Abstract
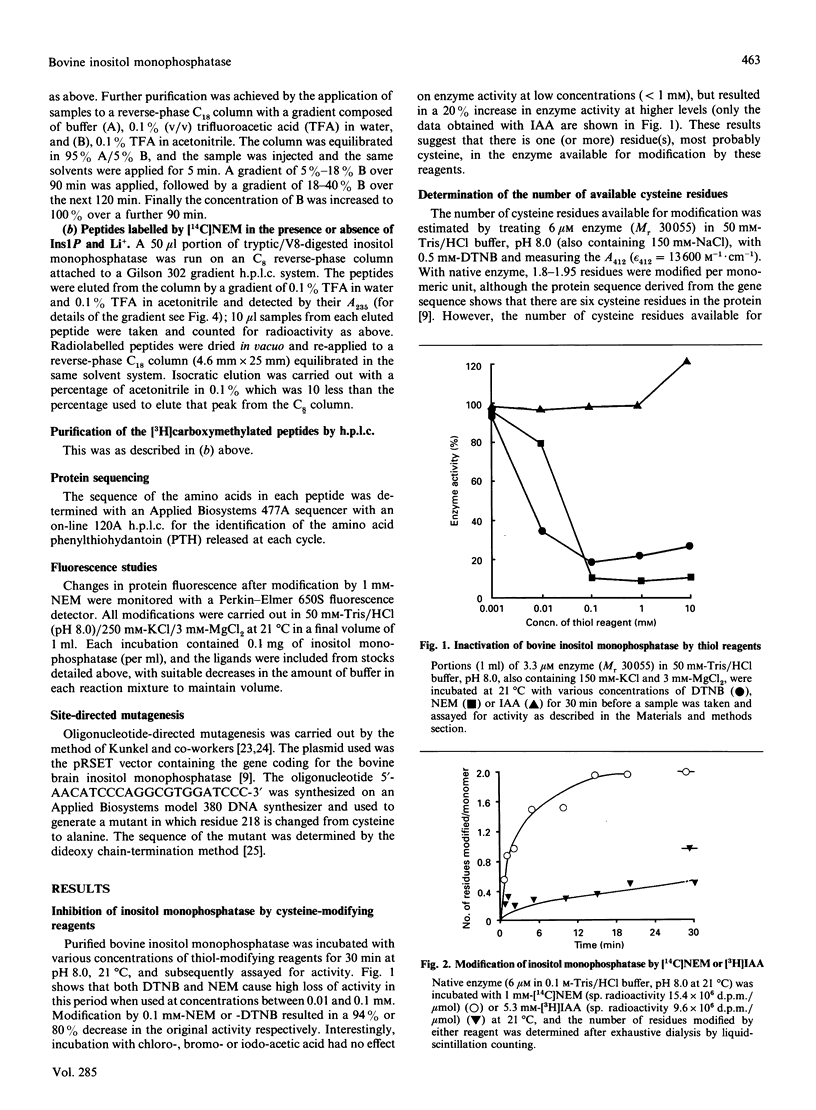
1. Bovine inositol monophosphatase reacts with thiol reagents such as 5,5'-dithiobis-(2-nitrobenzoic acid) (DTNB), N-ethylmaleimide (NEM) and iodoacetic acid (IAA). 2. Modification by NEM results in nearly total loss of enzyme activity, whereas modification by IAA causes a slight increase in activity. 3. The loss of activity caused by NEM can be prevented by the inclusion of Ins1P, or better Ins1P and LiCl in the reaction mixture. 4. Two equivalents of p-nitrothiobenzoate (NTB2-) are released from the native enzyme on reaction with DTNB, and six equivalents of NTB2- are released from the SDS-denatured enzyme, suggesting that none of the six cysteine residues per molecule of enzyme is involved in intra- or inter-molecular disulphide bridges. 5. Both NEM and IAA react with two cysteine residues (residues 141 and 184 in the sequence) in a mutually exclusive manner. 6. NEM also reacts stoichiometrically with residue 218. 7. The NEM-induced loss of enzyme activity is accompanied by a 15% decrease in protein fluorescence. 8. A mutant of the enzyme which has an Ala-218 replacement for Cys-218 has full activity and is not sensitive to NEM, showing that the modification of this cysteine by NEM causes inhibition of the native protein by steric effects and that Cys-218 is not essential for activity.
Full text
PDF

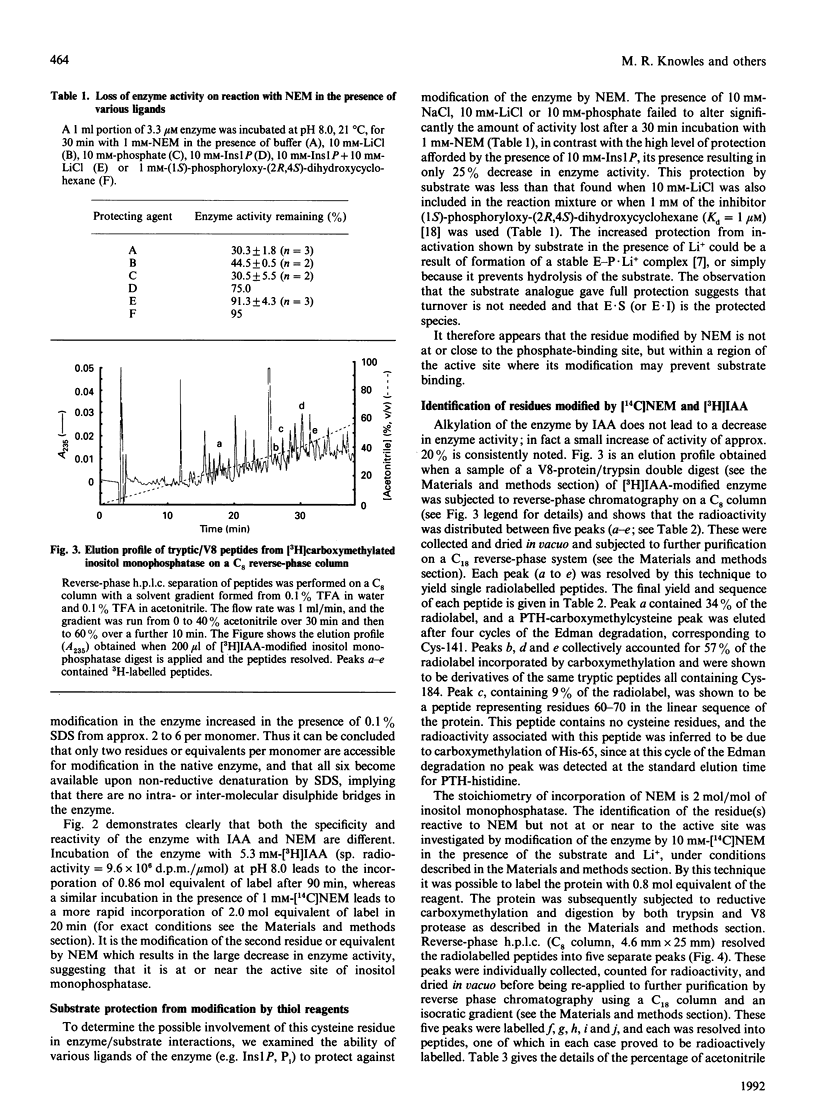


Selected References
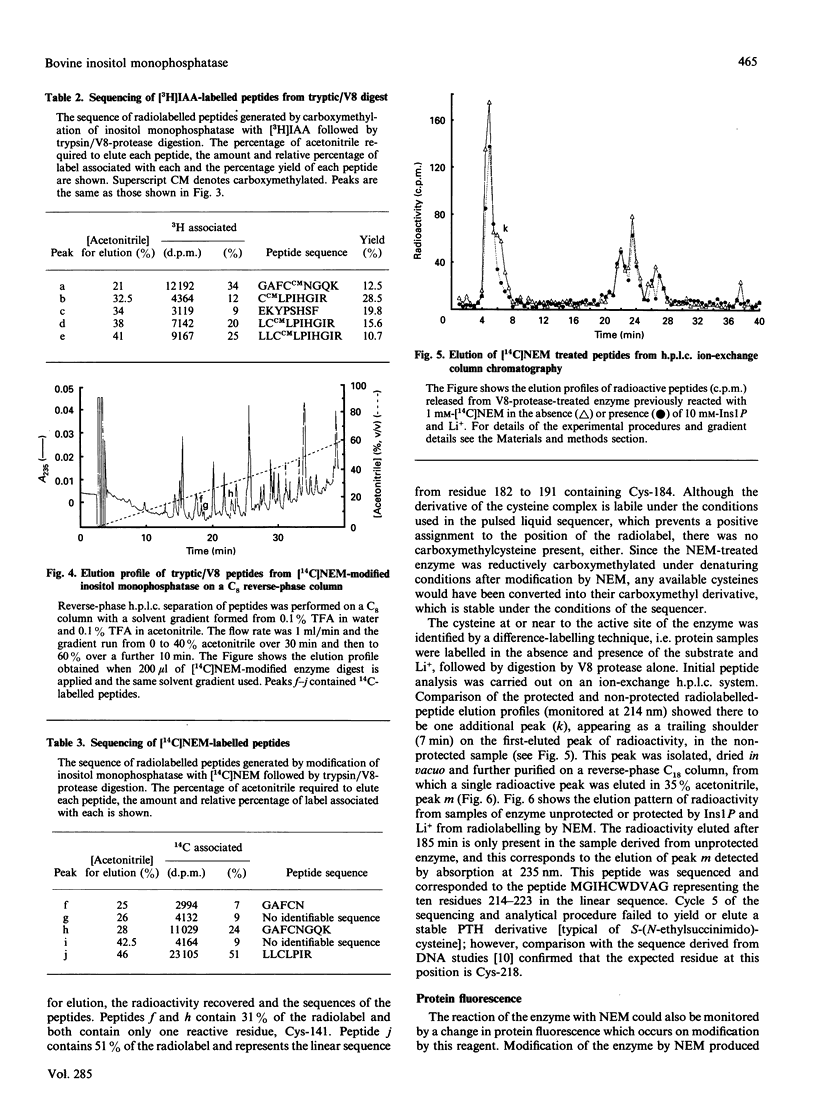
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Latif A. A. Calcium-mobilizing receptors, polyphosphoinositides, and the generation of second messengers. Pharmacol Rev. 1986 Sep;38(3):227–272. [PubMed] [Google Scholar]
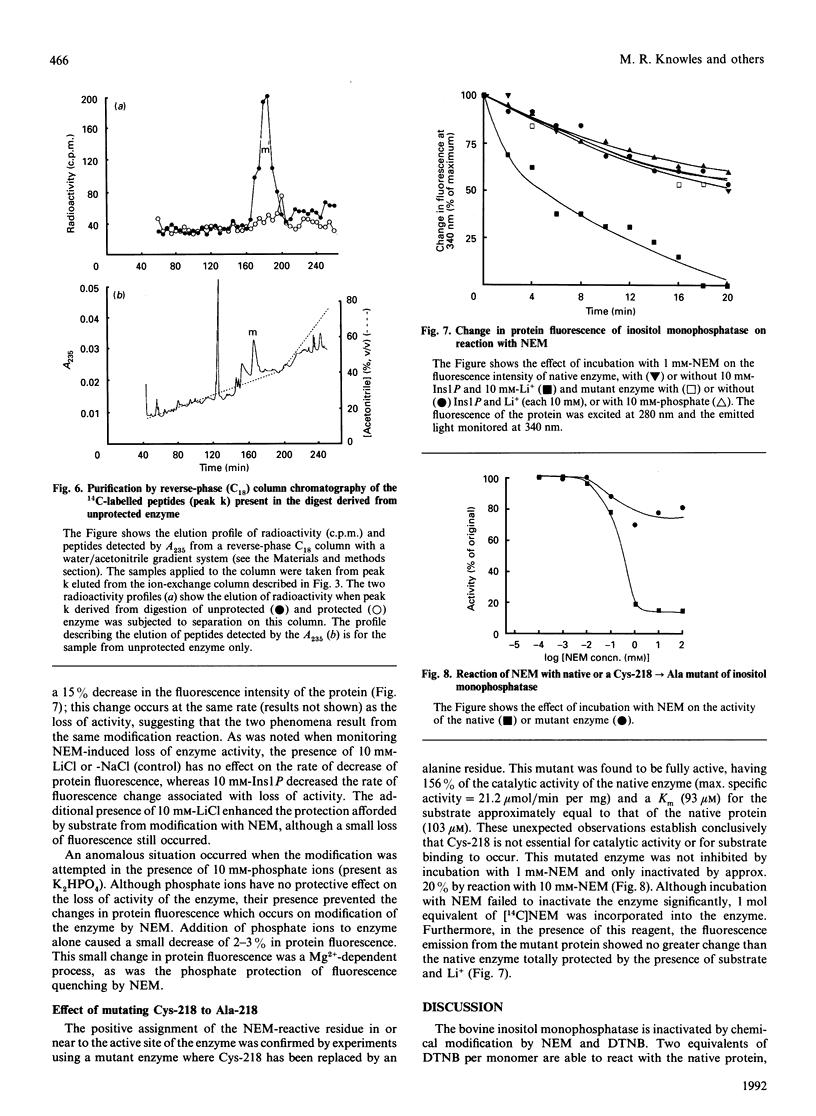
- Ashby B., Wootton J. C., Fincham J. R. Slow conformational changes of a Neurospora glutamate dehydrogenase studied by protein fluorescence. Biochem J. 1974 Nov;143(2):317–329. doi: 10.1042/bj1430317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge M. J., Downes C. P., Hanley M. R. Neural and developmental actions of lithium: a unifying hypothesis. Cell. 1989 Nov 3;59(3):411–419. doi: 10.1016/0092-8674(89)90026-3. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- COWGILL R. W. FLUORESCENCE AND THE STRUCTURE OF PROTEINS. V. IONIZATION OF TYROSYL RESIDUES. Biochim Biophys Acta. 1965 Jan 25;94:81–88. doi: 10.1016/0926-6585(65)90010-5. [DOI] [PubMed] [Google Scholar]
- Diehl R. E., Whiting P., Potter J., Gee N., Ragan C. I., Linemeyer D., Schoepfer R., Bennett C., Dixon R. A. Cloning and expression of bovine brain inositol monophosphatase. J Biol Chem. 1990 Apr 15;265(11):5946–5949. [PubMed] [Google Scholar]
- ELLMAN G. L. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959 May;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- Eisenberg F., Jr D-myoinositol 1-phosphate as product of cyclization of glucose 6-phosphate and substrate for a specific phosphatase in rat testis. J Biol Chem. 1967 Apr 10;242(7):1375–1382. [PubMed] [Google Scholar]
- Flashner M., Hollenberg P. F., Coon M. J. Mechanism of action of pyruvate kinase. Role of sulfhydryl groups in catalytic activity as determined by disulfide interchange. J Biol Chem. 1972 Dec 25;247(24):8114–8121. [PubMed] [Google Scholar]
- Ganzhorn A. J., Chanal M. C. Kinetic studies with myo-inositol monophosphatase from bovine brain. Biochemistry. 1990 Jun 26;29(25):6065–6071. doi: 10.1021/bi00477a026. [DOI] [PubMed] [Google Scholar]
- Gee N. S., Ragan C. I., Watling K. J., Aspley S., Jackson R. G., Reid G. G., Gani D., Shute J. K. The purification and properties of myo-inositol monophosphatase from bovine brain. Biochem J. 1988 Feb 1;249(3):883–889. doi: 10.1042/bj2490883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallcher L. M., Sherman W. R. The effects of lithium ion and other agents on the activity of myo-inositol-1-phosphatase from bovine brain. J Biol Chem. 1980 Nov 25;255(22):10896–10901. [PubMed] [Google Scholar]
- Jackson R. G., Gee N. S., Ragan C. I. Modification of myo-inositol monophosphatase by the arginine-specific reagent phenylglyoxal. Biochem J. 1989 Dec 1;264(2):419–422. doi: 10.1042/bj2640419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979 Nov 15;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- McAllister G., Whiting P., Hammond E. A., Knowles M. R., Atack J. R., Bailey F. J., Maigetter R., Ragan C. I. cDNA cloning of human and rat brain myo-inositol monophosphatase. Expression and characterization of the human recombinant enzyme. Biochem J. 1992 Jun 15;284(Pt 3):749–754. doi: 10.1042/bj2840749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragan C. I., Watling K. J., Gee N. S., Aspley S., Jackson R. G., Reid G. G., Baker R., Billington D. C., Barnaby R. J., Leeson P. D. The dephosphorylation of inositol 1,4-bisphosphate to inositol in liver and brain involves two distinct Li+-sensitive enzymes and proceeds via inositol 4-phosphate. Biochem J. 1988 Jan 1;249(1):143–148. doi: 10.1042/bj2490143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shears S. B. Metabolism of the inositol phosphates produced upon receptor activation. Biochem J. 1989 Jun 1;260(2):313–324. doi: 10.1042/bj2600313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot H., de Groot H., Noll T. Enzymic determination of inorganic phosphates, organic phosphates and phosphate-liberating enzymes by use of nucleoside phosphorylase-xanthine oxidase (dehydrogenase)-coupled reactions. Biochem J. 1985 Aug 15;230(1):255–260. doi: 10.1042/bj2300255. [DOI] [PMC free article] [PubMed] [Google Scholar]


