Abstract
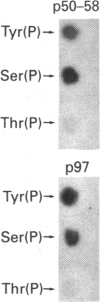
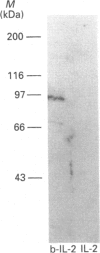
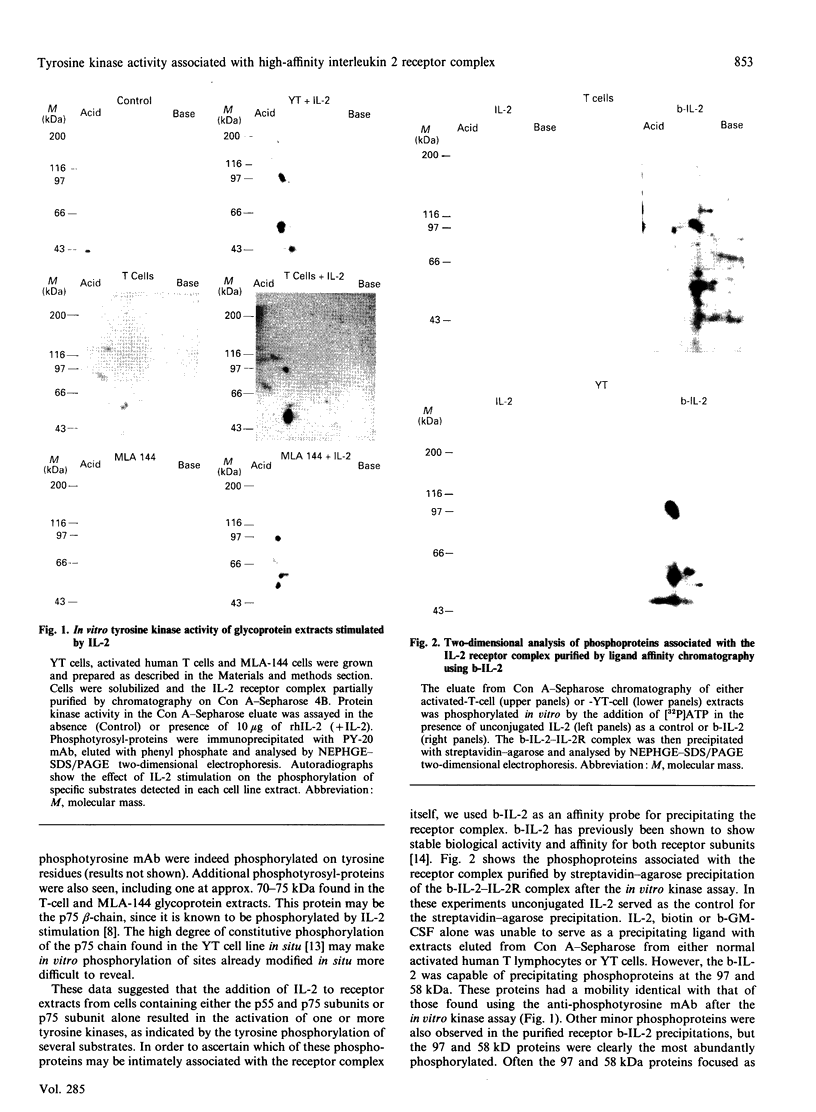
The IL-2 receptor complex is minimally composed of two genetically unrelated subunits of relative molecular masses 55 and 75 kDa respectively. Structural information deduced from the cDNA sequences of either subunit have not revealed significant information as to the basis of the mechanisms of IL-2 receptor signal transduction. Nevertheless, IL-2 stimulates the activation of one or more tyrosine kinases requiring the functional participation of the p75 member of the receptor complex. Here we have developed the methods to isolate the receptor complex with an associated tyrosine protein kinase. Extracts of membrane glycoproteins from activated normal human T lymphocytes and cell lines demonstrated catalytic activation of tyrosine kinase activity when stimulated with IL-2. Purification of the receptor complex with biotinylated IL-2 revealed the presence of two dominant phosphotyrosyl-proteins of approximate molecular masses 58 and 97 kDa. Denaturation gel electrophoresis followed by renaturation of proteins associated with the IL-2 receptor complex demonstrated that the 97 kDa protein had catalytic autophosphorylation activity. The results indicate that the 58 and 97 kDa phosphotyrosyl-proteins can be found to co-precipitate with the IL-2 receptor complex and that the 97 kDa protein was demonstrated to have protein kinase activity. The association of such kinases with receptors devoid of catalytic structure may represent a unique paradigm of growth-factor receptor mechanisms.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asao H., Takeshita T., Nakamura M., Nagata K., Sugamura K. Interleukin 2 (IL-2)-induced tyrosine phosphorylation of IL-2 receptor p75. J Exp Med. 1990 Mar 1;171(3):637–644. doi: 10.1084/jem.171.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazan J. F. A novel family of growth factor receptors: a common binding domain in the growth hormone, prolactin, erythropoietin and IL-6 receptors, and the p75 IL-2 receptor beta-chain. Biochem Biophys Res Commun. 1989 Oct 31;164(2):788–795. doi: 10.1016/0006-291x(89)91528-3. [DOI] [PubMed] [Google Scholar]
- Ben-David Y., Letwin K., Tannock L., Bernstein A., Pawson T. A mammalian protein kinase with potential for serine/threonine and tyrosine phosphorylation is related to cell cycle regulators. EMBO J. 1991 Feb;10(2):317–325. doi: 10.1002/j.1460-2075.1991.tb07952.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Andrea A. D., Fasman G. D., Lodish H. F. Erythropoietin receptor and interleukin-2 receptor beta chain: a new receptor family. Cell. 1989 Sep 22;58(6):1023–1024. doi: 10.1016/0092-8674(89)90499-6. [DOI] [PubMed] [Google Scholar]
- Farrar W. L., Ferris D. K. Two-dimensional analysis of interleukin 2-regulated tyrosine kinase activation mediated by the p70-75 beta subunit of the interleukin 2 receptor. J Biol Chem. 1989 Jul 25;264(21):12562–12567. [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Martin G. S. Thrombin stimulates the activities of multiple previously unidentified protein kinases in platelets. J Biol Chem. 1989 Dec 5;264(34):20723–20729. [PubMed] [Google Scholar]
- Ferris D. K., Willette-Brown J., Ortaldo J. R., Farrar W. L. IL-2 regulation of tyrosine kinase activity is mediated through the p70-75 beta-subunit of the IL-2 receptor. J Immunol. 1989 Aug 1;143(3):870–876. [PubMed] [Google Scholar]
- Glenney J. R., Jr, Zokas L., Kamps M. P. Monoclonal antibodies to phosphotyrosine. J Immunol Methods. 1988 May 9;109(2):277–285. doi: 10.1016/0022-1759(88)90253-0. [DOI] [PubMed] [Google Scholar]
- Goodwin R. G., Friend D., Ziegler S. F., Jerzy R., Falk B. A., Gimpel S., Cosman D., Dower S. K., March C. J., Namen A. E. Cloning of the human and murine interleukin-7 receptors: demonstration of a soluble form and homology to a new receptor superfamily. Cell. 1990 Mar 23;60(6):941–951. doi: 10.1016/0092-8674(90)90342-c. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Kono T., Kobayashi N., Kawahara A., Levin S. D., Perlmutter R. M., Taniguchi T. Interaction of the IL-2 receptor with the src-family kinase p56lck: identification of novel intermolecular association. Science. 1991 Jun 14;252(5012):1523–1528. doi: 10.1126/science.2047859. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Mori H., Doi T., Taniguchi T. A restricted cytoplasmic region of IL-2 receptor beta chain is essential for growth signal transduction but not for ligand binding and internalization. Cell. 1989 Dec 1;59(5):837–845. doi: 10.1016/0092-8674(89)90607-7. [DOI] [PubMed] [Google Scholar]
- Hatakeyama M., Tsudo M., Minamoto S., Kono T., Doi T., Miyata T., Miyasaka M., Taniguchi T. Interleukin-2 receptor beta chain gene: generation of three receptor forms by cloned human alpha and beta chain cDNA's. Science. 1989 May 5;244(4904):551–556. doi: 10.1126/science.2785715. [DOI] [PubMed] [Google Scholar]
- Howell B. W., Afar D. E., Lew J., Douville E. M., Icely P. L., Gray D. A., Bell J. C. STY, a tyrosine-phosphorylating enzyme with sequence homology to serine/threonine kinases. Mol Cell Biol. 1991 Jan;11(1):568–572. doi: 10.1128/mcb.11.1.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kypta R. M., Goldberg Y., Ulug E. T., Courtneidge S. A. Association between the PDGF receptor and members of the src family of tyrosine kinases. Cell. 1990 Aug 10;62(3):481–492. doi: 10.1016/0092-8674(90)90013-5. [DOI] [PubMed] [Google Scholar]
- Leonard W. J., Depper J. M., Crabtree G. R., Rudikoff S., Pumphrey J., Robb R. J., Krönke M., Svetlik P. B., Peffer N. J., Waldmann T. A. Molecular cloning and expression of cDNAs for the human interleukin-2 receptor. Nature. 1984 Oct 18;311(5987):626–631. doi: 10.1038/311626a0. [DOI] [PubMed] [Google Scholar]
- Michiel D. F., Garcia G. G., Evans G. A., Farrar W. L. Regulation of the interleukin 2 receptor complex tyrosine kinase activity in vitro. Cytokine. 1991 Sep;3(5):428–438. doi: 10.1016/1043-4666(91)90047-h. [DOI] [PubMed] [Google Scholar]
- Saltzman E. M., Thom R. R., Casnellie J. E. Activation of a tyrosine protein kinase is an early event in the stimulation of T lymphocytes by interleukin-2. J Biol Chem. 1988 May 25;263(15):6956–6959. [PubMed] [Google Scholar]
- Saragovi H., Malek T. R. Evidence for additional subunits associated to the mouse interleukin 2 receptor p55/p75 complex. Proc Natl Acad Sci U S A. 1990 Jan;87(1):11–15. doi: 10.1073/pnas.87.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharon M., Gnarra J. R., Leonard W. J. The beta-chain of the IL-2 receptor (p70) is tyrosine-phosphorylated on YT and HUT-102B2 cells. J Immunol. 1989 Oct 15;143(8):2530–2533. [PubMed] [Google Scholar]
- Taki S., Shimamura T., Abe M., Shirai T., Takahara Y. Biotinylation of human interleukin-2 for flow cytometry analysis of interleukin-2 receptors. J Immunol Methods. 1989 Aug 15;122(1):33–41. doi: 10.1016/0022-1759(89)90331-1. [DOI] [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]