Abstract
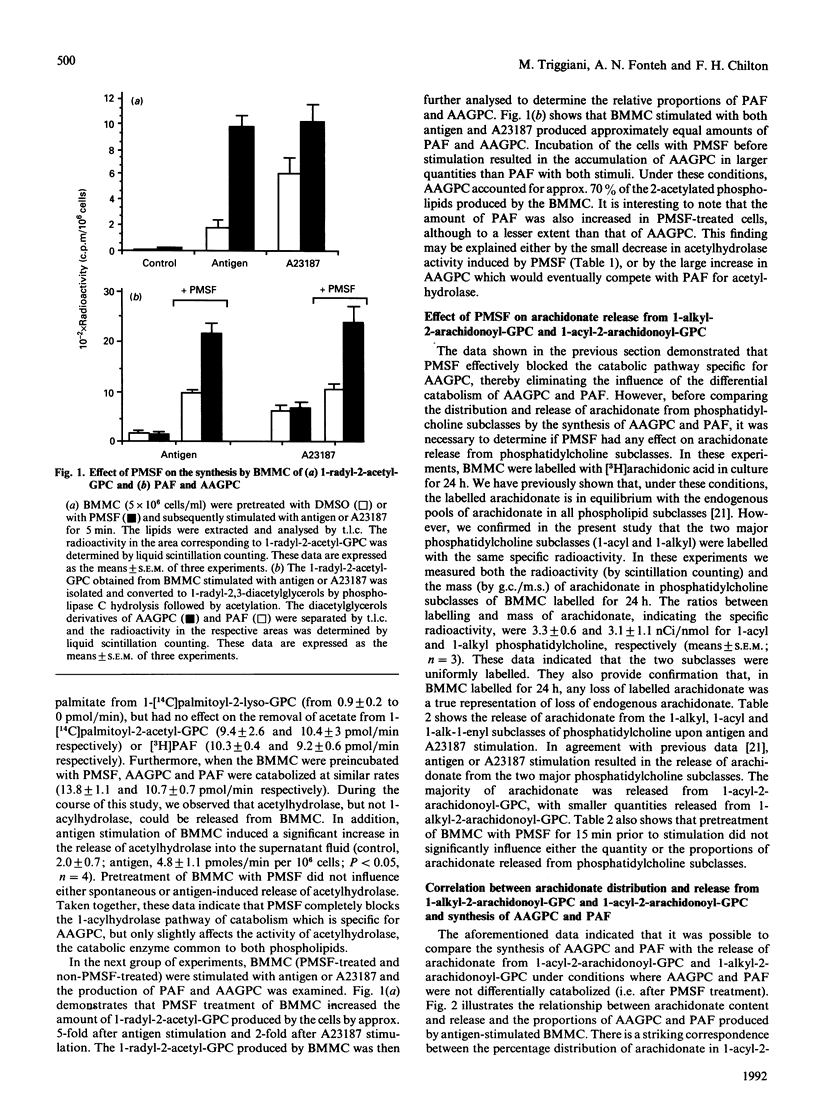
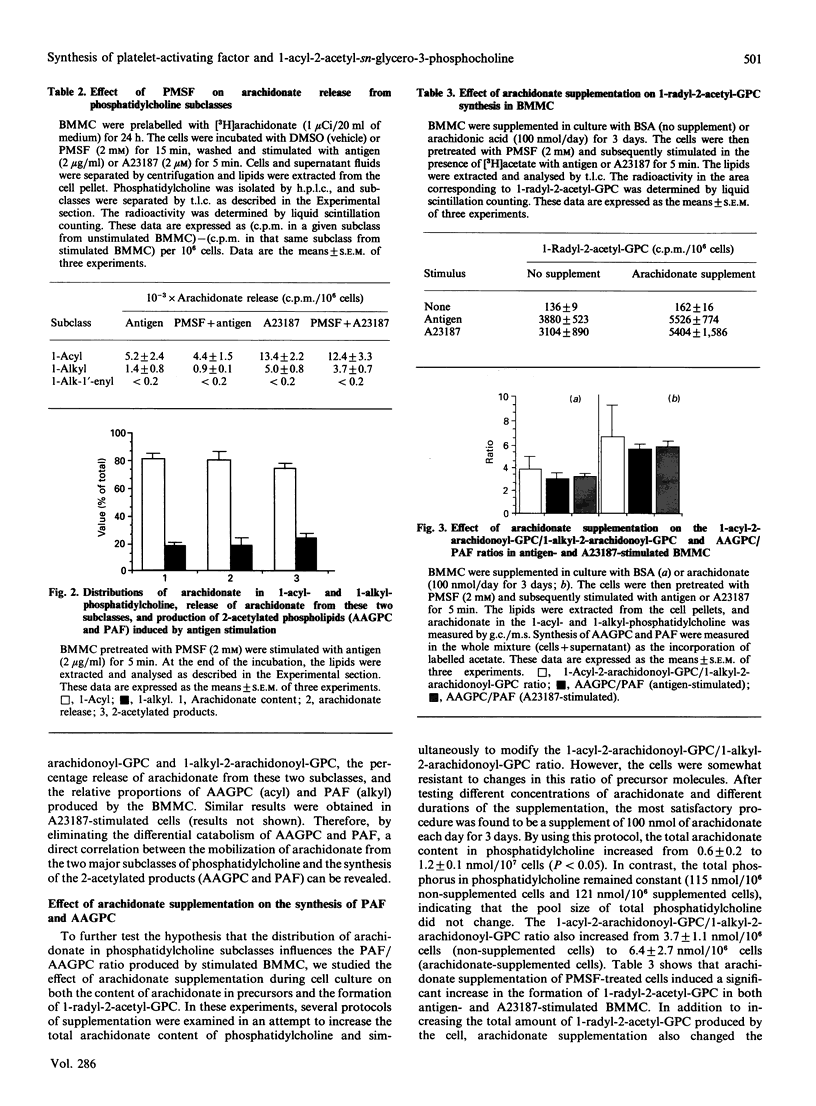
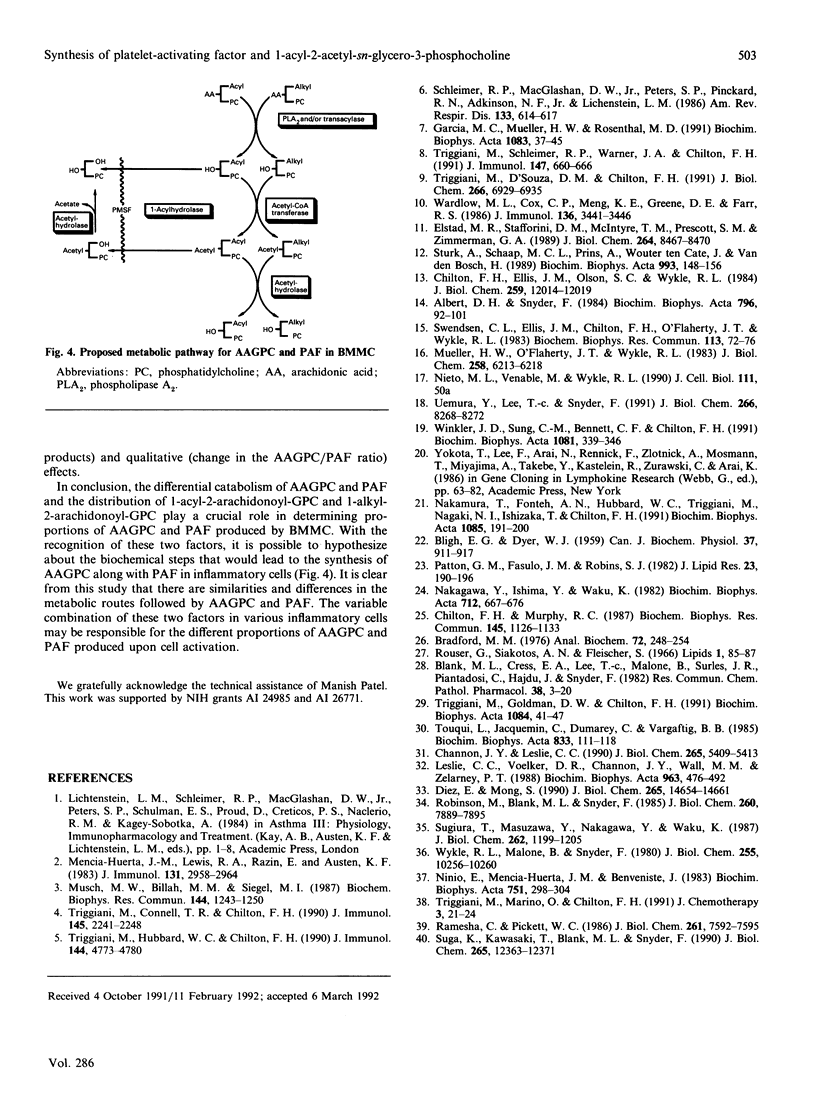
Recent studies have demonstrated that inflammatory cells can be divided into two groups depending on the type of 2-acetylated phospholipids [1-radyl-2-acetyl-sn-glycero-3-phosphocholine (GPC)] they produce: those that produce predominantly platelet-activating factor (PAF), and those that produce predominantly its 1-acyl analogue (1-acyl-2-acetyl-GPC; AAGPC) [Triggiani, Schleimer, Warner & Chilton (1991) J. Immunol. 147, 660-666]. The present study has examined the factors that regulate the production of these two molecules in mouse bone marrow-derived mast cells (BMMC). Initial experiments indicated that PAF and AAGPC were catabolized by BMMC in a differential manner via two pathways: the first, exclusive for AAGPC, involved a 1-acyl hydrolase that removed the long chain at the sn-1 position of the molecule, and the second, common to AAGPC and PAF, involved acetylhydrolase that removed the acetate at the sn-2 position of the two molecules. Experiments were next designed to identify conditions where the differential catabolism of AAGPC and PAF could be eliminated in order to uncover other factors that regulate the proportions of AAGPC and PAF produced. Phenylmethanesulphonyl fluoride (PMSF) completely blocked the 1-acylhydrolase activity while having little or no effect on the acetyl hydrolase activity, thereby eliminating the influence of the catabolic pathway unique to AAGPC. Moreover, PMSF did not alter the release of arachidonic acid from phospholipid subclasses. PMSF-treated BMMC produced larger quantities of AAGPC than of PAF. The AAGPC/PAF ratio detected in PMSF-treated BMMC was very similar to the ratio of arachidonate contained in and released from 1-acyl-/1-alkyl-linked phosphatidylcholine (PC). BMMC supplemented with arachidonic acid in culture for 3 days increased their total arachidonic acid content in PC as well as the ratio of 1-acyl-2-arachidonoyl-GPC to 1-alkyl-2-arachidonoyl-GPC. These changes resulted in parallel and significant increases in both the total amount of 1-radyl-2-acetyl-GPC and the AAGPC/PAF ration in BMMC. These data indicate that the AAGPC/PAF ratio produced by inflammatory cells is regulated by at least two factors: (1) differential catabolism of these two molecules, and (2) the distribution of arachidonate in 1-acyl- and 1-alkyl-2-arachidonyl-GPC. These observations support the concept of a common pathway for AAGPC and PAF biosynthesis in which the two precursor molecules are 1-acyl-2-arachidonoyl-GPC and 1-alkyl-2-arachidonoyl-GPC, respectively.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert D. H., Snyder F. Release of arachidonic acid from 1-alkyl-2-acyl-sn-glycero-3-phosphocholine, a precursor of platelet-activating factor, in rat alveolar macrophages. Biochim Biophys Acta. 1984 Oct 24;796(1):92–101. doi: 10.1016/0005-2760(84)90242-x. [DOI] [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Blank M. L., Cress E. A., Lee T. C., Malone B., Surles J. R., Piantadosi C., Hajdu J., Snyder F. Structural features of platelet activating factor (1-alkyl-2-acetyl-sn-glycero-3-phosphocholine) required for hypotensive and platelet serotonin responses. Res Commun Chem Pathol Pharmacol. 1982 Oct;38(1):3–20. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Channon J. Y., Leslie C. C. A calcium-dependent mechanism for associating a soluble arachidonoyl-hydrolyzing phospholipase A2 with membrane in the macrophage cell line RAW 264.7. J Biol Chem. 1990 Apr 5;265(10):5409–5413. [PubMed] [Google Scholar]
- Chilton F. H., Ellis J. M., Olson S. C., Wykle R. L. 1-O-alkyl-2-arachidonoyl-sn-glycero-3-phosphocholine. A common source of platelet-activating factor and arachidonate in human polymorphonuclear leukocytes. J Biol Chem. 1984 Oct 10;259(19):12014–12019. [PubMed] [Google Scholar]
- Chilton F. H., Murphy R. C. Stimulated production and natural occurrence of 1,2-diarachidonoylglycerophosphocholine in human neutrophils. Biochem Biophys Res Commun. 1987 Jun 30;145(3):1126–1133. doi: 10.1016/0006-291x(87)91554-3. [DOI] [PubMed] [Google Scholar]
- Diez E., Mong S. Purification of a phospholipase A2 from human monocytic leukemic U937 cells. Calcium-dependent activation and membrane association. J Biol Chem. 1990 Aug 25;265(24):14654–14661. [PubMed] [Google Scholar]
- Elstad M. R., Stafforini D. M., McIntyre T. M., Prescott S. M., Zimmerman G. A. Platelet-activating factor acetylhydrolase increases during macrophage differentiation. A novel mechanism that regulates accumulation of platelet-activating factor. J Biol Chem. 1989 May 25;264(15):8467–8470. [PubMed] [Google Scholar]
- Garcia M. C., Mueller H. W., Rosenthal M. D. C20 polyunsaturated fatty acids and phorbol myristate acetate enhance agonist-stimulated synthesis of 1-radyl-2-acetyl-sn-glycero-3-phosphocholine in vascular endothelial cells. Biochim Biophys Acta. 1991 Apr 24;1083(1):37–45. doi: 10.1016/0005-2760(91)90122-x. [DOI] [PubMed] [Google Scholar]
- Leslie C. C., Voelker D. R., Channon J. Y., Wall M. M., Zelarney P. T. Properties and purification of an arachidonoyl-hydrolyzing phospholipase A2 from a macrophage cell line, RAW 264.7. Biochim Biophys Acta. 1988 Dec 16;963(3):476–492. doi: 10.1016/0005-2760(88)90316-5. [DOI] [PubMed] [Google Scholar]
- Mencia-Huerta J. M., Lewis R. A., Razin E., Austen K. F. Antigen-initiated release of platelet-activating factor (PAF-acether) from mouse bone marrow-derived mast cells sensitized with monoclonal IgE. J Immunol. 1983 Dec;131(6):2958–2964. [PubMed] [Google Scholar]
- Mueller H. W., O'Flaherty J. T., Wykle R. L. Biosynthesis of platelet activating factor in rabbit polymorphonuclear neutrophils. J Biol Chem. 1983 May 25;258(10):6213–6218. [PubMed] [Google Scholar]
- Musch M. W., Billah M. M., Siegel M. I. Antigen-and ionophore-stimulated synthesis of platelet-activating factor by the cloned mast cell line, MC9. Biochem Biophys Res Commun. 1987 May 14;144(3):1243–1250. doi: 10.1016/0006-291x(87)91444-6. [DOI] [PubMed] [Google Scholar]
- Nakagawa Y., Waku K., Ishima Y. Changes in the composition of fatty chains of diacyl, alkylacyl and alkenylacyl ethanolamine and choline phosphoglycerides during the development of chick heart ventricular cells. High accumulation of 22-carbon fatty acid in ether phospholipids. Biochim Biophys Acta. 1982 Sep 14;712(3):667–676. [PubMed] [Google Scholar]
- Nakamura T., Fonteh A. N., Hubbard W. C., Triggiani M., Inagaki N., Ishizaka T., Chilton F. H. Arachidonic acid metabolism during antigen and ionophore activation of the mouse bone marrow derived mast cell. Biochim Biophys Acta. 1991 Sep 11;1085(2):191–200. doi: 10.1016/0005-2760(91)90094-x. [DOI] [PubMed] [Google Scholar]
- Ninio E., Mencia-Huerta J. M., Benveniste J. Biosynthesis of platelet-activating factor (PAF-acether). V. Enhancement of acetyltransferase activity in murine peritoneal cells by calcium ionophore A23187. Biochim Biophys Acta. 1983 May 16;751(3):298–304. doi: 10.1016/0005-2760(83)90287-4. [DOI] [PubMed] [Google Scholar]
- Patton G. M., Fasulo J. M., Robins S. J. Separation of phospholipids and individual molecular species of phospholipids by high-performance liquid chromatography. J Lipid Res. 1982 Jan;23(1):190–196. [PubMed] [Google Scholar]
- Ramesha C. S., Pickett W. C. Platelet-activating factor and leukotriene biosynthesis is inhibited in polymorphonuclear leukocytes depleted of arachidonic acid. J Biol Chem. 1986 Jun 15;261(17):7592–7595. [PubMed] [Google Scholar]
- Robinson M., Blank M. L., Snyder F. Acylation of lysophospholipids by rabbit alveolar macrophages. Specificities of CoA-dependent and CoA-independent reactions. J Biol Chem. 1985 Jul 5;260(13):7889–7895. [PubMed] [Google Scholar]
- Rouser G., Siakotos A. N., Fleischer S. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids. 1966 Jan;1(1):85–86. doi: 10.1007/BF02668129. [DOI] [PubMed] [Google Scholar]
- Schleimer R. P., MacGlashan D. W., Jr, Peters S. P., Pinckard R. N., Adkinson N. F., Jr, Lichtenstein L. M. Characterization of inflammatory mediator release from purified human lung mast cells. Am Rev Respir Dis. 1986 Apr;133(4):614–617. doi: 10.1164/arrd.1986.133.4.614. [DOI] [PubMed] [Google Scholar]
- Sturk A., Schaap M. C., Prins A., ten Cate J. W., van den Bosch H. Synthesis of platelet-activating factor by human blood platelets and leucocytes. Evidence against selective utilization of cellular ether-linked phospholipids. Biochim Biophys Acta. 1989 Dec 8;993(2-3):148–156. doi: 10.1016/0304-4165(89)90157-8. [DOI] [PubMed] [Google Scholar]
- Suga K., Kawasaki T., Blank M. L., Snyder F. An arachidonoyl (polyenoic)-specific phospholipase A2 activity regulates the synthesis of platelet-activating factor in granulocytic HL-60 cells. J Biol Chem. 1990 Jul 25;265(21):12363–12371. [PubMed] [Google Scholar]
- Sugiura T., Masuzawa Y., Nakagawa Y., Waku K. Transacylation of lyso platelet-activating factor and other lysophospholipids by macrophage microsomes. Distinct donor and acceptor selectivities. J Biol Chem. 1987 Jan 25;262(3):1199–1205. [PubMed] [Google Scholar]
- Swendsen C. L., Ellis J. M., Chilton F. H., 3rd, O'Flaherty J. T., Wykle R. L. 1-O-alkyl-2-acyl-sn-glycero-3-phosphocholine: a novel source of arachidonic acid in neutrophils stimulated by the calcium ionophore A23187. Biochem Biophys Res Commun. 1983 May 31;113(1):72–79. doi: 10.1016/0006-291x(83)90433-3. [DOI] [PubMed] [Google Scholar]
- Touqui L., Jacquemin C., Dumarey C., Vargaftig B. B. 1-O-alkyl-2-acyl-sn-glycero-3-phosphorylcholine is the precursor of platelet-activating factor in stimulated rabbit platelets. Evidence for an alkylacetyl-glycerophosphorylcholine cycle. Biochim Biophys Acta. 1985 Jan 9;833(1):111–118. doi: 10.1016/0005-2760(85)90258-9. [DOI] [PubMed] [Google Scholar]
- Triggiani M., Connell T. R., Chilton F. H. Evidence that increasing the cellular content of eicosapentaenoic acid does not reduce the biosynthesis of platelet-activating factor. J Immunol. 1990 Oct 1;145(7):2241–2248. [PubMed] [Google Scholar]
- Triggiani M., D'Souza D. M., Chilton F. H. Metabolism of 1-acyl-2-acetyl-sn-glycero-3-phosphocholine in the human neutrophil. J Biol Chem. 1991 Apr 15;266(11):6928–6935. [PubMed] [Google Scholar]
- Triggiani M., Goldman D. W., Chilton F. H. Biological effects of 1-acyl-2-acetyl-sn-glycero-3-phosphocholine in the human neutrophil. Biochim Biophys Acta. 1991 Jun 19;1084(1):41–47. doi: 10.1016/0005-2760(91)90053-k. [DOI] [PubMed] [Google Scholar]
- Triggiani M., Hubbard W. C., Chilton F. H. Synthesis of 1-acyl-2-acetyl-sn-glycero-3-phosphocholine by an enriched preparation of the human lung mast cell. J Immunol. 1990 Jun 15;144(12):4773–4780. [PubMed] [Google Scholar]
- Triggiani M., Schleimer R. P., Warner J. A., Chilton F. H. Differential synthesis of 1-acyl-2-acetyl-sn-glycero-3-phosphocholine and platelet-activating factor by human inflammatory cells. J Immunol. 1991 Jul 15;147(2):660–666. [PubMed] [Google Scholar]
- Uemura Y., Lee T. C., Snyder F. A coenzyme A-independent transacylase is linked to the formation of platelet-activating factor (PAF) by generating the lyso-PAF intermediate in the remodeling pathway. J Biol Chem. 1991 May 5;266(13):8268–8272. [PubMed] [Google Scholar]
- Wardlow M. L., Cox C. P., Meng K. E., Greene D. E., Farr R. S. Substrate specificity and partial characterization of the PAF-acylhydrolase in human serum that rapidly inactivates platelet-activating factor. J Immunol. 1986 May 1;136(9):3441–3446. [PubMed] [Google Scholar]
- Winkler J. D., Sung C. M., Bennett C. F., Chilton F. H. Characterization of CoA-independent transacylase activity in U937 cells. Biochim Biophys Acta. 1991 Feb 5;1081(3):339–346. doi: 10.1016/0005-2760(91)90291-o. [DOI] [PubMed] [Google Scholar]
- Wykle R. L., Malone B., Snyder F. Enzymatic synthesis of 1-alkyl-2-acetyl-sn-glycero-3-phosphocholine, a hypotensive and platelet-aggregating lipid. J Biol Chem. 1980 Nov 10;255(21):10256–10260. [PubMed] [Google Scholar]


