Key Teaching Points.
-
•
Ventricular pacing in patients with implantable cardioverter-defibrillator has potentially proarrhythmic effects.
-
•
Timely ventricular pacing owing to an automatic ventricular threshold testing and atrial undersensing played an important role in the occurrence of ventricular fibrillation (VF) by forming a short-long-short sequence.
-
•
In patients with long QT syndrome, discontinuation of an automatic ventricular threshold testing and appropriate lead management are essential to prevent iatrogenic VF.
Introduction
Long QT type 3 syndrome (LQT3) is an inherited arrhythmogenic condition that causes QT interval prolongation, life-threatening ventricular arrhythmias, atrial fibrillation (AF), cardiac sudden death (typically at rest or during sleep), and bradycardia.1,2 In LQT3 patients, an implantable cardioverter-defibrillator (ICD) should be considered for secondary prevention of cardiac sudden death.3,4 However, ventricular pacing in transvenous ICD patients was reported to have proarrhythmic effects because it produces abrupt changes in ventricular cycle length and short-long-short (S-L-S) sequences, which induces polymorphic ventricular tachycardia (VT) and ventricular fibrillation (VF).5,6 Herein, we report an LQT3 patient with AF who experienced multiple ICD shock therapies for polymorphic VT and VF induced by inappropriate ventricular pacing owing to automatic ventricular pacing threshold testing and atrial undersensing.
Case report
A 32-year-old man with LQT3 was admitted to our hospital because of ICD shock therapy. At age 3 years, he was diagnosed with LQT3 by genetic testing and electrocardiogram following loss of consciousness. At age 19 years, he was resuscitated from VF and received transvenous dual-chamber ICD implantation. At age 26 years, the ICD generator was replaced because of battery depletion. At age 27 years, he underwent pulmonary vein isolation for AF, which subsequently recurred. Genetic testing revealed an SCN5A exon28 c.5384A>G, p.Y1795C mutation. Pilsicainide (150 mg/d) had been prescribed because of QT interval shortening. The ICD (Evera XT DR; Medtronic, Inc, Minneapolis, MN) was programmed to 1 VF zone (rate >200 beats/min) with ICD shocks (35 J × 6) in AAI−DDD mode with a lower limit rate of 50 beats/min, a paced atrial-ventricular (AV) delay of 180 ms, and a sensed AV delay of 150 ms.
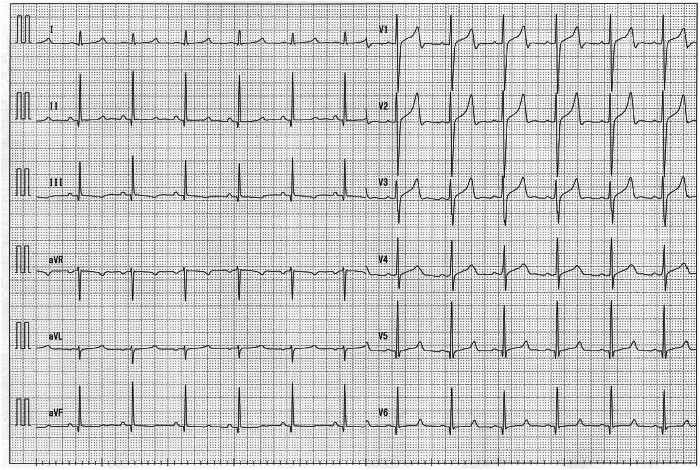
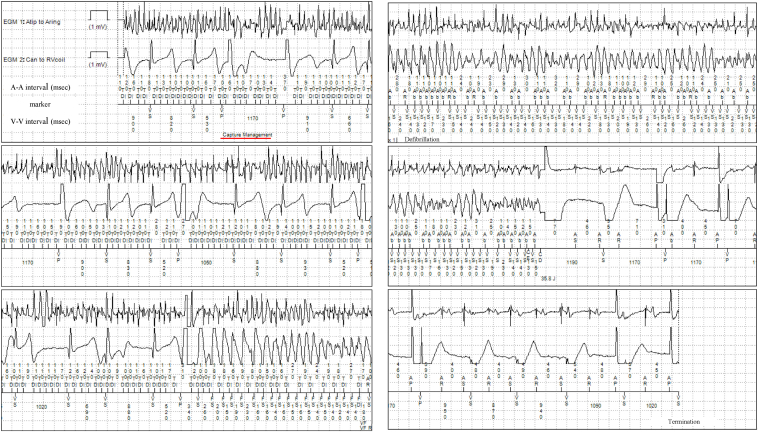
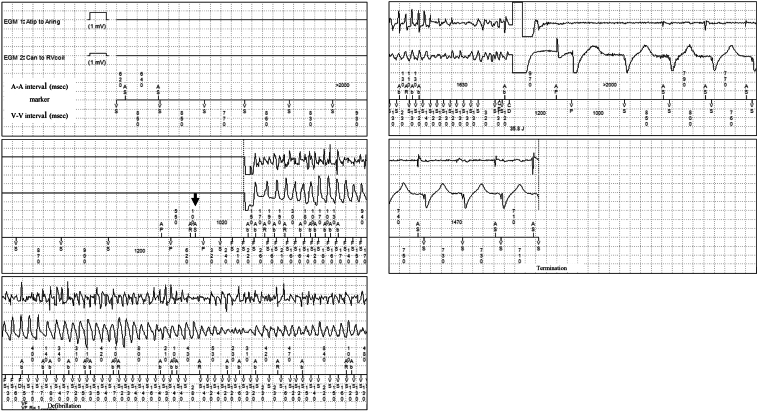
The 12-lead electrocardiogram on admission showed a prolonged QT corrected interval (QTc) of 484 ms calculated by Bazett’s formula, a prolonged ST segment, and a late-onset T wave, which are typical features of LQT3 (Figure 1). There were no electrolyte abnormalities that could cause further QT interval prolongation. ICD interrogation data on admission revealed that he had experienced multiple ICD shock therapies for polymorphic VT and VF over 6 years. There were 2 different modes of polymorphic VT and VF onset. One mode occurred from midnight to 3:30 AM while he was sleeping (Figure 2). VF was induced by ventricular pacing for automatic ventricular pacing threshold testing (Ventricular Capture Management; Medtronic, Inc). The ventricular pacing delivered on the last T wave, with a ventricular coupling interval of 520 ms under AF rhythm, formed an S-L-S sequence, which finally induced VF. VF and AF were terminated by ICD shock therapy. The other mode of onset occurred at approximately 5:00 AM (Figure 3). AF was undersensed and mode switch to non–atrial tracking mode failed. VF was induced by ventricular pacing following inappropriately sensed atrial activity. VF and AF were terminated by ICD shock therapy. The 24-hour Holter electrocardiography showed a QT interval of 613 ms and QTc of 560 ms at the minimum heart rate of 50 beats/min at night, which was more prolonged than that during the daytime.
Figure 1.
The 12-lead electrocardiogram on admission while the patient was taking pilsicainide (150 mg/d). The QT corrected interval (QTc) calculated by Bazett’s formula was 484 ms.
Figure 2.
The intracardiac electrocardiogram of ventricular fibrillation (VF) occurring at 3:30 AM. In the upper left intracardiac electrocardiogram (IEGM), the start of the automatic ventricular pacing threshold testing (Ventricular Capture Management; Medtronic, Inc, Minneapolis, MN) is highlighted with a red underline. Ventricular pacing for the automatic ventricular pacing threshold testing was conducted at a pacing coupling interval of 520 ms. In the lower left IEGM, VF was induced by ventricular pacing on the last T wave. A rapid irregular A-A interval indicated atrial fibrillation (AF). In the middle right IEGM, VF and AF were terminated by shock therapy (35.8 J).
Figure 3.
Because of atrial fibrillation (AF) undersensing, the atrial activity was determined as an atrial premature beat (black arrow) without mode switch to non–atrial tracking mode. Subsequent ventricular pacing triggered a short-long-short sequence, which led to ventricular fibrillation (VF). VF and AF were terminated by shock therapy (38.5 J).
The P-wave amplitude averaged 1.6 mV and the P-wave sensing was set at 0.6 mV, but the P wave was sometimes not captured abruptly even under the sinus rhythm. The ventricular pacing threshold had increased over time without significant change in the ventricular pacing impedance. Note that the atrial lead impedance often fell below 200 Ω (minimum 57 Ω), which indicated microdislodgment or malfunction of the atrial lead. To detect AF properly, the atrial sensing was changed to 0.6 mV, 0.3 mV, and 0.15 mV, none of which worked. The heart team decided to remove the leads and implant a new device (a subcutaneous ICD and a cardiac implantable monitor) as a class Ⅱb indication for lead extraction, in accordance with the 2017 HRS expert consensus.7 The automatic ventricular pacing threshold testing was switched off. To avoid unnecessary ventricular pacing owing to AF undersensing, the setting was changed from DDD mode to VVI mode until the lead extraction. To obtain further QT interval shortening, pilsicainide was changed to mexiletine (300 mg/d), which reduced the QTc interval to 445 ms in the daytime. The patient did not experience ICD shock therapies and the frequency of AF decreased over the following 6 months.
Discussion
We present a case of VF induced by inappropriate ventricular pacing in an LQT3 patient with AF. This case is an important reminder that timely ventricular pacing by automatic ventricular pacing threshold testing and atrial undersensing may increase the risk of iatrogenic VF in LQT3 patients with a prolonged QT interval.
VT/VF induced by ventricular pacing has been previously reported. For example, in a combined post hoc analysis of the PainFree Rx Ⅱ and EnTrust trials, ventricular pacing–associated VT/VF accounted for 29.8% of all VT/VF episodes.6 Additionally, pacing-facilitated VT/VF, which was defined as ventricular pacing involved in the S-L-S sequence, accounted for 35% of all VT/VF episodes. Therefore, the proarrhythmic effects of ventricular pacing cannot be ignored. Other studies have reported that algorithms designed to reduce ventricular pacing and atrial undersensing are possible causes of pacing-induced VF.8,9
In the present case, 3 different mechanisms were considered for the mode of VF onset. First, the timely ventricular pacing with a short coupling interval contributed to the induction of VF. A short-coupled ventricular pacing may generate the so-called R-on-T, and this region is thought to be vulnerable to ventricular pacing. This is because ventricular excitation encounters a refractory region, which leads to unidirectional block and development of a figure-of-8 reentry.10 In the initial VF shown in Figure 2, ventricular pacing was conducted at a pacing cycle length of 520 ms. After heart rate stability was confirmed, automatic ventricular pacing threshold testing (Ventricular Capture Management) was applied during AF. In the latter VF shown in Figure 3, atrial sensing and sensed AV delay were followed by ventricular pacing. This was because of AF undersensing, whereby the atrial activity was determined to be an atrial premature beat without mode switch to non–atrial tracking mode.
The second mechanism of VF induction involves the dispersion of the R-R interval of AF. AF is an independent risk factor for VF.11 In the present case, the R-R interval immediately before VF onset formed an S-L-S sequence caused by the irregular R-R AF intervals and ventricular pacing. The S-L-S sequence is considered more likely to induce VF because it can increase cardiac transmural dispersion of repolarization, which results in functional block and slow conduction.12 Furthermore, irregular ventricular activation of AF can lead to QT prolongation and QT dispersion, which makes it more vulnerable to ventricular pacing.13
The third mechanism was that the features of LQT3 can contribute to dispersion of the QT interval during AF. It was previously reported that LQT3 patients have a significant prolongation of QTc interval at night, which may be associated with the clinical occurrence of nocturnal events.14,15 In this case, despite a QTc of 484 ms in the daytime, the QTc was prolonged to 560 ms at night, indicating that this patient belongs to the high-risk group of cardiac events with diurnal variation in QTc.16 In general, since the automatic ventricular pacing threshold testing is conducted during the night, ventricular pacing with short coupling interval during the test is more likely to induce VF following R-on-T. Importantly, it is essential to evaluate the diurnal variation of QTc in LQT3 patients before conducting the automatic ventricular pacing threshold testing, and this test should not be applied to high-risk patients.
Lastly, LQT3 patients have been reported to show repolarization dispersion owing to action potential duration prolongation in both ventricle and atrium, leading to QT interval prolongation, polymorphic VT, VF, polymorphic atrial tachycardia, and early-onset AF.17 With regard to pharmacotherapy, Mazzanti and colleagues18 clearly state that in high-risk LQT3 patients with QTc >500 ms, mexiletine should be considered to avoid ventricular arrhythmias, and we changed the medication accordingly. Interestingly, in this case mexiletine, which shortens action potential duration, was effective not only for QT interval shortening but also for AF suppression. This fact was thought to reflect the electrophysiological substrate in LQT3 patients.
This case highlights the importance of individual assessment prior to application of automatic ventricular pacing threshold testing and periodic lead management. These 2 modes of onset of iatrogenic VF could have been avoided if clinicians had known the proarrhythmic effects of automatic ventricular threshold testing and the risk of ventricular pacing after AF undersensing.
Conclusion
In LQT3 patients with AF, timely ventricular pacing plays an important role in the occurrence of VF by forming an S-L-S sequence. Ventricular pacing should be minimized. Discontinuation of automatic ventricular pacing threshold testing and appropriate intervention for lead failure will prevent iatrogenic VF.
Disclosures
All authors declare no conflicts of interest related to this work.
Acknowledgments
We thank Edanz (https://jp.edanz.com/ac) for editing a draft of this manuscript.
Funding Sources
This report did not receive any specific grants from funding agencies in the public, commercial, or not-for-profit sectors.
References
- 1.Schwartz P.J., Priori S.G., Spazzolini C., et al. Genotype-phenotype correlation in long-QT syndrome: gene-specific triggers for life-threatening arrhythmias. Circulation. 2001;103:89–95. doi: 10.1161/01.cir.103.1.89. [DOI] [PubMed] [Google Scholar]
- 2.Plantonov P.G., McNitt S., Polonsky B., Rosero S.Z., Zareba W. Atrial fibrillation in long QT syndrome by genotype. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.119.007213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zeppenfeld K., Hansen J.T., Riva M., et al. 2022 ESC Guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death. Eur Heart J. 2022;43:3997–4126. doi: 10.1093/eurheartj/ehac262. [DOI] [PubMed] [Google Scholar]
- 4.Wilde A.M., Amin A.S. Clinical spectrum of SCN5A mutations long QT syndrome, Brugada syndrome, and cardiomyopathy. JACC Clin Electrophysiol. 2018;4:569–579. doi: 10.1016/j.jacep.2018.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Himmrich E., Przibille O., Zellerhoff C., et al. Proarrhythmic effect of pacemaker stimulation in patients with implanted cardioverter-defibrillators. Circulation. 2003;108:192–197. doi: 10.1161/01.CIR.0000080291.65638.CC. [DOI] [PubMed] [Google Scholar]
- 6.Sweeney M.O., Ruetz L.L., Belk P., Mullen T.J., Johnson J.W., Sheldon T. Bradycardia pacing-induced short-long-short sequences at the onset of ventricular tachyarrhythmias: a possible mechanism of proarrhythmia? J Am Coll Cardiol. 2007;50:614–622. doi: 10.1016/j.jacc.2007.02.077. [DOI] [PubMed] [Google Scholar]
- 7.Kusumoto F.M., Schoenfeld M.H., Wilkoff B.L., et al. 2017 HRS expert consensus statement on cardiovascular implantable electronic device lead management and extraction. Heart Rhythm. 2017;14:e503–e551. doi: 10.1016/j.hrthm.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 8.Day G.F., Padanilam B.J. Pacing threshold testing induced ventricular fibrillation following acute rate control of atrial fibrillation. J Cardiovasc Electrophysiol. 2009;20:1405–1407. doi: 10.1111/j.1540-8167.2009.01505.x. [DOI] [PubMed] [Google Scholar]
- 9.Halawa A., Aguilar M., Sweeney M.O. Syncope after successful implantation of atrioventricular synchronous leadless pacemaker caused by polymorphic ventricular tachycardia. HeartRhythm Case Rep. 2020;6:503–506. doi: 10.1016/j.hrcr.2020.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu M.B., Vandersickel N., Panfilov A.V., Qu Z. R-from-T as a common mechanism of arrhythmia initiation in long QT syndromes. Circ Arrhythm Electrophysiol. 2019;12 doi: 10.1161/CIRCEP.119.007571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bardai A., Blom M.T., Hoeijen D.A., Deutekom H.W.M., Brouwer H.J., Tan H.L. Atrial fibrillation is an independent risk factor for ventricular fibrillation. A large-scale population-based case-control study. Circ Arrhythm Electrophysiol. 2014;7:1033–1039. doi: 10.1161/CIRCEP.114.002094. [DOI] [PubMed] [Google Scholar]
- 12.Noda T., Shimizu W., Satomi K., et al. Classification and mechanism of Torsade de Pointes initiation in patients with congenital long QT syndrome. Eur Heart J. 2004;25:2149–2154. doi: 10.1016/j.ehj.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 13.Akoum N.W., Sanders N.A., Wasmund S.L., Hamdan M.H. Irregular ventricular activation results in QT prolongation and increased QT dispersion. J Cardiovasc Electrophysiol. 2011;22:1249–1252. doi: 10.1111/j.1540-8167.2011.02110.x. [DOI] [PubMed] [Google Scholar]
- 14.Stramba-Badiale M., Priori S.G., Napolitano C., et al. Gene-specific differences in circadian variation of ventricular repolarization in long QT syndrome: a key to sudden death during sleep? Ital Heart J. 2000;1:329–330. [PubMed] [Google Scholar]
- 15.Locati E.T. QT Interval Duration Remains a Major Risk Factor in Long QT Syndrome Patients. J Am Coll Cardiol. 2006;48:1053–1055. doi: 10.1016/j.jacc.2006.06.034. [DOI] [PubMed] [Google Scholar]
- 16.Priori S.G., Schwartz P.J., Napolitano C., et al. Risk stratification in the long-QT syndrome. N Engl J Med. 2003;348:1866–1874. doi: 10.1056/NEJMoa022147. [DOI] [PubMed] [Google Scholar]
- 17.Vijayakumar R., Silva J.N.A., Desouza K.A., et al. Electrophysiologic substrate in congenital long QT syndrome noninvasive mapping with electrocardiographic imaging (ECGI) Circulation. 2014;130:1936–1943. doi: 10.1161/CIRCULATIONAHA.114.011359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mazzanti A., Maragna R., Faragli A., et al. Gene-specific therapy with mexiletine reduces arrhythmic events in patients with long QT syndrome type 3. J Am Coll Cardiol. 2016;67:1053–1058. doi: 10.1016/j.jacc.2015.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]