bone cancer pain
The procedure was performed in accordance with earlier reports [43]. In summary, the abdominal cavity of SD rats (weighing 60 g) was administered with Walker 256 mammary gland cancer cells (2 × 107 cells/ml, 1 ml). Following 5–7 days, the ascites was removed, centrifuged for 3 min at 1200 rpm to retrieve the cells, and the pellet was rinsed three times with 10 ml of normal saline (NS) before being centrifuged again for 3 min. Utilizing hemocytometer, the cells were counted, diluted with NS to a final concentration of 1 × 108/ml, and stored on ice until administration into rats. Sodium pentobarbital (50 mg/kg, i.p.) was used to anesthetize SD rats. The left medullary cavity of the tibia was progressively injected with 4 μL of cells (4 × 105) or the equivalent amount of NS (Sham cohort) employing microinjection with a microinjection syringe. To inhibit the cancer cells from migrating along the injection route, we left the syringe in place for an additional 2 min. The rats in the naïve cohort did not get any therapy. The animals were put on a heating pad until they were awake again and then taken back to their cages.
2.3. Behavioral tests
Rats' responses to pain were assessed by Von Frey filament technique, as previously mentioned [44]. Rats were briefly housed for 30 min in a large cage made of clear plastic mesh to provide the conditions for the experiment. Rats' mechanical allodynia was found to react through the 50 % paw withdrawal threshold (PWT) in response to Von Frey filament stimulus employing "up & down" approach. From 1.0 to 26.0 g of Von Frey filaments were administered perpendicularly to the plantar surface of the hind paw with enough power to cause filaments to bend for one to 2 s. Next, lesser force filament was used if there was a reaction. When there was no reaction, a higher force was used until the exercise was conducted three times and the final Von Frey filaments were identified. In order to ensure the uniformity, the behavioral tests were conducted by the same two technicians in whole study following the double-blind principle. Briefly, one technician (No. 1) randomly placed the rats in the cage before the behavior test. The other technician (No. 2) conducted the behavior test, and the behavior data was analyzed by No.1 technician after the test.
2.4. Western blotting
Employing Western blotting, the expressions of TGFβ1, TGFβ receptors, and TRPV1 were identified. Rapid isolation and liquid nitrogen freezing of the left L4-6 DRG and left hind tibias followed by long-term storage at −80 °C till usage. Liquid nitrogen was employed to freeze the bone tissues, which were subsequently crushed into powder. After being lysed in RIPA buffer containing protease suppressors (Fude, Hangzhou, China), specimens from bone tissue and DRG were sonicated. A BCA Protein Assay kit was used to measure the total protein content (Cowin, Taizhou, China). On 8 % polyacrylamide gels from Beyotime in Shanghai, China, identical quantities of total proteins were separated before being transported to polyvinylidene difluoride membranes. The membranes were blocked in 5 % non-fat milk for 2 h at room temperature (RT), and then the primary antibodies against TGFβ1 (1:500, Immunoway, USA), TGFβRI (1:250, Santa Cruz, USA), TGFβRII (1:250, Santa Cruz, USA), and TRPV1 (1:500, GeneTex, USA) were incubated overnight at 4 °C. Membranes were cleaned in TBST before being preserved with HRP-conjugated secondary antibodies for 2 h at RT. As the loading control, GAPDH (Abcam, USA) was employed. The bands were photographed utilizing ChemiDoc XRs technology and viewed with ECL (Bio-Rad, CA, USA). The expression of the protein was compared to GAPDH.
2.5. Immunofluorescence staining
The bone tissues and the L4-6 DRG were fixed in 4 % paraformaldehyde overnight at 4 °C. The EDTA decalcification solution was replaced every week until the fine needle pierced bone tissue as the endpoint of decalcification. The bone tissues were then immersed in a 20 × volume EDTA decalcification solution at RT. Then, for 24–48 h at 4 °C for cryoprotection, submerged DRG and bone tissues in a 10–30 % gradient of sucrose in PBS. DRG and bone tissues were cut into 14 μm sections, which were then incubated with the primary antibodies TGFβRI (1:50), TGFβRII (1:50), TRPV1 (1:200), and TGFβ1 (1:200) for an overnight period at 4 °C. The secondary antibodies Alexa Fluor 488 and 555 were then added, and the incubation process was continued for an additional hour at RT. Fluorescence microscopy was employed to photograph the slides, and Image J was used to analyze the images.
2.6. Drug treatment
We acquired Metformin from Sigma Aldrich (St. Louis, MO, USA). TargetMol provided the TGFβRⅠ kinase suppressor SD208 for use (Boston, MA, USA). TGFβ1 was purchased from Peprotech (Rocky Hill, NJ, USA). Metformin was diluted with normal saline (NS) to achieve a final use concentration (20 mg/ml), and the injection volume of metformin or NS was 1.8–2.0 ml according to the body weight of rats (200 mg/kg). The intraperitoneal injections of Metformin or NS were given to the rats in the BCP + Metformin and BCP + NS cohorts single injection or once daily for seven days. According to the drug instruction, 7.1 mg SD208 was dissolved in 1 ml DMSO and further diluted in NS to 0.625 mg/ml 4 % sevoflurane was used to anesthetize SD rats, then SD208 (2.5 mg/kg, 0.72–0.8 ml) or TGFβ1 (10 μg/ml, 0.1 ml) was immediately delivered into local deep tissue around the tumor bone using 1 ml syringe at a rate of approximately 1 ml/min. The needle was injected vertically into the center of the tumor and then infiltrated into the surrounding area. After the drug was completely absorbed, the needle was pulled out. The rats were injected NS or SD208 once or once daily for a week around the malignant bone.
2.7. Body weight and blood glucose assay
Body weight of each overnight fasting rat was recorded. Then, blood was collected from the tail vein of rats. For measurement of serum glucose levels, serum was separated from the blood cells by centrifugation. Then, all serum samples were immediately stored at −20 °C until unified analyses. Serum glucose levels were determined by spectrophotometry using glucose oxidase method [45] (Unico 1200, Japan).
2.8. Statistics
All experiments (behavioral tests, drug administration, molecular detection) and data analyses were carried out following the double-blind principles. All results were displayed as the Mean ± SEM. The statistical analysis was done employing Graphpad 9.0. Before analysis, the normality of all the data was verified. Where applicable, one-way or two-way analysis of variance (ANOVA) followed by Tukey's post hoc test was conducted. P < 0.05 was regarded as a statistically significant value.
3. Results
3.1. Transplantation of Walker 256 cells induced mechanical allodynia in rats
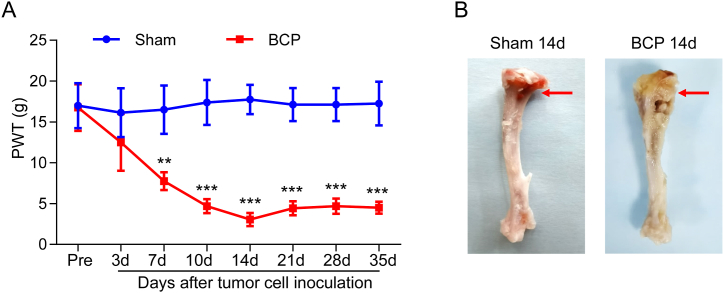
To create an animal model of BCP, we injected female SD rats with intra-tibial injections of Walker 256 mammary gland cancer cells. The ipsilateral hindpaw PWT of BCP rats significantly decreased at day 7 and peaked at day 14 (Fig. 1B), and the decline sustained for at least 35 days (Fig. 1A). The ipsilateral cancer rose significantly postmortem, but not in the sham tibia (Fig. 1B). The results show that the mechanical allodynia of rats was effectively increased by Walker 256 cell transplantation.
Fig. 1.
Walker 256 cell transplantation induced mechanical allodynia in rats. (A) The PWT was reduced at day 7 (***P < 0.001 vs Sham group, two-way ANOVA), and persisted for at least 35 d in the ipsilateral hindpaw (**P < 0.01 vs Sham group, n = 8, two-way ANOVA). (B) Photograph of the tumor-injected left hindpaw was showed in the rat after 14 days when inoculation. No effect was seen on the NS-injected left hindpaw.
3.2. Tumor cell inoculation increased the expression of TGFβ1, TGFβRI and TRPV1
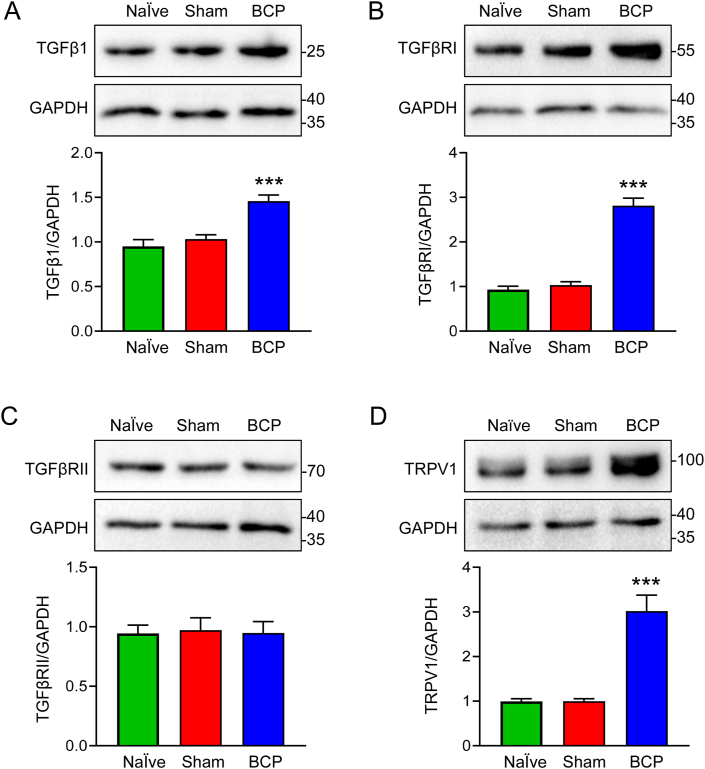
TGFβ is thought to be produced as a result of bone loss and cancer growth [[46], [47], [48]]. Here, our findings demonstrated that the progressed cancer-bearing rats' bone tissues had elevated in TGFβ1 protein at day 14 after tumor cell inoculation (Fig. 2A). TGFβ binds the transmembrane serine/threonine receptor type Ⅰ (TGFβRI) and TGFβRII [49]. We observed TGFβRI and TGFβRII expression in L4-6 DRG ipsilateral to the impacted bone in cancer-bearing rats. TGFβRI was highly elevated at day 14 after tumor cell inoculation (Fig. 2B). The three cohorts did not differ in TGFβRII expression at day 14 after tumor cell inoculation (Fig. 2C). We further detected TRPV1 expression, a significant increase of TRPV1 in L4-6 DRG ipsilateral to the tumor-inoculated bone was showed in Fig. 2D. These results suggested that tumor cell inoculation upregulated the expression of TGFβ1, TGFβRI and TRPV1 in BCP rats. The original blots of Fig. 2 were placed in Supplementary Fig. 1.
Fig. 2.
The expression of TGFβ1, TGFβRⅠ and TRPV1 were increased in BCP rats. (A) Western blotting analysis revealed a significant increase in TGFβ1 expression in the affected bone tissues of BCP rats at 14 days after transplantation compared with Naïve or Sham rats (***P < 0.001 vs Naïve or Sham group, n = 5, one-way ANOVA). (B) The expression of TGFβRⅠ in the ipsilateral L4-6 DRG was clearly enhanced in BCP rats at 14 days after transplantation compared with Naïve or Sham rats (***P < 0.001 vs Naïve or Sham group, n = 5, one-way ANOVA). (C) There are no significant differences in TGFβRII expression in the ipsilateral L4-6 DRG at 14 days after transplantation among the three groups (P > 0.05 vs Naïve or Sham group, n = 5, one-way ANOVA). (D) Western blotting analysis revealed a significant increase in TRPV1 expression in the ipsilateral L4-6 DRG at 14 days after transplantation (***P < 0.001 vs Naïve and Sham group, n = 5, one-way ANOVA).
3.3. TGFβRⅠ inhibition attenuated the mechanical allodynia in a short-term effect
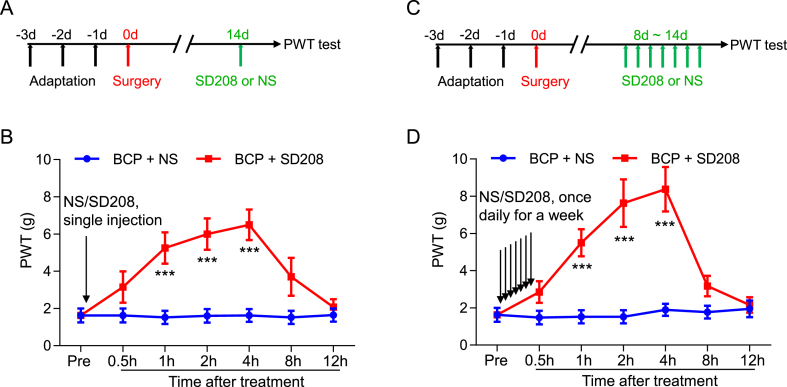
To further examine whether TGFβRI are involved in mechanical allodynia induced by bone cancer metastasis, we local injected (around tumor bone) the TGFβRⅠ antagonist SD208 (2.5 mg/kg) at day 14 after transplantation. Notably, the PWT of BCP rats was substantially elevated, starting from 1 to 4 h after injection (Fig. 3A and B). Interestingly, the PWT was further increased after a week consecutive daily injection of SD208 from day 8–14 after transplantation, while the duration of efficacy was not extended (Fig. 3C and D). These results indicated that increased TGFβRⅠ expression in L4-6 DRG contributes to the mechanical allodynia of BCP rats.
Fig. 3.
Short-term analgesic effect of TGFβRⅠ inhibitor on PWT in BCP rats. (A) The schematic diagram showed the timeline of SD208 administration and behavioral test. (B) Local injection (around tumor bone) of SD208 (2.5 mg/kg) at 14 days after transplantation obviously elevated the PWT of BCP rats, it began at 1 h and lasted for up to 4 h after SD208 injection (**P < 0.01, ***P < 0.001 vs Control group, n = 8, two-way ANOVA). (C) The schematic diagram showed the timeline of SD208 administration and behavioral test. (D) Continuous daily local injection of SD208 for a week from day 8 after transplantation significantly increased the PWT of BCP rats, it began at 1 h and lasted for up to 4 h after the last time of SD208 injection (***P < 0.001 vs Control group, n = 8, two-way ANOVA).
3.4. Metformin treatment relieved the mechanical allodynia in a long-term effect
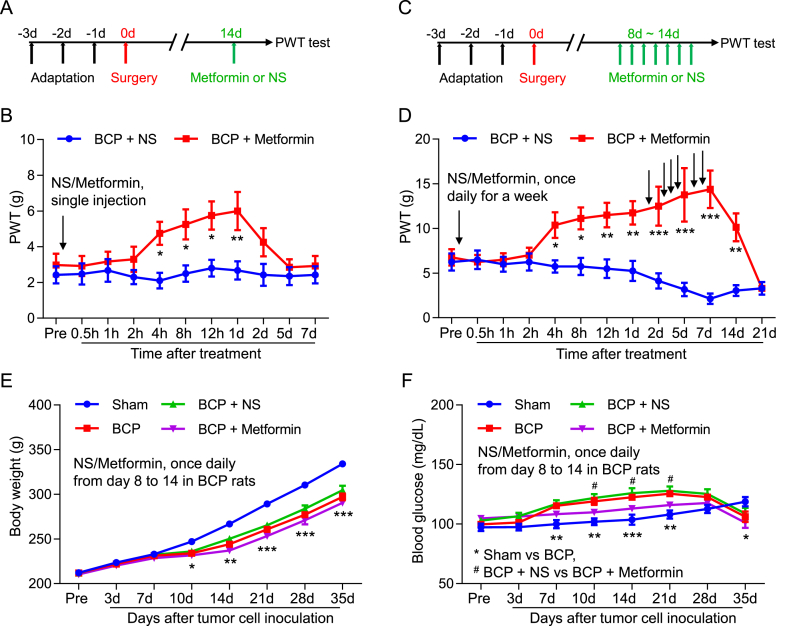
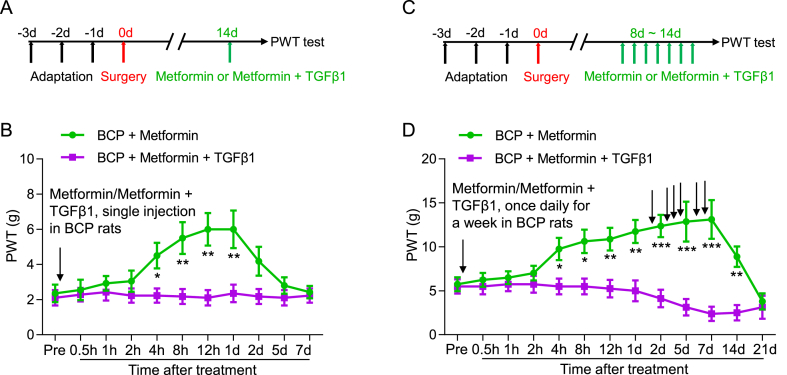
To study the analgesic effect of metformin on bone cancer pain. The intraperitoneal administrations of Metformin or NS were given to the rats in the BCP + Metformin and BCP + NS cohorts at day 14 after transplantation. We showed that the PWT in BCP + Metformin was significantly increased, starting from 4 h to 1 d after injection (Fig. 4A and B). Interestingly, the PWT was further increased after a week consecutive daily injection of Metformin from day 8–14 after transplantation, and the duration of efficacy was further extended, starting from 4 h to 14 d after continuous Metformin treatment (Fig. 4C and D). These results revealed that continuous Metformin treatment has a long-term analgesic effect on mechanical allodynia in BCP rats. Next, we examined the effects of continuous metformin treatment on body weight and blood glucose in rats. The results showed that rats with bone cancer pain decreased body weight relative to the sham group, but proper dose metformin treatment did not significantly affect body weight (Fig. 4E). Rats with bone cancer pain have elevated blood glucose relative to the sham group, but blood glucose decreases in advanced cancer stages, and metformin treatment reduces bone cancer-induced blood glucose elevation (Fig. 4F).
Fig. 4.
Long-term analgesic effect of continuous Metformin treatment on PWT in BCP rats. (A) The schematic diagram showed the timeline of Metformin administration and behavioral test. (B) The PWT of BCP rats treated with Metformin (i.p., 200 mg/kg) at 14 days after transplantation was significantly increased, compared with the BCP rats injected with normal saline, it began at 4 h and lasted for up to 1 d after Metformin treatment (*P < 0.05, **P < 0.01 vs BCP + NS, n = 8, two-way ANOVA). (C) The schematic diagram showed the timeline of Metformin administration and behavioral test. (D) The PWT of BCP rats treated with Metformin from 8 to 14 days after transplantation was significantly increased, compared with the BCP rats injected with normal saline, it began at 4 h and lasted for up to 14 d after continuous Metformin treatment (*P < 0.05, **P < 0.01, ***P < 0.001 vs BCP + NS, n = 8, two-way ANOVA). (E) Body weight of Sham, BCP, BCP + NS, BCP + Metformin groups (*P < 0.05, **P < 0.01, ***P < 0.001, Sham vs BCP, n = 8, two-way ANOVA). (F) Blood glucose of Sham, BCP, BCP + NS, BCP + Metformin groups (**P < 0.01, ***P < 0.001, Sham vs BCP, #P < 0.05, BCP + NS vs BCP + Metformin, n = 8, two-way ANOVA).
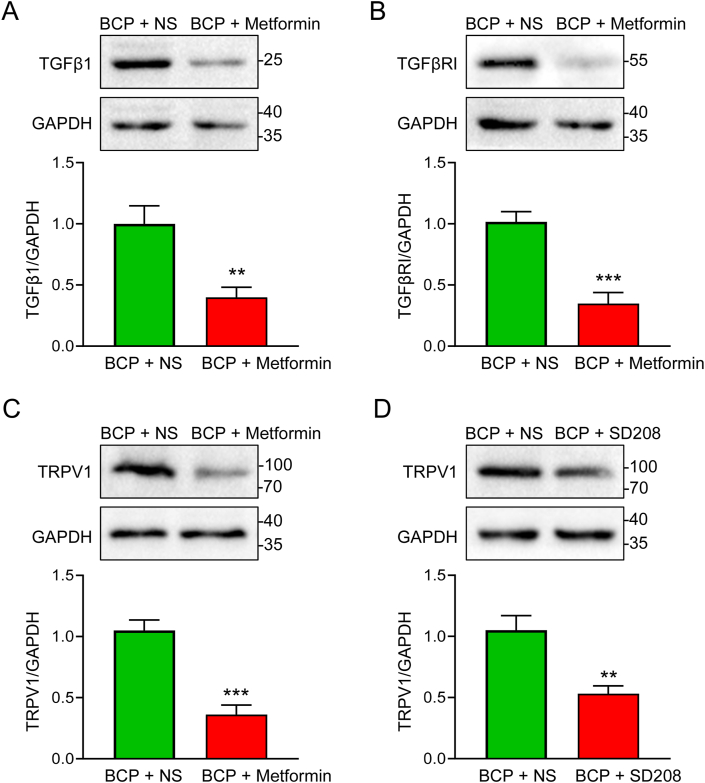
3.5. Metformin reduced the expression of TGFβ1, TGFβRI and TRPV1
To further explored the role of TGFβ1-TGFβRⅠ signaling in Metformin-mediated analgesic effect on BCP rats, we determined TGFβ1 levels in the metastatic bone tissue of rats following the last Metformin supplementation for seven consecutive days. Western blotting analysis revealed that continuous Metformin treatment reduced TGFβ1 expression in BCP rats (Fig. 5A). Furthermore, continuous Metformin treatment substantially decreased TGFβRⅠ and TRPV1 expression in the ipsilateral L4-6 DRG of BCP rats (Fig. 5B and C). To discover the causality of TRPV1 and TGFβRⅠ signaling in Metformin-mediated analgesia of BCP rats, we detected TRPV1 expression in the ipsilateral L4-6 DRG of BCP rats following the last SD208 injection for seven consecutive days. Interestingly, the TRPV1 expression was decreased along with the TGFβRⅠ inhibition by SD208 (Fig. 5D). These results suggested that continuous Metformin treatment may reduce TRPV1 expression by inhibiting TGFβ1-TGFβRⅠ signaling. The original blots of Fig. 5 were placed in Supplementary Fig. 2.
Fig. 5.
Continuous Metformin treatment reduced the expression of TGFβ1, TGFβRⅠ and TRPV1 in BCP rats. (A) Western blotting analysis revealed a decrease in the protein level of TGFβ1 in the affected bone tissues treated with Metformin (**P < 0.01 vs BCP + NS, n = 5, two-tailed t-test). (B) Western blotting analysis displayed a decrease in the protein level of TGFβRI in the ipsilateral L4-6 DRG treated with Metformin (**P < 0.01 vs BCP + NS, n = 5, two-tailed t-test). (C) Western blotting analysis illustrated a decrease in the protein level of TRPV1 in the ipsilateral L4-6 DRG treated with Metformin (**P < 0.01 vs BCP + NS, n = 5, two-tailed t-test). (D) Western blotting analysis showed a decrease in the protein level of TRPV1 in the ipsilateral L4-6 DRG treated with TGFβRI inhibitor (SD208, 2.5 mg/kg) (**P < 0.01 vs BCP + NS, n = 5, two-tailed t-test).
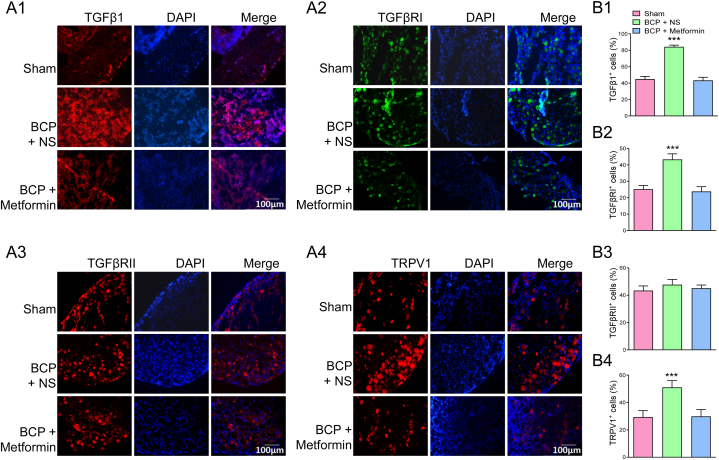
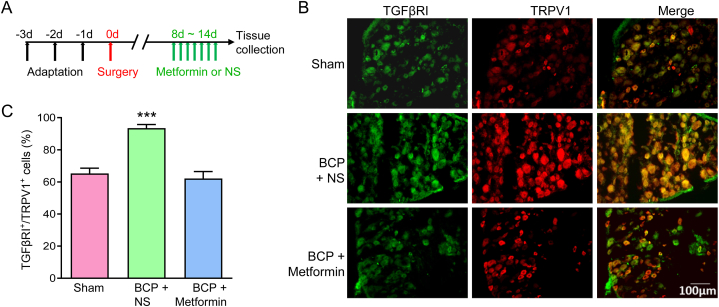
These results were further verified by immunofluorescence assay. IF assay showed that TGFβ1, TGFβRⅠ and TRPV1 were widely distributed in BCP + NS group, but their expressions were rarely detected in the BCP + Metformin rats, and the TGFβRⅠI did not change significantly (Fig. 6A and B). Furthermore, double staining using IF revealed the co-localization of TRPV1 with TGFβRⅠ in L4-6 DRG (Fig. 7A and B). Moreover, their co-localization expression was obviously reduced after Metformin treatment (Fig. 7C).
Fig. 6.
Continuous Metformin treatment reversed the expression of TGFβ1, TGFβRⅠ and TRPV1 in situ. (A1) Immunofluorescence assay showed that the number of TGFβ1 positive cells was increased in the affected bone tissues of BCP rats, and it was rarely detected in BCP rats treated with Metformin (Bar = 100 μm). (A2) Immunofluorescence assay displayed that the number of TGFβRI positive cells was increased in L4-6 DRG of BCP rats, and it was rarely detected in BCP rats treated with Metformin (Bar = 100 μm). (A3) Immunofluorescence assay indicated that there was no obvious difference in TGFβRII expression in L4-6 DRG from Sham, BCP and Metformin treatment rats. (A4) The TRPV1 expression was increased in L4-6 DRG of BCP rats, and Metformin treatment clearly reduced TRPV1 expression in situ. (B1–B4) Proportion of TGFβ1, TGFβRI, TGFβRII and TRPV1 positive cells (***P < 0.001 vs Sham and Metformin group, n = 5, one-way ANOVA).
Fig. 7.
Continuous Metformin treatment reversed the co-expression of TGFβRI and TRPV1 in L4-6 DRG. (A) The schematic diagram showed the timeline of Metformin administration and molecular detection. (B) Immunofluorescence assay showed the co-localization of TGFβRI and TRPV1 in DRG neurons from Sham, BCP and Metformin treatment rats (Bar = 100 μm). (C) The proportion of TGFβRI positive and TRPV1 positive co-labeled cells in DRG neurons from Sham, BCP and Metformin treatment rats (***P < 0.001 vs Sham and Metformin group, n = 5, one-way ANOVA).
3.6. TGFβ1 counteracted the analgesic effect of metformin in BCP rats
TGFβ1 acts as a ligand and agonist for TGFβRI. To investigate whether Metformin relieves bone cancer pain via the TGFβ1-TGFβRI pathway. Recombinant TGFβ1 was injected into local deep tissue around the tumor bone to activate TGFβRI, while Metformin was injected intraperitoneally into BCP rats at day 14 or from day 8–14 after transplantation. The results showed that Metformin + TGFβ1 counteracted the analgesic effect of metformin, suggesting that metformin exerts its analgesic effect through inhibition of the TGFβ1-TGFβRI signaling (Fig. 8A–D).
Fig. 8.
TGFβ1 abolished the analgesic effect of metformin in BCP rats. (A) The schematic diagram showed the timeline of Metformin and TGFβ1 administration and behavioral test. (B) The PWT of BCP rats treated with Metformin + TGFβ1 at 14 days after transplantation was significantly lowered, compared with the BCP rats injected with Metformin (*P < 0.05, **P < 0.01, n = 8, two-way ANOVA). (C) The schematic diagram showed the timeline of Metformin and TGFβ1 administration and behavioral test. (D) The PWT of BCP rats treated with Metformin + TGFβ1 from 8 to 14 days after transplantation was significantly decreased, compared with the BCP rats injected with Metformin (*P < 0.05, **P < 0.01, ***P < 0.001, n = 8, two-way ANOVA).
4. Conclusion
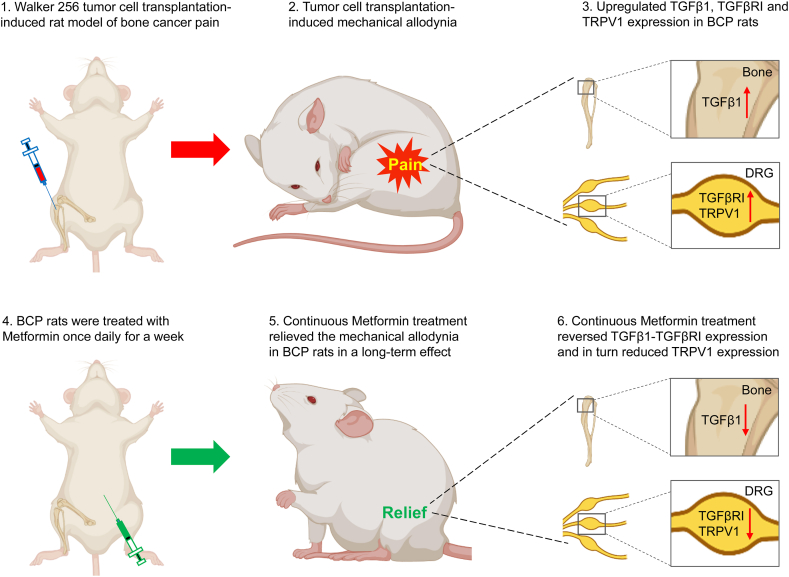
We identified that Metformin could reduce the mechanical allodynia of BCP rats, the analgesia might be mediated by TGFβ1-TGFβRⅠ-TRPV1 signaling pathway (Fig. 9). These findings imply that Metformin-mediated therapy for BCP may be effective and reliable.
Fig. 9.
Working Model. Walker 256 tumor cell transplantation induced bone cancer pain, and upregulated TGFβ1, TGFβRI and TRPV1 expression in BCP rats. Metformin could reduce the mechanical allodynia of BCP rats, the analgesia might be mediated by TGFβ1-TGFβRⅠ-TRPV1 signaling pathway.
5. Discussion
Bone cancer pain has a severe impact on patients' quality of life. One of the most challenging cancer pains is BCP. There is a critical urgency for safe and enforced BCP therapies. Metformin is a widely used first-line diabetes medicine. In addition to diabetes, it has been demonstrated to treat several types of chronic pain [[50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64]]. The exact mechanism of the analgesic effect of metformin in BCP rats is not fully understood, we showed that Metformin decreased TGFβ1-TGFβRI expression and in turn reduced TRPV1 expression, TGFβ1 was upregulated in the metastatic bone tissue of BCP rats, this finding supports the previous reports that TGFβ1 is a novel target of Metformin [65,66]. Overall, these findings suggested that TGFβRⅠ has a regulatory role in the expression of TRPV1 in L4-6 DRG of BCP rats, and that the TGFβ1-TRPV1 signaling may be involved in the analgesic action of Metformin in BCP rats.
TGFβ1 is known to be widely released during the bone resorption process. It was reported that TGFβ1 signaling related to BCP via the elevation and sensitization of TRPV1 in primary sensory neurons [42]. TRPV1 is a nonselective cation channel that is involved in osteoblast cell proliferation and bone mass repair [67], and can be activated by extracellular protons, zoledronic acid and capsaicin [68]. In the investigation, we verified that TGFβRⅠ inhibition by SD208 could obviously reduce the upregulation of TRPV1 in BCP rats, further suggesting the important role of TGFβ1-TGFβRⅠ signaling in the regulation of TRPV1 in BCP rats. More importantly, Metformin treatment could markedly downregulate TGFβ1, TGFβRⅠ and TRPV1 expression in BCP rats. The effect of Metformin on bone locally might be another important mechanism (such as reducing TGFβ1 expression in bone tissues after continuous Metformin injection) in relieving bone cancer pain. The role of TGFβ1 was further verified in other report which showed that peripheral injection of TGFβ1 directly induced thermal hyperalgesia in rats, and extracellular application of TGFβ1 significantly potentiated TRPV1 currents and increased Ca2+ in DRG neurons [42]. Additionally, the protective effect of Metformin on bone mass was reported in previous studies, Metformin could increase the bone-forming activity of osteoblasts [69,70], and inhibit the bone-resorbing activity of osteoclasts [71].
Both TGFβRI antagonist SD208 and Metformin relieves bone cancer pain by reducing TRPV1 in the ipsilateral L4-6 DRG, Metformin has a long-term analgesic effect with continuous treatment relative to TGFβRⅠ inhibitor SD208, but has a slower onset of action. Different treatment effects may be due to the following reasons. Firstly, the injection method is different, SD208 was delivered into local deep tissue around the tumor bone, whereas metformin is injected intraperitoneally for wider absorption and distribution. Secondly, the mechanism of action is different, SD208 directly inhibits the TGFβRI kinase activity, thereby blocking the downstream signaling cascade of TGFβ1. This includes the inhibition of Smad2/3 phosphorylation and subsequent transcriptional activities related to pain and inflammation [[72], [73], [74]]. Metformin, primarily known for its anti-diabetic effects, influences multiple pathways. In addition to inhibiting TGFβRI-TRPV1 signaling, Metformin can activate AMP-activated protein kinase (AMPK), which has various downstream effects, including anti-inflammatory and analgesic properties [75]. Thirdly, the pharmacokinetics are different, the pharmacokinetics of SD208, including absorption, distribution, metabolism, and excretion, are optimized for targeting the TGFβRI pathway. The selectivity of SD208 for TGFβRI ensures that the inhibition is confined to the TGFβRI pathway, potentially resulting in fewer off-target effects but also limiting the range of therapeutic effects. Metformin has a well-characterized pharmacokinetic profile that includes oral administration, slow absorption, and wide distribution, influencing its overall impact on the body. Metformin's effects are systemic due to its influence on metabolic pathways. Metformin enters the blood vessel and blood-brain barrier, thereby exerting similar therapeutic effect on the central nervous system [76], so intraperitoneal injection allows metformin to affect the spinal cord and brain similarly to the DRG, this explains the distribution and effect of metformin in DRG, spinal cord and brain. The extent of pain relief might differ due to the broader systemic effects of Metformin compared to the more focused action of SD208. While both SD208 and Metformin relieve bone cancer pain by targeting the TGFβRI-TRPV1 signaling, their different mechanisms, pharmacokinetics, and systemic effects lead to distinct therapeutic outcomes. SD208 provides a more specific inhibition of TGFβRI, while Metformin's broader impact on metabolism and other pathways results in a wider range of effects, potentially offering additional benefits but also posing different risks and side effects. Understanding these differences is crucial for optimizing therapeutic strategies for bone cancer pain.
This study also has some limitations that need to be considered. Firstly, the detailed mechanism of metformin-induced TGFβ1 downregulation in tumor-bearing bone tissues is not clear and deserves further study. Secondly, the exact mechanism of TGFβRⅠ inhibition-induced TRPV1 downregulation in Metformin-mediated analgesia in BCP rats remains uncertain and needs to be explored in the future investigation. Thirdly, the sample size of this study is relatively small and more samples, models and methods are needed to confirm the role of metformin in the treatment of bone cancer pain.
Ethics approval
This work was performed in accordance with the guidelines of the International Association for the Study of Pain (IASP). The protocol was approved by the Institutional Animal Care and Use Committee of Soochow University (SYXK 2022-0043).
Funding
This work was supported by grants from the National Natural Science Foundation of China (82201575), the Suzhou Science and Technology Project for Youth (KJXW2020059) and from the Zhangjiagang Science and Technology Project (ZKS2112).
Data availability statement
The datasets used or analyzed during the current study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Fang Zhou: Writing – original draft, Investigation, Data curation, Conceptualization. He-Ya Qian: Validation, Investigation, Data curation. Ke Wang: Validation, Investigation. Yong-Juan Gu: Validation, Investigation. Pei-Lin Liu: Validation, Investigation. Ling Zhang: Writing – review & editing. Long Chen: Writing – review & editing. Yu Song: Writing – review & editing. Ya-Nan Chen: Writing – review & editing. Hai-Long Zhang: Writing – review & editing, Supervision, Project administration, Funding acquisition, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e34991.
Contributor Information
Ya-Nan Chen, Email: chenyanan99@126.com.
Hai-Long Zhang, Email: hlzhang76@suda.edu.cn.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Scarborough B.M., Smith C.B. Optimal pain management for patients with cancer in the modern era. CA Cancer J Clin. 2018;68(3):182–196. doi: 10.3322/caac.21453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aielli F., Ponzetti M., Rucci N. Bone metastasis pain, from the bench to the bedside. Int. J. Mol. Sci. 2019;20(2) doi: 10.3390/ijms20020280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zajaczkowska R., et al. Bone pain in cancer patients: mechanisms and current treatment. Int. J. Mol. Sci. 2019;20(23) doi: 10.3390/ijms20236047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen H.W., et al. The circular RNA circSlc7a11 promotes bone cancer pain pathogenesis in rats by modulating LLC-WRC 256 cell proliferation and apoptosis. Mol. Cell. Biochem. 2021;476(4):1751–1763. doi: 10.1007/s11010-020-04020-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Okui T., et al. The HMGB1/RAGE axis induces bone pain associated with colonization of 4T1 mouse breast cancer in bone. J Bone Oncol. 2021;26 doi: 10.1016/j.jbo.2020.100330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jidveian Popescu M., et al. Depression and anxiety in recurrent giant cell tumor of bone. Rom. J. Morphol. Embryol. 2020;61(4):1057–1065. doi: 10.47162/RJME.61.4.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang K., et al. PD-1 blockade inhibits osteoclast formation and murine bone cancer pain. J. Clin. Invest. 2020;130(7):3603–3620. doi: 10.1172/JCI133334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kapoor R., et al. Cancer induced bone pain: current management and future perspectives. Med. Oncol. 2021;38(11):134. doi: 10.1007/s12032-021-01587-7. [DOI] [PubMed] [Google Scholar]
- 9.Yan Z., et al. Acupuncture as a complementary therapy for cancer-induced bone pain: a systematic review and meta-analysis. Front Pain Res (Lausanne) 2022;3 doi: 10.3389/fpain.2022.925013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zheng X.Q., et al. Neurophysiological mechanisms of cancer-induced bone pain. J. Adv. Res. 2022;35:117–127. doi: 10.1016/j.jare.2021.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vernieri C., et al. Impact of metformin use and diabetic status during adjuvant fluoropyrimidine-oxaliplatin chemotherapy on the outcome of patients with resected colon cancer: a tosca study subanalysis. Oncol. 2019;24(3):385–393. doi: 10.1634/theoncologist.2018-0442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cejuela M., et al. Metformin and breast cancer: where are we now? Int. J. Mol. Sci. 2022;23(5) doi: 10.3390/ijms23052705. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Triggle C.R., et al. Metformin: is it a drug for all reasons and diseases? Metabolism. 2022;133 doi: 10.1016/j.metabol.2022.155223. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y.M., et al. Anxiolytic effect of antidiabetic metformin is mediated by AMPK activation in mPFC inhibitory neurons. Mol. Psychiatr. 2023 Sep;28(9):3955–3965. doi: 10.1038/s41380-023-02283-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang J., et al. Metformin modulates microbiota-derived inosine and ameliorates methamphetamine-induced anxiety and depression-like withdrawal symptoms in mice. Biomed. Pharmacother. 2022;149 doi: 10.1016/j.biopha.2022.112837. [DOI] [PubMed] [Google Scholar]
- 16.Keshavarzi S., et al. Protective role of metformin against methamphetamine induced anxiety, depression, cognition impairment and neurodegeneration in rat: the role of CREB/BDNF and Akt/GSK3 signaling pathways. Neurotoxicology. 2019;72:74–84. doi: 10.1016/j.neuro.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 17.Yu H., et al. Association of metformin and depression in patients with type 2 diabetes. J. Affect. Disord. 2022;318:380–385. doi: 10.1016/j.jad.2022.09.015. [DOI] [PubMed] [Google Scholar]
- 18.El Massry M., et al. Metformin: a growing journey from glycemic control to the treatment of alzheimer's disease and depression. Curr. Med. Chem. 2021;28(12):2328–2345. doi: 10.2174/0929867327666200908114902. [DOI] [PubMed] [Google Scholar]
- 19.Fang W., et al. Metformin ameliorates stress-induced depression-like behaviors via enhancing the expression of BDNF by activating AMPK/CREB-mediated histone acetylation. J. Affect. Disord. 2020;260:302–313. doi: 10.1016/j.jad.2019.09.013. [DOI] [PubMed] [Google Scholar]
- 20.Foretz M., Guigas B., Viollet B. Metformin: update on mechanisms of action and repurposing potential. Nat. Rev. Endocrinol. 2023;19(8):460–476. doi: 10.1038/s41574-023-00833-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J., et al. Metformin limits osteoarthritis development and progression through activation of AMPK signalling. Ann. Rheum. Dis. 2020;79(5):635–645. doi: 10.1136/annrheumdis-2019-216713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Russe O.Q., et al. Activation of the AMP-activated protein kinase reduces inflammatory nociception. J. Pain. 2013;14(11):1330–1340. doi: 10.1016/j.jpain.2013.05.012. [DOI] [PubMed] [Google Scholar]
- 23.Na H.S., et al. Metformin attenuates monosodium-iodoacetate-induced osteoarthritis via regulation of pain mediators and the autophagy-lysosomal pathway. Cells. 2021;10(3) doi: 10.3390/cells10030681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baeza-Flores G.D.C., et al. Metformin: a prospective alternative for the treatment of chronic pain. Front. Pharmacol. 2020;11 doi: 10.3389/fphar.2020.558474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qian H.Y., et al. Metformin attenuates bone cancer pain by reducing TRPV1 and ASIC3 expression. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.713944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chen S., et al. Association between metformin use and risk of total knee arthroplasty and degree of knee pain in knee osteoarthritis patients with diabetes and/or obesity: a retrospective study. J. Clin. Med. 2022;11(16) doi: 10.3390/jcm11164796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weng W., et al. Metformin relieves neuropathic pain after spinal nerve ligation via autophagy flux stimulation. J. Cell Mol. Med. 2019;23(2):1313–1324. doi: 10.1111/jcmm.14033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan P., et al. Metformin attenuates sevoflurane-induced neurogenesis damage and cognitive impairment: involvement of the Nrf2/G6PD pathway. Metab. Brain Dis. 2023;38(6):2037–2053. doi: 10.1007/s11011-023-01218-2. [DOI] [PubMed] [Google Scholar]
- 29.Teng Z., et al. Long-term use of metformin is associated with reduced risk of cognitive impairment with alleviation of cerebral small vessel disease burden in patients with type 2 diabetes. Front. Aging Neurosci. 2021;13 doi: 10.3389/fnagi.2021.773797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shao T., et al. Metformin improves cognitive impairment in patients with schizophrenia: associated with enhanced functional connectivity of dorsolateral prefrontal cortex. Transl. Psychiatry. 2023;13(1):315. doi: 10.1038/s41398-023-02616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang J., et al. Metformin protects against myocardial ischemia-reperfusion injury and cell pyroptosis via AMPK/NLRP3 inflammasome pathway. Aging (Albany NY) 2020;12(23):24270–24287. doi: 10.18632/aging.202143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ma T., et al. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature. 2022;603(7899):159–165. doi: 10.1038/s41586-022-04431-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Coll A.P., et al. GDF15 mediates the effects of metformin on body weight and energy balance. Nature. 2020;578(7795):444–448. doi: 10.1038/s41586-019-1911-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang Y., et al. Metformin ameliorates chronic colitis-related intestinal fibrosis via inhibiting TGF-beta1/smad3 signaling. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.887497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohammad H.M.F., et al. Metformin suppresses LRG1 and TGFbeta1/ALK1-induced angiogenesis and protects against ultrastructural changes in rat diabetic nephropathy. Biomed. Pharmacother. 2023;158 doi: 10.1016/j.biopha.2022.114128. [DOI] [PubMed] [Google Scholar]
- 36.Shi L., et al. Metformin improves burn wound healing by modulating microenvironmental fibroblasts and macrophages. Cells. 2022;11(24) doi: 10.3390/cells11244094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hao Y., Baker D., Ten Dijke P. TGF-beta-Mediated epithelial-mesenchymal transition and cancer metastasis. Int. J. Mol. Sci. 2019;20(11) doi: 10.3390/ijms20112767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu S., et al. Analysis of genomics and immune infiltration patterns of epithelial-mesenchymal transition related to metastatic breast cancer to bone. Transl Oncol. 2021;14(2) doi: 10.1016/j.tranon.2020.100993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie F., et al. TGF-beta signaling in cancer metastasis. Acta Biochim. Biophys. Sin. 2018;50(1):121–132. doi: 10.1093/abbs/gmx123. [DOI] [PubMed] [Google Scholar]
- 40.Xia X., et al. Neutrophil extracellular traps promote metastasis in gastric cancer patients with postoperative abdominal infectious complications. Nat. Commun. 2022;13(1):1017. doi: 10.1038/s41467-022-28492-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhao M., Mishra L., Deng C.X. The role of TGF-beta/SMAD4 signaling in cancer. Int. J. Biol. Sci. 2018;14(2):111–123. doi: 10.7150/ijbs.23230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Q., et al. Peripheral TGF-beta1 signaling is a critical event in bone cancer-induced hyperalgesia in rodents. J. Neurosci. 2013;33(49):19099–19111. doi: 10.1523/JNEUROSCI.4852-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou Y.L., et al. Enhanced binding capability of nuclear factor-kappaB with demethylated P2X3 receptor gene contributes to cancer pain in rats. Pain. 2015;156(10):1892–1905. doi: 10.1097/j.pain.0000000000000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhou Y.Q., et al. Nrf2 activation ameliorates mechanical allodynia in paclitaxel-induced neuropathic pain. Acta Pharmacol. Sin. 2020;41(8):1041–1048. doi: 10.1038/s41401-020-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pournaghi P., et al. An investigation on body weights, blood glucose levels and pituitary-gonadal axis hormones in diabetic and metformin-treated diabetic female rats. Vet. Res. Forum. 2012;3(2):79–84. [PMC free article] [PubMed] [Google Scholar]
- 46.Liu S., Ren J., Ten Dijke P. Targeting TGFbeta signal transduction for cancer therapy. Signal Transduct Target Ther. 2021;6(1):8. doi: 10.1038/s41392-020-00436-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stuelten C.H., Zhang Y.E. Transforming growth factor-beta: an agent of change in the tumor microenvironment. Front. Cell Dev. Biol. 2021;9 doi: 10.3389/fcell.2021.764727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun X., et al. Suppression of breast cancer-associated bone loss with osteoblast proteomes via Hsp90ab1/moesin-mediated inhibition of TGFbeta/FN1/CD44 signaling. Theranostics. 2022;12(2):929–943. doi: 10.7150/thno.66148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vander Ark A., Cao J., Li X. TGF-beta receptors: in and beyond TGF-beta signaling. Cell. Signal. 2018;52:112–120. doi: 10.1016/j.cellsig.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 50.Shiers S., et al. Neuropathic pain creates an enduring prefrontal cortex dysfunction corrected by the type II diabetic drug metformin but not by gabapentin. J. Neurosci. 2018;38(33):7337–7350. doi: 10.1523/JNEUROSCI.0713-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu M., et al. Metformin relieves bortezomib-induced neuropathic pain by regulating AMPKa2-mediated autophagy in the spinal dorsal horn. Neurochem. Res. 2022;47(7):1878–1887. doi: 10.1007/s11064-022-03571-7. [DOI] [PubMed] [Google Scholar]
- 52.Li H., et al. Exploration of metformin as novel therapy for osteoarthritis: preventing cartilage degeneration and reducing pain behavior. Arthritis Res. Ther. 2020;22(1):34. doi: 10.1186/s13075-020-2129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Inyang K.E., et al. The antidiabetic drug metformin prevents and reverses neuropathic pain and spinal cord microglial activation in male but not female mice. Pharmacol. Res. 2019;139:1–16. doi: 10.1016/j.phrs.2018.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hacimuftuoglu A., et al. The analgesic effect of metformin on paclitaxel-induced neuropathic pain model in rats: by considering pathological results. J Cancer Res Ther. 2020;16(1):34–39. doi: 10.4103/jcrt.JCRT_1455_16. [DOI] [PubMed] [Google Scholar]
- 55.Ge A., et al. Effects of metformin on the expression of AMPK and STAT3 in the spinal dorsal horn of rats with neuropathic pain. Mol. Med. Rep. 2018;17(4):5229–5237. doi: 10.3892/mmr.2018.8541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dasuni Wasana P.W., et al. Metformin and curcumin co-encapsulated chitosan/alginate nanoparticles as effective oral carriers against pain-like behaviors in mice. Int J Pharm. 2023;640 doi: 10.1016/j.ijpharm.2023.123037. [DOI] [PubMed] [Google Scholar]
- 57.Dasuni Wasana P.W., et al. Curcumin and metformin synergistically modulate peripheral and central immune mechanisms of pain. Sci. Rep. 2022;12(1):9713. doi: 10.1038/s41598-022-13647-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Das V., et al. Early treatment with metformin in a mice model of complex regional pain syndrome reduces pain and edema. Anesth. Analg. 2020;130(2):525–534. doi: 10.1213/ANE.0000000000004057. [DOI] [PubMed] [Google Scholar]
- 59.Carvalho E.S.A.P., et al. The effect of the anti-diabetic drug metformin on musculoskeletal pain: a cross-sectional study with 21,889 individuals from the UK biobank. Eur. J. Pain. 2021;25(6):1264–1273. doi: 10.1002/ejp.1747. [DOI] [PubMed] [Google Scholar]
- 60.Carvalho E.S.A.P., et al. The modifier effect of physical activity, body mass index, and age on the association of metformin and chronic back pain: a cross-sectional analysis of 21,899 participants from the UK Biobank. PLoS One. 2023;18(2) doi: 10.1371/journal.pone.0282205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cao X.J., et al. Metformin attenuates diabetic neuropathic pain via AMPK/NF-kappaB signaling pathway in dorsal root ganglion of diabetic rats. Brain Res. 2021;1772 doi: 10.1016/j.brainres.2021.147663. [DOI] [PubMed] [Google Scholar]
- 62.Cao J., et al. [Metformin alleviates pathologic pain in mice with radiation dermatitis by inhibiting p38MAPK/NF-kappaB signaling pathway] Nan Fang Yi Ke Da Xue Xue Bao. 2023;43(10):1815–1820. doi: 10.12122/j.issn.1673-4254.2023.10.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Augusto P.S.A., et al. Metformin antinociceptive effect in models of nociceptive and neuropathic pain is partially mediated by activation of opioidergic mechanisms. Eur. J. Pharmacol. 2019;858 doi: 10.1016/j.ejphar.2019.172497. [DOI] [PubMed] [Google Scholar]
- 64.Afshari K., et al. Anti-inflammatory effects of Metformin improve the neuropathic pain and locomotor activity in spinal cord injured rats: introduction of an alternative therapy. Spinal Cord. 2018;56(11):1032–1041. doi: 10.1038/s41393-018-0168-x. [DOI] [PubMed] [Google Scholar]
- 65.Zheng Y., et al. Metformin inhibits ovarian cancer growth and migration in vitro and in vivo by enhancing cisplatin cytotoxicity. Am J Transl Res. 2018;10(10):3086–3098. [PMC free article] [PubMed] [Google Scholar]
- 66.Yoshida J., et al. Metformin inhibits TGF-beta1-induced epithelial-mesenchymal transition and liver metastasis of pancreatic cancer cells. Oncol. Rep. 2020;44(1):371–381. doi: 10.3892/or.2020.7595. [DOI] [PubMed] [Google Scholar]
- 67.Scala R., et al. Zoledronic acid modulation of TRPV1 channel currents in osteoblast cell line and native rat and mouse bone marrow-derived osteoblasts: cell proliferation and mineralization effect. Cancers. 2019;11(2) doi: 10.3390/cancers11020206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scala R., et al. Bisphosphonates targeting ion channels and musculoskeletal effects. Front. Pharmacol. 2022;13 doi: 10.3389/fphar.2022.837534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jang W.G., et al. Metformin induces osteoblast differentiation via orphan nuclear receptor SHP-mediated transactivation of Runx2. Bone. 2011;48(4):885–893. doi: 10.1016/j.bone.2010.12.003. [DOI] [PubMed] [Google Scholar]
- 70.Schurman L., et al. Metformin reverts deleterious effects of advanced glycation end-products (AGEs) on osteoblastic cells. Exp. Clin. Endocrinol. Diabetes. 2008;116(6):333–340. doi: 10.1055/s-2007-992786. [DOI] [PubMed] [Google Scholar]
- 71.Mai Q.G., et al. Metformin stimulates osteoprotegerin and reduces RANKL expression in osteoblasts and ovariectomized rats. J. Cell. Biochem. 2011;112(10):2902–2909. doi: 10.1002/jcb.23206. [DOI] [PubMed] [Google Scholar]
- 72.Lai T.H., et al. The presence of TGFbeta3 in human ovarian intrafollicular fluid and its involvement in thromboxane generation in follicular granulosa cells through a canonical TGFbetaRI, smad2/3 signaling pathway and COX-2 induction. Int. J. Mol. Sci. 2024;25(10) doi: 10.3390/ijms25105558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shu J., et al. Daidzein suppresses TGF-beta1-induced cardiac fibroblast activation via the TGF-beta1/SMAD2/3 signaling pathway. Eur. J. Pharmacol. 2022;919 doi: 10.1016/j.ejphar.2022.174805. [DOI] [PubMed] [Google Scholar]
- 74.Schmidt K.E., et al. Serum-induced proliferation of human cardiac stem cells is modulated via TGFbetaRI/II and SMAD2/3. Int. J. Mol. Sci. 2024;25(2) doi: 10.3390/ijms25020959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Steinberg G.R., Carling D. AMP-activated protein kinase: the current landscape for drug development. Nat. Rev. Drug Discov. 2019;18(7):527–551. doi: 10.1038/s41573-019-0019-2. [DOI] [PubMed] [Google Scholar]
- 76.Cao G., et al. Mechanism of metformin regulation in central nervous system: progression and future perspectives. Biomed. Pharmacother. 2022;156 doi: 10.1016/j.biopha.2022.113686. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used or analyzed during the current study are available from the corresponding author upon reasonable request.