Abstract
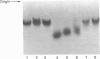
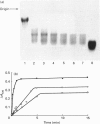
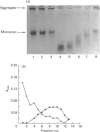
Degradation of cartilage proteoglycans was investigated under neutral conditions (pH 7.5) by using pig kidney calpain II (EC 3.4.22.17; Ca(2+)-dependent cysteine proteinase). Aggregate and monomer degradation reached a maximum in 5 min at 30 degrees C when the substrate/enzyme ratio was less than 1000:1. The mode of degradation was limited proteolysis of the core protein; the size of the products was larger than that of papain-digested products and comparable with that of trypsin-digested products. The hyaluronic acid-binding region was lost from the major glycosaminoglycan-bearing region after incubation with calpain II. Calpains thus may affect the form of proteoglycans in connective tissue. Ca(2+)-dependent proteoglycan degradation was unique in that proteoglycans adsorb large amounts of Ca2+ ions rapidly before activation of calpain II: 1 mg of pig cartilage proteoglycan monomer adsorbed 1.3-1.6 mu equiv. of Ca2+ ions before activation of calpain II, which corresponds to half the sum of anion groups in glycosaminoglycan side chains. This adsorption of Ca2+ was lost after solvolysis of proteoglycan monomer with methanol/50 mM-HCl, which was used to desulphate glycosaminoglycans. Therefore cartilage proteoglycans are not merely the substrates of proteolysis, but they may regulate the activation of Ca(2+)-dependent enzymes including calpains through tight chelation of Ca2+ ions between glycosaminoglycan side chains.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adachi E., Mukaiyama T., Sasai K., Hayashi T., Kawashima S., Kasai Y., Hayashi M., Hashimoto P. H. Immunohistochemical evidence of the extracellular localization of calcium-activated neutral protease (CANP) in rabbit skeletal muscle, lung and aorta. Arch Histol Cytol. 1990 Oct;53(4):413–422. doi: 10.1679/aohc.53.413. [DOI] [PubMed] [Google Scholar]
- BITTER T., MUIR H. M. A modified uronic acid carbazole reaction. Anal Biochem. 1962 Oct;4:330–334. doi: 10.1016/0003-2697(62)90095-7. [DOI] [PubMed] [Google Scholar]
- Barrett M. J., Goll D. E., Thompson V. F. Effect of substrate on Ca2(+)-concentration required for activity of the Ca2(+)-dependent proteinases, mu- and m-calpain. Life Sci. 1991;48(17):1659–1669. doi: 10.1016/0024-3205(91)90126-v. [DOI] [PubMed] [Google Scholar]
- Beckerle M. C., Burridge K., DeMartino G. N., Croall D. E. Colocalization of calcium-dependent protease II and one of its substrates at sites of cell adhesion. Cell. 1987 Nov 20;51(4):569–577. doi: 10.1016/0092-8674(87)90126-7. [DOI] [PubMed] [Google Scholar]
- Berkowitz S. D., Nozaki H., Titani K., Murachi T., Plow E. F., Zimmerman T. S. Evidence that calpains and elastase do not produce the von Willebrand factor fragments present in normal plasma and IIA von Willebrand disease. Blood. 1988 Aug;72(2):721–727. [PubMed] [Google Scholar]
- Buckwalter J. A. Proteoglycan structure in calcifying cartilage. Clin Orthop Relat Res. 1983 Jan-Feb;(172):207–232. [PubMed] [Google Scholar]
- Caplan A. I., Fiszman M. Y., Eppenberger H. M. Molecular and cell isoforms during development. Science. 1983 Sep 2;221(4614):921–927. doi: 10.1126/science.6348946. [DOI] [PubMed] [Google Scholar]
- Dziewiatkowski D. D. Binding of calcium by proteoglycans in vitro. Calcif Tissue Int. 1987 May;40(5):265–269. doi: 10.1007/BF02555259. [DOI] [PubMed] [Google Scholar]
- Ehrlich M. G., Armstrong A. L., Neuman R. G., Davis M. W., Mankin H. J. Patterns of proteoglycan degradation by a neutral protease from human growth-plate epiphyseal cartilage. J Bone Joint Surg Am. 1982 Dec;64(9):1350–1354. [PubMed] [Google Scholar]
- Harafuji H., Ogawa Y. Re-examination of the apparent binding constant of ethylene glycol bis(beta-aminoethyl ether)-N,N,N',N'-tetraacetic acid with calcium around neutral pH. J Biochem. 1980 May;87(5):1305–1312. doi: 10.1093/oxfordjournals.jbchem.a132868. [DOI] [PubMed] [Google Scholar]
- Heinegård D., Sommarin Y., Hedbom E., Wieslander J., Larsson B. Assay of proteoglycan populations using agarose-polyacrylamide gel electrophoresis. Anal Biochem. 1985 Nov 15;151(1):41–48. doi: 10.1016/0003-2697(85)90050-8. [DOI] [PubMed] [Google Scholar]
- Hunter G. K., Wong K. S., Kim J. J. Binding of calcium to glycosaminoglycans: an equilibrium dialysis study. Arch Biochem Biophys. 1988 Jan;260(1):161–167. doi: 10.1016/0003-9861(88)90437-7. [DOI] [PubMed] [Google Scholar]
- Kimata K., Kimura J. H., Thonar E. J., Barrach H. J., Rennard S. I., Hascall V. C. Swarm rat chondrosarcoma proteoglycans. Purification of aggregates by zonal centrifugation of preformed cesium sulfate gradients. J Biol Chem. 1982 Apr 10;257(7):3819–3826. [PubMed] [Google Scholar]
- Kitahara A., Sasaki T., Kikuchi T., Yumoto N., Yoshimura N., Hatanaka M., Murachi T. Large-scale purification of porcine calpain I and calpain II and comparison of proteolytic fragments of their subunits. J Biochem. 1984 Jun;95(6):1759–1766. [PubMed] [Google Scholar]
- Kunicki T. J., Montgomery R. R., Schullek J. Cleavage of human von Willebrand factor by platelet calcium-activated protease. Blood. 1985 Feb;65(2):352–356. [PubMed] [Google Scholar]
- Kunicki T. J., Mosesson M. W., Pidard D. Cleavage of fibrinogen by human platelet calcium-activated protease. Thromb Res. 1984 Jul 15;35(2):169–182. doi: 10.1016/0049-3848(84)90212-3. [DOI] [PubMed] [Google Scholar]
- Murakami T., Hatanaka M., Murachi T. The cytosol of human erythrocytes contains a highly Ca2+-sensitive thiol protease (calpain I) and its specific inhibitor protein (calpastatin). J Biochem. 1981 Dec;90(6):1809–1816. doi: 10.1093/oxfordjournals.jbchem.a133659. [DOI] [PubMed] [Google Scholar]
- Nguyen Q., Murphy G., Roughley P. J., Mort J. S. Degradation of proteoglycan aggregate by a cartilage metalloproteinase. Evidence for the involvement of stromelysin in the generation of link protein heterogeneity in situ. Biochem J. 1989 Apr 1;259(1):61–67. doi: 10.1042/bj2590061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oegema T. R., Jr, Hascall V. C., Dziewiatkowski D. D. Isolation and characterization of proteoglycans from the swarm rat chondrosarcoma. J Biol Chem. 1975 Aug 10;250(15):6151–6159. [PubMed] [Google Scholar]
- Oike Y., Kimata K., Shinomura T., Nakazawa K., Suzuki S. Structural analysis of chick-embryo cartilage proteoglycan by selective degradation with chondroitin lyases (chondroitinases) and endo-beta-D-galactosidase (keratanase). Biochem J. 1980 Oct 1;191(1):193–207. doi: 10.1042/bj1910193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettipher E. R., Henderson B., Hardingham T., Ratcliffe A. Cartilage proteoglycan depletion in acute and chronic antigen-induced arthritis. Arthritis Rheum. 1989 May;32(5):601–607. doi: 10.1002/anr.1780320514. [DOI] [PubMed] [Google Scholar]
- Roughley P. J., Barrett A. J. The degradation of cartilage proteoglycans by tissue proteinases. Proteoglycan structure and its susceptibility to proteolysis. Biochem J. 1977 Dec 1;167(3):629–637. doi: 10.1042/bj1670629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapolsky A. I., Woessner J. F., Jr, Howell D. S. A photometric assay for protease digestion of the proteoglycan subunit. Anal Biochem. 1975 Aug;67(2):649–654. doi: 10.1016/0003-2697(75)90339-5. [DOI] [PubMed] [Google Scholar]
- Sasaki T., Kikuchi T., Yumoto N., Yoshimura N., Murachi T. Comparative specificity and kinetic studies on porcine calpain I and calpain II with naturally occurring peptides and synthetic fluorogenic substrates. J Biol Chem. 1984 Oct 25;259(20):12489–12494. [PubMed] [Google Scholar]
- Sato C., Nishizawa K., Nakayama T., Nakamura H., Yoshimura N., Takano E., Murachi T. Rapid proteolysis of brain MAP-1 related cytoskeleton-associated 350kd protein by purified calpain. Cell Struct Funct. 1986 Sep;11(3):253–257. doi: 10.1247/csf.11.253. [DOI] [PubMed] [Google Scholar]
- Schmaier A. H., Smith P. M., Purdon A. D., White J. G., Colman R. W. High molecular weight kininogen: localization in the unstimulated and activated platelet and activation by a platelet calpain(s). Blood. 1986 Jan;67(1):119–130. [PubMed] [Google Scholar]
- Shimizu K., Hamamoto T., Hamakubo T., Lee W. J., Suzuki K., Nakagawa Y., Murachi T., Yamamuro T. Immunohistochemical and biochemical demonstration of calcium-dependent cysteine proteinase (calpain) in calcifying cartilage of rats. J Orthop Res. 1991 Jan;9(1):26–36. doi: 10.1002/jor.1100090105. [DOI] [PubMed] [Google Scholar]
- Suzuki K., Shimizu K., Hamamoto T., Nakagawa Y., Hamakubo T., Yamamuro T. Biochemical demonstration of calpains and calpastatin in osteoarthritic synovial fluid. Arthritis Rheum. 1990 May;33(5):728–732. doi: 10.1002/art.1780330516. [DOI] [PubMed] [Google Scholar]
- Takano E., Murachi T. Purification and some properties of human erythrocyte calpastatin. J Biochem. 1982 Dec;92(6):2021–2028. doi: 10.1093/oxfordjournals.jbchem.a134134. [DOI] [PubMed] [Google Scholar]
- Thornton D. J., Nieduszynski I. A., Oates K., Sheehan J. K. Electron-microscopic and electrophoretic studies of bovine femoral-head cartilage proteoglycan fractions. Biochem J. 1986 Nov 15;240(1):41–48. doi: 10.1042/bj2400041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward C., Davidson E. A. Structure-function relationships of protein polysaccharide complexes: specific ion-binding properties. Proc Natl Acad Sci U S A. 1968 May;60(1):201–205. doi: 10.1073/pnas.60.1.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita K., Ueda I., Kobata A. Sulfated asparagine-linked sugar chains of hen egg albumin. J Biol Chem. 1983 Dec 10;258(23):14144–14147. [PubMed] [Google Scholar]
- Yoshimura N., Kikuchi T., Sasaki T., Kitahara A., Hatanaka M., Murachi T. Two distinct Ca2+ proteases (calpain I and calpain II) purified concurrently by the same method from rat kidney. J Biol Chem. 1983 Jul 25;258(14):8883–8889. [PubMed] [Google Scholar]