Abstract
Introduction
Critically ill patients are at risk of suboptimal beta-lactam antibiotic (beta-lactam) exposure due to the impact of altered physiology on pharmacokinetics. Suboptimal concentrations can lead to treatment failure or toxicity. Therapeutic drug monitoring (TDM) involves adjusting doses based on measured plasma concentrations and individualising dosing to improve the likelihood of improving exposure. Despite its potential benefits, its adoption has been slow, and data on implementation, dose adaptation and safety are sparse. The aim of this trial is to assess the feasibility and fidelity of implementing beta-lactam TDM-guided dosing in the intensive care unit setting.
Methods and analysis
A beta-lactam antibiotic Dose AdaPtation feasibility randomised controlled Trial using Therapeutic Drug Monitoring (ADAPT-TDM) is a single-centre, unblinded, feasibility randomised controlled trial aiming to enroll up to 60 critically ill adult participants (≥18 years). TDM and dose adjustment will be performed daily in the intervention group; the standard of care group will undergo plasma sampling, but no dose adjustment. The main outcomes include: (1) feasibility of recruitment, defined as the number of participants who are recruited from a pool of eligible participants, and (2) fidelity of TDM, defined as the degree to which TDM as a test is delivered as intended, from accurate sample collection, sample processing to result availability. Secondary outcomes include target attainment, uptake of TDM-guided dosing and incidence of neurotoxicity, hepatotoxicity and nephrotoxicity.
Ethics and dissemination
This study has been approved by the Alfred Hospital human research ethics committee, Office of Ethics and Research Governance (reference: Project No. 565/22; date of approval: 22/11/2022). Prospective consent will be obtained and the study will be conducted in accordance with the Declaration of Helsinki. The finalised manuscript, including aggregate data, will be submitted for publication in a peer reviewed journal. ADAPT-TDM will determine whether beta-lactam TDM-guided dose adaptation is reproducible and feasible and provide important information required to implement this intervention in a phase III trial.
Trial registration number
Australian New Zealand Clinical Trials Registry, ACTRN12623000032651.
Keywords: CLINICAL PHARMACOLOGY, INFECTIOUS DISEASES, Randomized Controlled Trial, Implementation Science
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This is a randomised controlled trial investigating therapeutic drug monitoring (TDM)-guided dose adaptation in the intensive care unit.
The study assesses the feasibility of TDM-guided dosing by quantifying the recruitment and randomisation rates.
Fidelity of the intervention will be monitored to assess compliance with the TDM workflow and acceptability of dosing recommendation to clinicians.
Single-centre study with a small sample size limits generalisability and assessment of clinical outcomes.
This is an unblinded, open label study.
Introduction
Sepsis in the intensive care unit (ICU) carries a heavy burden of mortality and morbidity.1 Timely administration of the right antibiotic at the right dose can be life-saving.2 Beta-lactam antibiotics (beta-lactams) are most commonly used in the management of infection in the ICU.3 Beta-lactams have a strong exposure–response relationship, that is, improved bacterial killing is achieved with appropriate drug exposure at the site of infection.4 The bactericidal effect is time-dependent in that they exert their antimicrobial action when the free drug concentration (f) remains above the minimum inhibitory concentration (MIC) of the infecting organism for a particular duration of time (T). This index, fT>MIC, is the pharmacokinetic/pharmacodynamic (PK/PD) parameter that underpins the efficacy of the beta-lactam class.4 The time duration for which the concentration needs to remain above the MIC varies within the beta-lactam class, being 40% for carbapenems such as meropenem, 50%–60% for penicillins and monobactams and 70% for cephalosporins. These parameters were derived from in vitro and in vivo animal model studies.5
Septic patients have marked alterations in their physiology, leading to significant alterations in the distribution, metabolism and elimination (PK) of beta-lactam antibiotics.6 These patients are more likely to have complex comorbidities such as immune compromise, infections with highly virulent, less susceptible organisms such as Pseudomonas aeruginosa or deep-seated infections such as endocarditis.7 PK alterations coupled with comorbidities lead to suboptimal (subtherapeutic or supratherapeutic) exposure of beta-lactams when ‘fixed, one-size-fits-all’ dosing is used. Subtherapeutic exposure can lead to treatment failure, while supratherapeutic exposure can lead to toxicity. Moreover, there have been increasing recommendations recently to aim for higher PK/PD targets of 100% fT>MIC and even 2–4×MIC, to promote effective bacterial killing and reduce the emergence of resistance. Several PK studies have demonstrated the wide variability in beta-lactam exposure in critically ill patients.6 8 In our observational study of concentrations achieved with guideline-based doses of meropenem and piperacillin, we showed that nearly 30% of patients with critical illness do not achieve 50% fT>MIC in our ICU, underscoring the need for optimised dosing.9
Therapeutic drug monitoring (TDM) provides a way to quantify antibiotic exposure. TDM of beta-lactam antibiotics involves the measurement of concentrations achieved in the bloodstream after administration of a particular dose and adjusting the dose if these concentrations are considered subtherapeutic or supratherapeutic.10 TDM facilitates individualised dosing targeted at the patient’s unique pathophysiological and infection characteristics. Despite widely being considered safe, it has been hypothesised that the toxicity associated with beta-lactam antibiotics may be an under-recognised phenomenon in the ICU.11 TDM allows the use of higher doses to achieve desired target concentrations while also monitoring for potential toxicity.
Previously, we completed a systematic review and meta-analysis of the beta-lactam literature (n=11 studies, 4 randomised controlled trials (RCTs) and 7 observational studies) and demonstrated improved target attainment and microbiological and clinical cure with beta-lactam TDM.12 However, the risk of bias in the included studies was high.12 The studies were limited by inclusion of all patients (with or without sepsis), inclusion of a variety of beta-lactams which have intrinsic differences in their PD characteristics and delays to TDM and dose optimisation.12 RCT data on the impact of early beta-lactam TDM in targeted populations (eg, bloodstream infections, proven sepsis) are required. While there are recommendations on PK/PD targets (quality of evidence: low), there is no standard guidance on TDM-based dose adjustments (combined with glycopeptides and aminoglycosides), and there is significant variability in practice, with some centres using PK calculators, some using dosing software and others using linear algorithms of dose/frequency/infusion increment or reduction.12
A beta-lactam antibiotic Dose AdaPtation feasibility randomised controlled Trial using Therapeutic Drug Monitoring is a feasibility RCT, the primary aims being to evaluate the feasibility of recruitment and early randomisation (within 24–48 hours of study antibiotic commencement) and the fidelity of TDM implementation.
We hypothesise that early randomisation to beta-lactam TDM in the ICU is feasible, that TDM processes can be applied as intended and that TDM-guided dose adaptation is acceptable to clinicians. Our secondary aims include providing preliminary data on the impact of TDM on target attainment, clinical and microbiological cure and safety. This trial will provide the information required for a larger phase III beta-lactam TDM study.
Methods
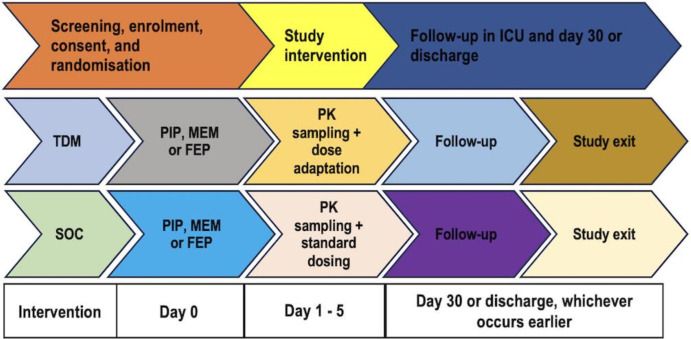
This single-centre RCT will be conducted in the ICU at The Alfred Hospital (Alfred Health, Melbourne, Victoria, Australia). The trial is expected to run from 15 July 2023 to 16 February 2024. An overview of the study is provided in figure 1. The protocol has been prepared as per the Standard Protocol Items: Recommendations for Interventional Trials 2013 reporting guidelines.13 The trial has been prospectively registered with the Australian New Zealand Clinical Trials Registry (ACTRN12623000032651).
Figure 1. Study overview. SOC, standard of care; TDM, therapeutic drug monitoring.
Participants
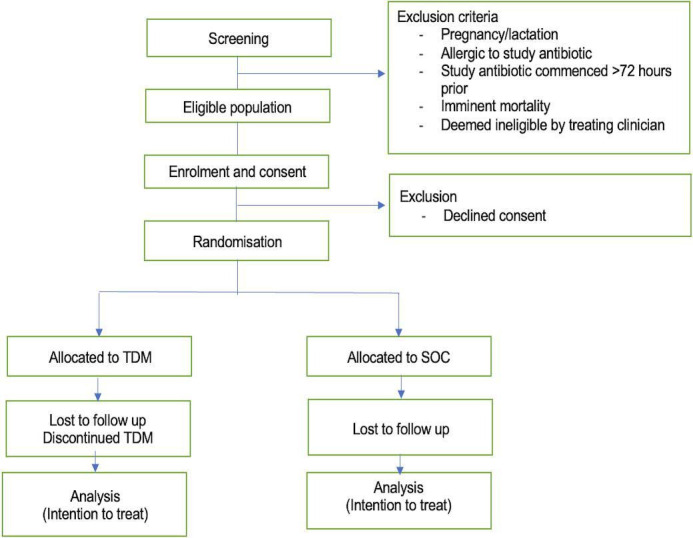
Adult patients (≥18 years) in whom sepsis secondary to a bacterial infection is proven or strongly suspected and are receiving the study beta-lactam (cefepime, piperacillin/tazobactam or meropenem) will be recruited. The participants will be identified based on antibiotic therapy and sepsis documentation in their medical chart. Written informed consent will be obtained from participants or their substitute medical treatment decision-makers. Pregnant or lactating patients, those who are allergic to the study beta-lactam, those who have been on the study beta-lactam for >72 hours, those who are considered inappropriate for participation by the treating clinician and those in whom death is imminent, will be excluded.
Interventions
Participants will be randomly allocated to the TDM arm (TDM-guided dosing) or to standard of care (SOC, usual dosing as per ICU guidelines).
SOC arm
SOC participants will receive study beta-lactam dosing as per established ICU antibiotic dosing guidelines (table 1). This dosing regimen is based on standard practice and does not involve TDM-guided adjustment. Daily plasma drug concentrations will be obtained in this arm, and the treating team will be blinded to these results. Dose modification in this arm will occur based on changes in renal function as per standard practice.14Any dose modifications will be documented, along with clinical the reasoning for the decision.
Table 1. Intensive care unit empiric antibiotic dosing guideline.
| Antibiotic | Dose | Frequency | |
| Meropenem | 2 g | 8 hourly | These are initial intravenous doses for sepsis/septic shock in critically ill patients with normal renal function (creatinine clearance>50 mL/min). Seek pharmacist advice for less severe infections, reduced renal function and/or maintenance dosing. |
| Piperacillin/tazobactam | 4.5 g | 6 hourly | |
| Cefepime | 2 g | 8 hourly |
TDM arm
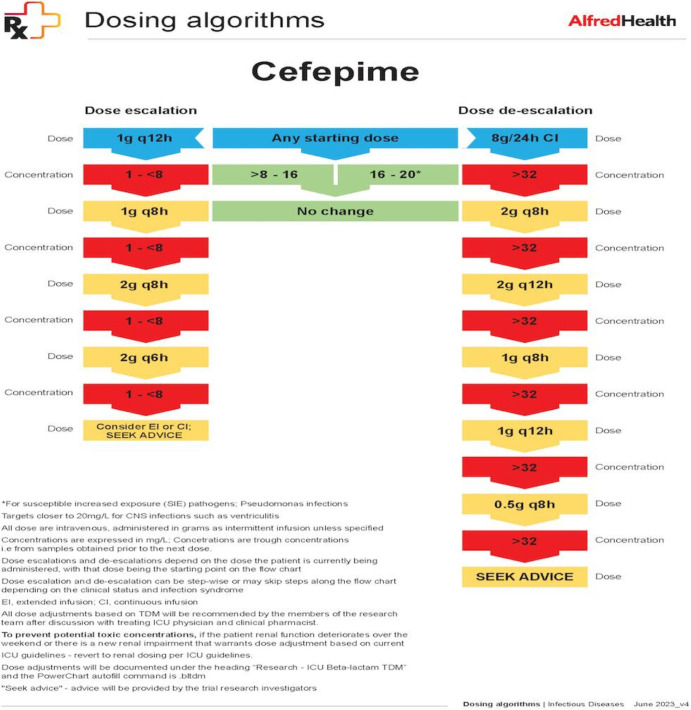
Participants in the TDM arm will undergo daily TDM for 5 days with same-day result availability and dose adaptation as required. Samples for participants in both arms will be obtained at the end of the dosing interval prior to the next dose (trough concentration). Initial doses will be administered as per local ICU guidelines (table 1). At steady state (after three to four doses), the first TDM will be performed. Subsequent doses will be adjusted based on the concentrations obtained in the TDM arm. The cefepime dosing algorithm is illustrated in figure 2. Dosing algorithms for meropenem and piperacillin can be found in online supplemental figure 1. Subsequent TDM samples will be collected to assess target attainment and guide ongoing dose changes. The choice of study beta-lactam and any recommended dosing changes will not be mandated by the study and will be at the discretion of the treating team.
Figure 2. Cefepime dosing algorithm. ICU, intensive care unit; TDM, therapeutic drug monitoring; CNS, central nervous system.
Sample acquisition
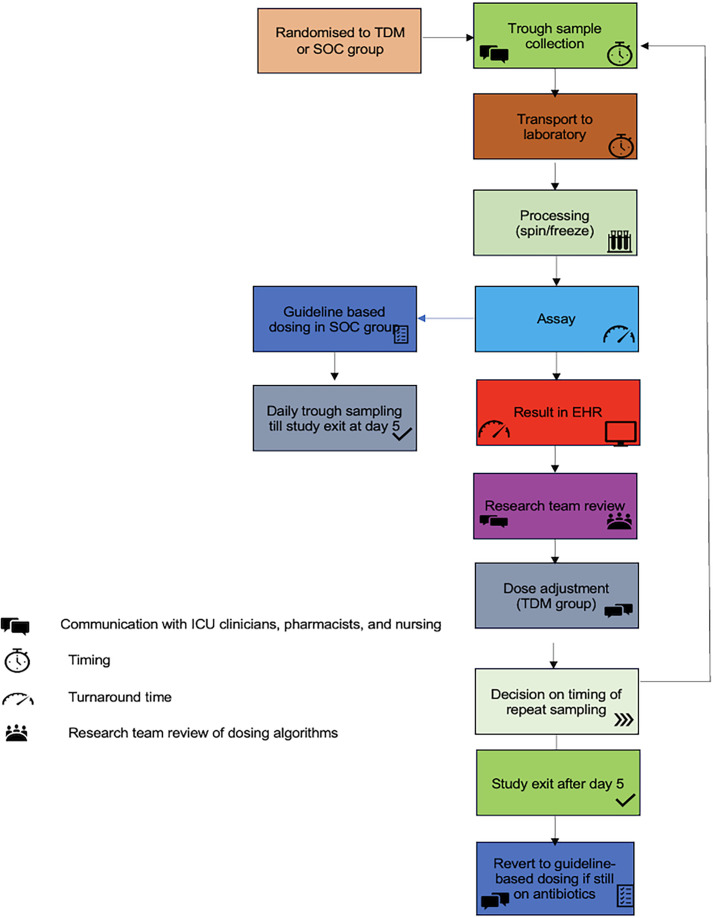
Enrolled patients will be given a study identification number (ID). This study ID will be their unique reference for all data collection. Blood samples from both groups will be obtained daily via existing vascular access (central venous catheter or arterial line). The timing of sample collection will vary depending on the beta-lactam used and the dosing interval (figure 2). Trough samples will be collected (figure 3). To the extent possible, this will be timed to coincide with usual blood collection in the ICU, precluding additional venepuncture. The samples will be tested daily for the interventional arm, and results will be available within working hours.
Figure 3. Process flow. EHR, electronic health record; ICU, intensive care unit; SOC, standard of care; TDM, therapeutic drug monitoring.
A prepackaged pathology sample collection bag labelled with the study ID containing labelled specimen collection tubes will be used to procure samples. Samples will be transported to the laboratory (on site) as per local procedures. Test results will be entered into the electronic medical record under a password-protected section accessible only to members of the research team. The whole workflow of beta-lactam TDM will be aimed to match the process flow of other routine tests and antimicrobial TDM. The timing of sample collection, sample arrival to the laboratory, result availability, dose recommendation and the acceptance of dose recommendation will be recorded. This process will be mapped against that specified in the protocol (figure 3).
Assays: analytical method
This study will use an in-house assay developed specifically for beta-lactam TDM. Assay development followed the required standards for Good Laboratory Practice. Plasma beta-lactam concentrations will be measured by ultrahigh performance liquid chromatography–tandem mass spectrometry (LC-MS/MS) on a Waters UPLC-TQD machine (Wilmslow, UK). The assay has been evaluated to meet the National Pathology Accreditation Advisory Council standards. In brief, plasma from EDTA whole blood samples will be separated by centrifugation and stored at −20°C until analysis. Following thawing, acetonitrile containing isotopically labelled internal standards will be added to the samples and centrifuged. The supernatant will then be further diluted 5 times in purified Type 1 water (Merck/Milli-Q) prior to analysis. 5 µL will be injected onto a Kinetex 2.7 µm biphenyl column (50×2.1 mm, Phenomenex, Sydney, Australia) and chromatographically separated by a 4.2 min gradient elution with mobile phase A (2 mM ammonium acetate in water and 0.1% formic acid) and mobile phase B (2 mM ammonium acetate in methanol and 0.1% formic acid). The MS/MS is operated in positive electrospray ionisation mode, and analytes are measured by multiple reaction monitoring. Two ion transitions are used per analyte.
Calibration standards with an analytical range of 1.0–100.0 mg/L were prepared by spiking certified reference materials into blank plasma. Aliquots were stored at −80°C. Standards and commercially sourced matrix matched bi-level control materials (Chromsystems, Germany) were prepared for use with each analytical run. Control imprecision is <7.5% for all analytes.
Dose adaptation
Doses will be adjusted as per the dosing algorithm (figure 2), while taking into account any clinical parameters such as microbiological characteristics, the infection site, renal function, the initiation of renal replacement therapy or recovery from acute kidney injury. The duration of intervention will depend on the duration of therapy, and at least five samples per participants are planned. The PK/PD target for beta-lactams is 100% fT>1–4×MIC. The PD target for piperacillin, cefepime and meropenem will be the European Committee on Antimicrobial Susceptibility Testing (EUCAST) epidemiological cut-off value of Pseudomonas species for empiric therapy or that of the infecting pathogen, if identified on culture from clinical samples such as blood, sputum or pus from the site of infection.15 Target concentrations (minimum and maximum trough concentrations) and maximum doses over 24 hours (administered via intermittent bolus or prolonged infusions) are outlined in table 2 and figure 2.
Table 2. Target trough concentrations for empiric therapy.
| ECOFF, Pseudomonas spp | Minimum trough concentration (mg/L) | Maximum trough concentration (mg/L) | Maximumdose/24 hours |
| PIP: 16 mg/L | 16–64 | 160 | 24 g |
| MEM: 2 mg/L | 4–20 | 20 | 8 g |
| FEP: 8 mg/L | 8–16 | 20 | 8 g |
ECOFF, epidemiological cut-off value; FEPcefepimeFEP, cefepimeMEM, meropenemMEMmeropenemmg/L, milligram/litre; PIPpiperacillinPIP, piperacillin
Dosing software program TDMx
TDMx (www.tdmx.eu) is a web-based, open-access antimicrobial dose optimisation tool, developed by the University of Hamburg, Germany, that provides dose optimisation advice based on inputs such as dose and schedule, covariates (renal function, weight, height) and drug concentrations.16 The software provides probabilistic (a priori) dosing simulations and Bayesian predictive dosing (a posteriori). TDMx outputs will be obtained and compared with the dosing protocol (figure 2). TDMx currently supports dosing decisions for meropenem and piperacillin and provides real-time dosing advice (online supplemental appendix A). No identifiable data will be entered into TDMx.
Outcomes
The main study outcomes are listed in table 3: (1) feasibility of recruitment, defined as the number of participants who are recruited from a pool of eligible participants, and (2) fidelity of TDM, defined as the degree to which TDM as a test is delivered as intended, from accurate sample collection, sample processing to result availability.
Table 3. Primary and secondary outcomes and outcome measures.
| Outcome | Definition/outcome measure |
| Primary outcomes | |
| Feasibility of recruitment | Enrolment of a sufficient number of eligible participants within the specified timeframe of the study (6 months).This will include:
|
| Feasibility and fidelity of TDM | The degree to which the test (TDM) is delivered as intended:
|
| Secondary outcomes | |
| Target attainment | The proportion of days where 100% fT>MIC is attained (for every individual patient), as assessed by daily plasma samples.
|
| Organ dysfunction | Delta SOFA
|
| ICU length of stay |
|
| Clinical cure |
|
| Microbiological cure |
|
| ICU mortality |
|
| 30-day mortality |
|
| ICU-free days to day 30 |
|
| Duration of antibiotic therapy |
|
| Safety of the intervention |
|
| Piperacillin dose recommendation as per TDMx |
|
| Dose recommendation per TDMx for meropenem |
|
AKI, acute kidney injury; DILI, drug-induced liver injury; EEGElectroencephalogramfT>MIC, free concentration for the duration of time over the minimum inhibitory concentration; ICU, intensive care unit; KDIGO, Kidney Disease Improving Global Outcomes; SOC, standard of care; SOFAsequential organ failure assessmentSOFA, sequential organ failure assessment score; TDM, therapeutic drug monitoring; TDMx, open access dosing software program
Secondary outcome measures are summarised in table 3. They include (1) target attainment and patient-related outcomes; (2) uptake of TDM; (3) dose and duration of antibiotic therapy; (4) adverse events; and (5) TDMx recommendations for meropenem and piperacillin dosing.
Participant timeline
Study participant timeline is outlined in figure 4.
Figure 4. Participant timeline. FEP, cefepime; MEM, meropenem; PIP, piperacillin; PK, pharmacokinetic; SOC, standard of care; TDM, therapeutic drug monitoring.
Sample size
As this is an exploratory study, we were guided by the sample size recommendations for pilot studies17,19 and the number of eligible patients anticipated to present to the Alfred Hospital’s ICU over a 6-month period. The approximate number of sepsis diagnoses in the ICU is 300 over a 6-month period. We aim for a sample size of 60 patients, 30 in each arm. The recruitment rate for TDM studies in ICU is approximately 20%–25%.20 Based on this, a sample size of 60 is achievable over the proposed study period. Block randomisation will occur within the beta-lactam type and each arm will aim to include 10 patients of each beta-lactam of interest (piperacillin, cefepime and meropenem).
Recruitment
Critically ill patients with suspected or proven bacterial infection in the ICU will be screened for eligibility by the study investigator. Treating clinicians of those patients who are eligible will be contacted for recruitment. Patients who are considered appropriate will then be approached and verbal and written information will be provided. Written, informed consent will be obtained from the substitute medical treatment decision-makers of participants who are eligible, but unable to consent for themselves.
Assignment of interventions
Once informed consent has been obtained, participants will be randomly assigned to the TDM or SOC arm. For this, a computer-generated randomisation list has been prepared by the study statistician. Patients will be allocated in 1:1 ratio in blocks of four, stratified by beta-lactams. Randomisation will be executed using REDCap. With the exception of the concentration result in the SOC arm, all other study procedures are unblinded. After eligibility has been confirmed and the patient (or carer) has provided informed consent, the patient will be considered enrolled in the study.
Data collection, management and analysis
Deidentified data will be entered into a secure REDCap database. Reidentifiable data will be maintained on a separate database that will be maintained on the hospital premises in a locked ICU research office. Access to these data will be restricted to named study investigators only who are employees of Alfred Health. All data will be maintained in password-protected computers. Hard copies of consent forms will be maintained in a locked office on site. Only the study investigators will have access to this office. Data analysis will be performed on deidentified data. Deidentified data will be made available on reasonable request in writing to the principal investigator. Published data will not be reidentifiable or linked to individual patients. Study procedures are outlined in online supplemental table 1.
Statistical methods
Feasibility of recruitment and fidelity of TDM will be reported as proportions with 95% CIs. Secondary outcomes will be reported by group and compared initially using univariate tests such as the χ2 and Mann-Whitney U tests. Time to event analysis methods will be used for clinical and microbiological cure, duration of antibiotic therapy and mortality outcomes. Per cent agreement of protocol dosing algorithm with TDMx recommendations will be reported using a kappa statistic. Effect size will be quantified with logistic regression or Cox models with 95% CI. Statistical significance will be defined as a p value≤0.05. Analysis will be conducted using Stata V.18.0 (StataCorp, College Station, Texas, USA).
Monitoring
Regular review will be conducted after every 20 patients recruited by the study investigators and oversight for this study will be provided by a TDM advisory committee comprised of individuals not directly involved in any operational aspect. Assessments will include any breaches in confidentiality and protocol violations. Although the study beta-lactams have a wide therapeutic index, they are known to cause allergic reactions and organ dysfunction (hepatic and renal impairment, encephalopathy and seizures) table 3.21,23 All adverse events will be reviewed and will be included in the final report regardless of whether they were deemed related to study intervention. Study investigators will be responsible for maintaining oversight of protocol adherence with governance from the TDM advisory committee.
Follow-up and post study care
Study exit will be documented in the participant’s EHR and verbally communicated to the treating team that no further TDM will be performed. After study exit, dose adjustments will occur based on renal function as per standard practice. All participants will be followed up via chart review till day 30 or discharge, whichever occurs earlier.
Patient and public involvement
No patient or public involvement was sought in the conception or design of this study. However, we conducted key stakeholder interviews to understand the barriers and enablers to the implementation of beta-lactam TDM prior to designing this pilot RCT.24 Stakeholders included nurses, ICU and infectious disease physicians, microbiologists, pharmacists and laboratory scientists.24
Ethics and dissemination
This study will be conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. This study has been approved by the Alfred Hospital human research ethics committee, Office of Ethics and Research Governance (reference: Project No. 565/22; date of approval: 22/11/2022). We will obtain written informed consent from all participants. In the event that a participant is unable to consent, we will obtain written informed consent from their medical treatment decision-maker. The results of this study will be presented at international and national conferences. The finalised manuscript with aggregate data will be submitted for publication in peer reviewed journals.
Implications for future research
The findings from this trial will guide the design and execution of a larger, multicentre RCT. Factors such as recruitment rates, protocol adherence and observed effect sizes will enable sample size estimation and outcome measures for this trial.
The fidelity of TDM process will provide further opportunities to refine and optimise the TDM protocols ensuring their clinical relevance. The findings of this study will also provide preliminary information on resource allocation and highlight areas where additional training might be needed for ICU staff.
Discussion
This trial is designed as a single-centre feasibility RCT. Its completion will inform a future phase III multicentre RCT. It will provide information on the acceptability of TDM-guided dosing and the implementation of beta-lactam TDM as a routine clinical test in the ICU.
supplementary material
Acknowledgements
The authors would like to acknowledge Mr Gavin Hawkins, Senior Graphic Designer and Brand Coordinator, Public Affairs and Communications, Alfred Health, Melbourne, Victoria, for his support in development of the design of the dosing algorithms.
Footnotes
Funding: This research was supported by The Alfred Research Trust Small Project Grant (Award number: T11928). The assays were developed with the support of Alfred Pathology Special Purpose Fund, Alfred Health, Melbourne, Victoria, Australia (Award number: NA) . Neither of these bodies had any input into trial design or protocol development. They will not be involved in data analysis or manuscript preparation.
Prepublication history and additional supplemental material for this paper are available online. To view these files, please visit the journal online (https://doi.org/10.1136/bmjopen-2023-083635).
Provenance and peer review: Not commissioned; externally peer reviewed.
Patient consent for publication: Not applicable.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Contributor Information
Rekha Pai Mangalore, Email: rekhapai16@yahoo.co.in.
Ming Gene Chai, Email: gene.chai@alfred.org.au.
Jeffrey Pope, Email: j.pope@alfred.org.au.
Sue J Lee, Email: sue.lee@monash.edu.
Alexander Padiglione, Email: a.padiglione@alfred.org.au.
Arne Diehl, Email: arne.diehl@gmail.com.
Llyod Roberts, Email: l.roberts@alfred.org.au.
Kirsty Sim, Email: kirsty.sim@alfred.org.au.
Philip Rawson-Harris, Email: p.rawsonharris@alfred.org.au.
Sebastian Wicha, Email: sebastian.wicha@uni-hamburg.de.
Hans G Schneider, Email: H.Schneider@alfred.org.au.
Trish N Peel, Email: Trisha.Peel@monash.edu.
Adam Jenney, Email: a.jenney@alfred.org.au.
Darshini Ayton, Email: darshini.ayton@monash.edu.
Anton Y Peleg, Email: anton.peleg@monash.edu.
Andrew A Udy, Email: andrew@udy.com.
References
- 1.Li L SN, Rathnayake K, Westbrook JI. Epidemiology of sepsis in Australian public hospitals. 2020
- 2.Dryden M, Johnson AP, Ashiru-Oredope D, et al. Using antibiotics responsibly: right drug, right time, right dose, right duration. J Antimicrob Chemother. 2011;66:2441–3. doi: 10.1093/jac/dkr370. [DOI] [PubMed] [Google Scholar]
- 3.Dulhunty JM, Paterson D, Webb SAR, et al. Antimicrobial utilisation in 37 Australian and New Zealand intensive care units. Anaesth Intensive Care. 2011;39:231–7. doi: 10.1177/0310057X1103900212. [DOI] [PubMed] [Google Scholar]
- 4.Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis. 1998;26:1–10. doi: 10.1086/516284. [DOI] [PubMed] [Google Scholar]
- 5.Craig WA. Antibiotic selection factors and description of a hospital-based outpatient antibiotic therapy program in the USA. Eur J Clin Microbiol Infect Dis. 1995;14:636–42. doi: 10.1007/BF01690745. [DOI] [PubMed] [Google Scholar]
- 6.Roberts JA, Ulldemolins M, Roberts MS, et al. Therapeutic drug monitoring of beta-lactams in critically ill patients: proof of concept. Int J Antimicrob Agents. 2010;36:332–9. doi: 10.1016/j.ijantimicag.2010.06.008. [DOI] [PubMed] [Google Scholar]
- 7.Garland A, Olafson K, Ramsey CD, et al. Epidemiology of critically ill patients in intensive care units: a population-based observational study. Crit Care. 2013;17:R212. doi: 10.1186/cc13026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roberts JA, Paul SK, Akova M, et al. DALI: defining antibiotic levels in intensive care unit patients: are current Β-lactam antibiotic doses sufficient for critically ill patients. Clin Infect Dis. 2014;58:1072–83. doi: 10.1093/cid/ciu027. [DOI] [PubMed] [Google Scholar]
- 9.Pai Mangalore R, Padiglione AA, Martin E, et al. Insufficient plasma concentrations of empiric anti-pseudomonal beta-lactam antibiotics in critically ill patients with suspected sepsis. Pharmacy Practice and Res. 2020;50:345–50. doi: 10.1002/jppr.1639. [DOI] [Google Scholar]
- 10.Huttner A, Harbarth S, Hope WW, et al. Therapeutic drug monitoring of the Β-lactam antibiotics: what is the evidence and which patients should we be using it for? J Antimicrob Chemother. 2015;70:3178–83. doi: 10.1093/jac/dkv201. [DOI] [PubMed] [Google Scholar]
- 11.Roger C, Louart B. Beta-lactams toxicity in the intensive care unit: an underestimated collateral damage. Microorganisms. 2021;9:1505. doi: 10.3390/microorganisms9071505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pai Mangalore R, Ashok A, Lee SJ, et al. Beta-lactam antibiotic therapeutic drug monitoring in critically ill patients: a systematic review and meta-analysis. Clin Infect Dis. 2022;75:1848–60. doi: 10.1093/cid/ciac506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chan A-W, Tetzlaff JM, Gøtzsche PC, et al. SPIRIT 2013 explanation and elaboration: guidance for protocols of clinical trials. BMJ. 2013;346:e7586. doi: 10.1136/bmj.e7586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Therapeutic Guidelines Melbourne: Therapeutic Guidelines Limited. [3-Aug-2023]. https://www.tg.org.au Available. Accessed.
- 15.European Committee on Antimicrobial Susceptibility Testing Data from the EUCAST MIC distribution website. [3-Aug-2023]. http://www.eucast.org Available. Accessed.
- 16.Wicha SG, Kees MG, Solms A, et al. TDMx: a novel web-based open-access support tool for optimising antimicrobial dosing regimens in clinical routine. Int J Antimicrob Agents. 2015;45:442–4. doi: 10.1016/j.ijantimicag.2014.12.010. [DOI] [PubMed] [Google Scholar]
- 17.Teare MD, Dimairo M, Shephard N, et al. Sample size requirements to estimate key design parameters from external pilot randomised controlled trials: a simulation study. Trials. 2014;15:264. doi: 10.1186/1745-6215-15-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Julious SA. Sample size of 12 per group rule of thumb for a pilot study. Pharm Stat. 2005;4:287–91. doi: 10.1002/pst.185. [DOI] [Google Scholar]
- 19.Whitehead AL, Julious SA, Cooper CL, et al. Estimating the sample size for a pilot randomised trial to minimise the overall trial sample size for the external pilot and main trial for a continuous outcome variable. Stat Methods Med Res. 2016;25:1057–73. doi: 10.1177/0962280215588241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagel S, Bach F, Brenner T, et al. Effect of therapeutic drug monitoring-based dose optimization of piperacillin/tazobactam on sepsis-related organ dysfunction in patients with sepsis: a randomized controlled trial. Intensive Care Med. 2022;48:311–21. doi: 10.1007/s00134-021-06609-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–84. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 22.David S, Hamilton JP. Drug-induced liver injury. US Gastroenterol Hepatol Rev. 2010;6:73–80. [PMC free article] [PubMed] [Google Scholar]
- 23.Li H-T, Lee C-H, Wu T, et al. Clinical, electroencephalographic features and prognostic factors of cefepime-induced neurotoxicity: a retrospective study. Neurocrit Care. 2019;31:329–37. doi: 10.1007/s12028-019-00682-y. [DOI] [PubMed] [Google Scholar]
- 24.Pai Mangalore R, Udy AA, Peel TN, et al. Implementation strategies addressing stakeholder-perceived barriers and enablers to the establishment of a beta-lactam antibiotic therapeutic drug monitoring program: a qualitative analysis. Ther Drug Monit. 2024;46:351–62. doi: 10.1097/FTD.0000000000001162. [DOI] [PubMed] [Google Scholar]