Abstract
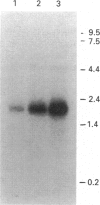
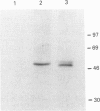
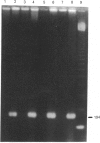
A cDNA encoding UDP-GlcNAc-dolichyl-phosphate N-acetylglucosaminephosphotransferase (GPT; EC 2.7.8.15), an enzyme that catalyses the first step in the synthesis of dolichol-linked oligosaccharides, was isolated from mRNA prepared from mouse mammary glands. The cDNA contains an open reading frame that codes for a protein of 410 amino acids with a predicted molecular mass of 46.472 kDa. Mouse GPT has two copies of a putative dolichol-recognition sequence that has so far been identified in all eukaryotic enzymes which interact with dolichol, and four consensus sites for asparagine-linked glycosylation. It shows a high degree of conservation with yeast and hamster GPTs at the amino acid level. The mouse GPT cDNA recognized a single mRNA species of about 2 kb in mouse mammary glands when used as a probe in Northern blot analysis. An antiserum raised against a 15-residue peptide, derived from the predicted amino acid sequence of the cloned mouse cDNA, specifically precipitated the activity of GPT from solubilized mouse mammary gland microsomes, and detected a protein of about 48 kDa on Western blot. This size is in good agreement with that predicted from the cDNA sequence, and also with that (46 and 50 kDa) of purified bovine GPT. With the use of a panel of mouse/hamster somatic-cell hybrids and a specific probe derived from the 3'-non-coding region of the mouse cDNA, the GPT gene was mapped to mouse chromosome 17.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeijon C., Hirschberg C. B. Topography of initiation of N-glycosylation reactions. J Biol Chem. 1990 Aug 25;265(24):14691–14695. [PubMed] [Google Scholar]
- Albright C. F., Orlean P., Robbins P. W. A 13-amino acid peptide in three yeast glycosyltransferases may be involved in dolichol recognition. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7366–7369. doi: 10.1073/pnas.86.19.7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armant D. R., Kaplan H. A., Lennarz W. J. N-linked glycoprotein biosynthesis in the developing mouse embryo. Dev Biol. 1986 Jan;113(1):228–237. doi: 10.1016/0012-1606(86)90125-9. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Botteri F. M., Ballmer-Hofer K., Rajput B., Nagamine Y. Disruption of cytoskeletal structures results in the induction of the urokinase-type plasminogen activator gene expression. J Biol Chem. 1990 Aug 5;265(22):13327–13334. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Degen S. J., Rajput B., Reich E. The human tissue plasminogen activator gene. J Biol Chem. 1986 May 25;261(15):6972–6985. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hartog K. O., Bishop B. Genomic sequence coding for tunicamycin resistance in yeast. Nucleic Acids Res. 1987 Apr 24;15(8):3627–3627. doi: 10.1093/nar/15.8.3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes G. R., Lucas J. J. Stimulation of lipid-linked oligosaccharide assembly during oviduct differentiation. J Biol Chem. 1983 Dec 25;258(24):15095–15100. [PubMed] [Google Scholar]
- Jackson M. R., Nilsson T., Peterson P. A. Identification of a consensus motif for retention of transmembrane proteins in the endoplasmic reticulum. EMBO J. 1990 Oct;9(10):3153–3162. doi: 10.1002/j.1460-2075.1990.tb07513.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaushal G. P., Elbein A. D. Purification and properties of UDP-GlcNAc:dolichyl-phosphate GlcNAc-1-phosphate transferase. Activation and inhibition of the enzyme. J Biol Chem. 1985 Dec 25;260(30):16303–16309. [PubMed] [Google Scholar]
- Kean E. L. Topographical orientation in microsomal vesicles of the N-acetylglucosaminyltransferases which catalyze the biosynthesis of N-acetylglucosaminylpyrophosphoryldolichol and N-acetylglucosaminyl-N-acetylglucosaminylpyrophosphoryldolichol. J Biol Chem. 1991 Jan 15;266(2):942–946. [PubMed] [Google Scholar]
- Kornfeld R., Kornfeld S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985;54:631–664. doi: 10.1146/annurev.bi.54.070185.003215. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5'-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987 Oct 26;15(20):8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kukuruzinska M. A., Robbins P. W. Protein glycosylation in yeast: transcript heterogeneity of the ALG7 gene. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2145–2149. doi: 10.1073/pnas.84.8.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lalley P. A., McKusick V. A. Report of the Committee on Comparative Mapping. Cytogenet Cell Genet. 1985;40(1-4):536–566. doi: 10.1159/000132187. [DOI] [PubMed] [Google Scholar]
- Lehrman M. A., Zhu X. Y., Khounlo S. Amplification and molecular cloning of the hamster tunicamycin-sensitive N-acetylglucosamine-1-phosphate transferase gene. The hamster and yeast enzymes share a common peptide sequence. J Biol Chem. 1988 Dec 25;263(36):19796–19803. [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mohana Rao J. K., Argos P. A conformational preference parameter to predict helices in integral membrane proteins. Biochim Biophys Acta. 1986 Jan 30;869(2):197–214. doi: 10.1016/0167-4838(86)90295-5. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Pain D., Kanwar Y. S., Blobel G. Identification of a receptor for protein import into chloroplasts and its localization to envelope contact zones. Nature. 1988 Jan 21;331(6153):232–237. doi: 10.1038/331232a0. [DOI] [PubMed] [Google Scholar]
- Rajput B., Marshall A., Killary A. M., Lalley P. A., Naylor S. L., Belin D., Rickles R. J., Strickland S. Chromosomal assignments of genes for tissue plasminogen activator and urokinase in mouse. Somat Cell Mol Genet. 1987 Sep;13(5):581–586. doi: 10.1007/BF01534500. [DOI] [PubMed] [Google Scholar]
- Rine J., Hansen W., Hardeman E., Davis R. W. Targeted selection of recombinant clones through gene dosage effects. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6750–6754. doi: 10.1073/pnas.80.22.6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scocca J. R., Krag S. S. Sequence of a cDNA that specifies the uridine diphosphate N-acetyl-D-glucosamine:dolichol phosphate N-acetylglucosamine-1-phosphate transferase from Chinese hamster ovary cells. J Biol Chem. 1990 Nov 25;265(33):20621–20626. [PubMed] [Google Scholar]
- Shailubhai K., Dong-Yu B., Saxena E. S., Vijay I. K. Purification and characterization of UDP-N-acetyl-D-glucosamine:dolichol phosphate N-acetyl-D-glucosamine-1-phosphate transferase involved in the biosynthesis of asparagine-linked glycoproteins in the mammary gland. J Biol Chem. 1988 Nov 5;263(31):15964–15972. [PubMed] [Google Scholar]
- Starr C. M., Lucas J. J. Regulation of dolichyl phosphate-mediated protein glycosylation: estrogen effects on glucosyl transfers in oviduct membranes. Arch Biochem Biophys. 1985 Feb 15;237(1):261–270. doi: 10.1016/0003-9861(85)90277-2. [DOI] [PubMed] [Google Scholar]
- Steffens D. L., Gross R. W. Sequencing of cloned DNA using bacteriophage lambda gt11 templates. Biotechniques. 1989 Jul-Aug;7(7):674–680. [PubMed] [Google Scholar]
- Theune S., Fung J., Todd S., Sakaguchi A. Y., Naylor S. L. PCR primers for human chromosomes: reagents for the rapid analysis of somatic cell hybrids. Genomics. 1991 Mar;9(3):511–516. doi: 10.1016/0888-7543(91)90418-e. [DOI] [PubMed] [Google Scholar]
- Vijay I. K., Oka T. Developmental regulation of glycosyltransferases involved in biosynthesis of asparagine-linked glycoproteins in mouse mammary gland. Eur J Biochem. 1986 Jan 2;154(1):57–62. doi: 10.1111/j.1432-1033.1986.tb09358.x. [DOI] [PubMed] [Google Scholar]
- Welply J. K., Lau J. T., Lennarz W. J. Developmental regulation of glycosyltransferases involved in synthesis of N-linked glycoproteins in sea urchin embryos. Dev Biol. 1985 Jan;107(1):252–258. doi: 10.1016/0012-1606(85)90393-8. [DOI] [PubMed] [Google Scholar]
- Zhu X. Y., Lehrman M. A. Cloning, sequence, and expression of a cDNA encoding hamster UDP-GlcNAc:dolichol phosphate N-acetylglucosamine-1-phosphate transferase. J Biol Chem. 1990 Aug 25;265(24):14250–14255. [PubMed] [Google Scholar]
- von Heijne G. Signal sequences. The limits of variation. J Mol Biol. 1985 Jul 5;184(1):99–105. doi: 10.1016/0022-2836(85)90046-4. [DOI] [PubMed] [Google Scholar]