Abstract
Background:
Alliance A021501 is the first randomized trial to evaluate stereotactic body radiation therapy (SBRT) for borderline resectable pancreatic ductal adenocarcinoma (PDAC) after neoadjuvant chemotherapy. In this post hoc study, we reviewed the quality of radiation therapy (RT) delivered.
Methods:
SBRT (6.6 Gy x 5) was intended, although hypofractionated RT (5 Gy x 5) (HIGRT) was permitted if SBRT specifications could not be met. Institutional credentialing through the National Cancer Institute-funded Imaging and Radiation Oncology Core (IROC) was required. Rigorous RT quality assurance (RT QA) was mandated, including pretreatment review by a radiation oncologist. Revisions were required for unacceptable deviations. Additionally, we performed a post hoc RT QA analysis in which contours and plans were reviewed by 3 radiation oncologists and assigned a score (1, 2, 3) based on adequacy. A score of 1 indicated no deviation, a 2 indicated minor deviation, and a 3 indicated a major deviation that could be clinically significant. Clinical outcomes were compared by treatment modality and by case score.
Results:
Forty patients were registered to receive RT (1 planned but not treated) at 27 centers (18 academic, 9 community). Twenty-three centers were appropriately credentialed for moving lung/liver targets, while 4 were approved for static head and neck only. Thirty-two of 39 patients (82.1%) were treated with SBRT, and 7 (17.9%) with HIGRT. Five cases (13%) required revision prior to treatment. On post hoc review, 23 patients (59.0%) were noted to have suboptimal contours or plan coverage, 12 (30.8%) were scored a 2 and 11 cases (28.2%) were scored a 3. There were no apparent differences in failure patterns or surgical outcomes based on treatment technique or post hoc case score. Details related to on-treatment imaging were not recorded.
Conclusion:
Despite rigorous QA, we encountered variability in simulation, contouring, plan coverage, and dose on trial. While clinical outcomes did not appear to be impacted, findings from this analysis serve to inform subsequent PDAC SBRT trial designs and QA requirements.
Introduction
The role of neoadjuvant radiation therapy (RT) for patients with borderline resectable (BR) pancreatic ductal adenocarcinoma (PDAC) remains controversial. Multiple studies have shown that neoadjuvant chemotherapy or chemoradiotherapy, can provide clinical benefit, but the optimal regimen remains uncertain and the subject of ongoing investigation.1–3 RT for patients with PDAC is complex, and failure to adhere to RT protocol requirements has been shown to negatively impact trial outcomes.4,5
Alliance for Clinical Trials in Oncology A021501 was the first randomized trial to investigate the role of neoadjuvant hypofractionated RT in patients with BR PDAC.6 In this phase II trial, patients with non-metastatic BR adenocarcinoma of the pancreatic head or uncinate process were randomized at diagnosis to receive modified FOLFIRINOX (mFFX) alone versus mFFX followed by 5-fraction RT. Although the primary outcome measure was 18-month overall survival (OS) the mFFX + RT arm closed early, per protocol-specific interim analysis, given the low proportion of patients who underwent an R0 resection. Of the 56 patients randomized to the mFFX + RT arm, 40 patients (71.4%) actually received RT and 19 (33.9%) underwent subsequent surgical resection.
Rigorous quality assurance (QA) was mandated for all patients undergoing RT on the Alliance A021501 trial. Instructions for sites included online tutorials, central RT contouring and plan review. This post hoc study was undertaken with the following objectives: (1) review adherence to protocol RT QA specifications; (2) describe the quality of RT contours and plans; (3) determine associations between RT quality and clinical outcomes; (4) identify opportunities to improve RT planning and delivery to inform the design of future studies.
Methods
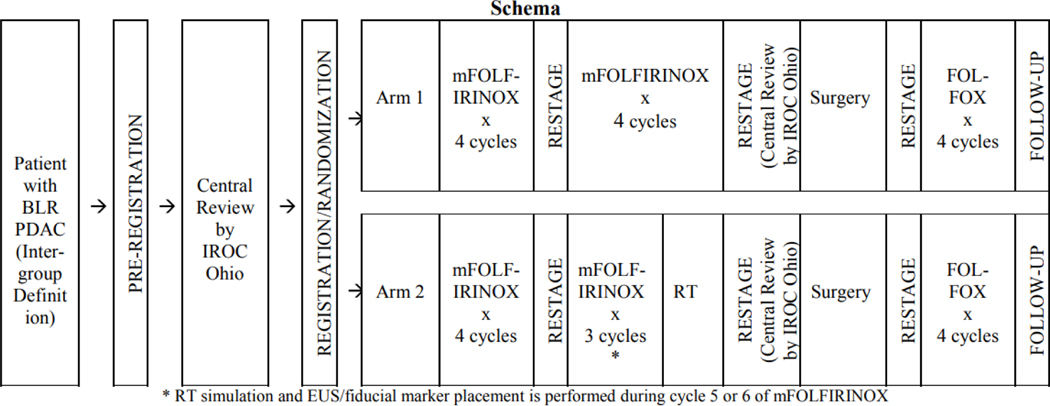
Patients were randomized 1:1 either to receive 8 cycles of mFFX followed by surgery or to 7 cycles of mFFX plus 5-fraction RT followed by surgery (Figure 1). The National Cancer Institute (NCI) requires institutions delivering RT on NCI sponsored trials to be credentialed by the Imaging and Radiation Oncology Core (IROC) Houston for the RT treatment modality used.7 Institutions treating with SBRT had to pass phantom irradiation testing using a moving lung/liver phantom, which requires robust motion management techniques.8 For institutions previously credentialed for intensity modulated radiation therapy (IMRT), prior irradiation of a static head and neck phantom was accepted. Patients could be treated at either an academic center, defined by affiliation with a university or an NCI-designated cancer center, or in a community-based setting, defined as lacking an educational affiliation.
Figure 1:
Alliance A021501 Trial Schema
Abbreviations: BLR, borderline resectable; PDAC, pancreatic ductal adenocarcinoma; IROC, Imaging and Radiation Oncology Core; FOLFIRINOX, leucovorin, fluorouracil, irinotecan, oxaliplatin; RT, radiation therapy
Detailed instructions for RT delivery were provided in the protocol (Supplement 1). In addition, an online tutorial for radiation planning and treatment was made available through EduCase, as was a practice case to review contouring. It was intended that patients be treated using SBRT. Requirements for SBRT (Supplement 1) included no tumor invasion of bowel or stomach, no active gastric or duodenal ulcers, ability to meet SBRT organ-at-risk (OAR) constraints, fiducial marker placement, appropriate motion management, 4 dimensional (4D) CT simulation, and daily image guidance. If requirements for SBRT could not be met, then hypofractionated image-guided RT (HIGRT) was permitted.
Per protocol, patients were simulated supine with arms above the head in a custom immobilization device. Patients were required to be nil per os (NPO) 3 hours prior to CT simulation and treatment. Administration of intravenous (IV) and oral contrast at the time of CT simulation was recommended, but was not required. 4DCT was recommended if available and respiratory motion management techniques were required if tumor motion was noted to be >5 mm. The target volume definitions for SBRT and HIGRT included the GTV (gross tumor on breathing scan), iGTV (GTV, accounting for motion), tumor vessel interface (TVI) including the segment of portal vein (PV), superior mesenteric vein (SMV), superior mesenteric artery (SMA), common hepatic artery (CHA), and/or celiac artery that is in direct contact with tumor, iTVI (TVI, accounting for motion), and PRVgi (gastrointestinal planning risk volume). There were three planning target volumes (PTV) for patients receiving SBRT, and only one for patients receiving HIGRT. PTV1 (iGTV + iTVI) was to receive 25 Gy in 5 fractions and applied to both SBRT and HIGRT cases. PTV2 (PTV1 – PRVgi) and PTV3 (iTVI + 3mm) were to receive 33 Gy and 36 Gy, respectively, in 5 fractions via a simultaneous integrated boost only for cases treated with SBRT. The PTV 36 Gy was allowed to receive up to 40 Gy maximum dose. Additional details regarding target volume creation and dose prescriptions are described in Supplement 1. Strict compliance criteria for prescription dose coverage as well as OAR constraints were also provided.
Regardless whether SBRT or HIGRT was used, a review of the contours, treatment plan, and adherence to protocol compliance criteria was required for all patients prior to the start of RT that was performed by an experienced radiation oncologist (XX) from IROC in collaboration with XY on select cases. Any plans not meeting prespecified compliance criteria were required to be revised and resubmitted prior to treatment initiation. Cases requiring revision were reviewed as part of the current QA analysis.
For this analysis, post hoc review of all RT cases was carried out by 3 radiation oncologists (ROs) with expertise in treating PDAC (XY, XW, XZ). At least 2 ROs reviewed target volume contours, OAR contours, and dose coverage for each case and then independently assigned each a score of 1, 2, or 3. If there was disagreement, a majority consensus had to be reached before a score was assigned. Criteria used for scoring included adequacy of organ at risk contours (were organs contoured accurately and completely), adequacy of target volume contours with particular attention to inclusion of the entire involved blood vessel in cases involving a TVI contour ensuring minimal overlap with OAR contours, and adequate coverage of the PTV such that at least 95% of the PTV was covered by 95% of the dose as specified in the protocol (Supplement). A score of 1 indicated that the post hoc reviewer did not recommend any adjustments to the contours or treatment plan as submitted. A score of 2 indicated a minor deviation that was unlikely to be clinically meaningful. Finally, a score of 3 indicated a major deviation that could be clinically meaningful.
Recurrence was determined radiographically on follow-up imaging and did not require biopsy for confirmation unless imaging findings were equivocal. Locoregional recurrence was defined as a new soft tissue mass in the tumor bed/residual pancreas, around the mesenteric vasculature, or in regional lymph nodes. Distant recurrence was defined primarily as a new hypodensity in the liver, lungs, or peritoneum, or at other less common sites (adrenal, brain) if biopsy proven. The patterns of failure were categorized as local (within the tumor bed or residual pancreas), regional (within the lymph nodes or vasculature), distant, or a combination. Surgical outcomes included: patient taken to the operating room, surgery completed as planned, and resection margin status. Only 2 patients on the RT arm experienced grade 3 or higher toxicity, thus we are not reporting on QA parameters with respect to toxicity in the present analysis.
Statistical Methods
Among patients enrolled to A021501 trial, only those received RT were included in this post hoc analysis. Details regarding the power and sample size analysis used for the original trial are described in detail in the original publication.9 However, the original trial design was not powered to address the aims in the current post-hoc analyses. Thus statistical analyses were mainly descriptive and the comparisons among the subgroups within this cohort, by treatment technique (SBRT versus HIGRT) and by case score (1 and 2 versus 3), were limited by small numbers. Patient demographics, treatment and facility characteristics, and results of post-hoc case review were summarized by median (IQR) and frequency (percentage) for continuous and categorical variables, respectively. Time-to-event endpoints (e.g., overall survival) was summarized by the Kaplan-Meier estimates. Categorical outcomes (e.g., patterns of failure and surgical outcomes) were compared between patients receiving SBRT and those receiving HIGRT, or with post hoc case score (1 versus 2 versus 3) by Fisher’s exact tests. Log-rank test was used to compare time-to-event endpoint between subgroups. P-value less than 0.05 was considered statistically significant.
Each participant signed an IRB-approved, protocol-specific informed consent document in accordance with federal and institutional guidelines. Data collection and statistical analyses were conducted by the Alliance Statistics and Data Management Center. Data quality was ensured by review of data by the Alliance Statistics and Data Management Center and by the study chairperson following Alliance policies. All analyses were based on the study database frozen on September 1st, 2021.
Results
Fifty-six patients were randomized to the RT arm between February 17th, 2017 and May 9th, 2019. Median follow-up for the study was 42.9 months. Of these 56 patients, only 40 patients completed all 7 cycles of mFFX and were registered to start RT. The RT arm of the trial was closed on August 13, 2018. Of note, one patient was enrolled in the RT arm before this arm was closed but received RT after treatment arm closure and thus was included in the primary analysis of A201501, but was excluded from the QA analysis. Therefore, 39 patients were included in the current analysis. Further details regarding enrolled subjects analyzed in the present study is shown in the Consort Diagram (Supplement).
Patient, tumor, and treatment characteristics are shown in Table 1. The median age was 67 years and most patients were female (53.8%). Although 35 patients were planned to receive SBRT and 5 were planned to receive HIGRT per the original publication, ultimately, 32/39 (82.1%) were treated with SBRT, as intended, and 7/39 (17.9%) were treated with HIGRT. Two patients initially planned for SBRT actually received HIGRT, one due to insurance denial and one because fiducial markers were not placed as required by the study protocol for SBRT.. The changes in planning technique were not reported at the time of the original publication. The median GTV size was 28.5 cc (range 1.0–79.0 cc). The maximum dose from SBRT was 47.15 Gy and from HIGRT was 28.25 Gy. Most patients were simulated with IV contrast alone (n=17, 43.6%) and 8 patients (20.5%) were simulated with both IV and oral contrast. Five methods of motion management were utilized: abdominal compression (n=13, 33.3%), free breathing with increased margin (n=9, 23.1%), gating during free breathing (n=1, 2.6%), gating with breath-hold (n=9, 23.1%), and motion-tracking on a CyberKnife (n=2, 5.1%).
Table 1:
Patient, treatment, and facility characteristics
| Characteristic | Total Patients (N=39) |
|---|---|
| Median Age, years (IQR) | 67.0 (60.0–70.0) |
| Gender | N (%) |
| Male | 18 (46.2) |
| Female | 21 (53.8) |
| GTV and OAR Volumes | Size (IQR) |
| Median GTV size in cc | 28.5 (14.0–38.0) |
| Median V20 Small bowel in cc | 0.0 (0.0–3.0) |
| Median V20 Duodenum in cc | 14.0 (4.0–18.0) |
| Contrast | N (%) |
| Oral Only | 9 (23.1) |
| Intravenous Only | 17 (43.6) |
| Both | 8 (20.5) |
| Neither | 5 (12.8) |
| Unknown | 5 (12.8) |
| Motion Management Technique | N (%) |
| Abdominal Compression | 13 (33.3) |
| Free breathing + margin | 9 (23.1) |
| Gating with free breathing | 1 (2.6) |
| Gating with breath-hold | 9 (23.1) |
| Tracking | 2 (5.1) |
| Unknown | 5 (12.8) |
| Treatment Technique | N (%) |
| SBRT | 32 (82.1) |
| HIGRT | 7 (17.9) |
| Number of Patients on Trial | N (%) |
| One | 19 (70.4) |
| More than one | 8 (29.6) |
| Academic vs Community | N (%) |
| Academic | 18 (66.7) |
| Community | 9 (33.3) |
| Approved for Moving Lung/Liver | N (%) |
| No | 4 (14.8) |
| Yes | 23 (85.2) |
Abbreviations: N, number; IQR, inter-quartile range; GTV, gross tumor volume; OAR, organ-at-risk; V20, volume receiving 20 Gray; SBRT, stereotactic body radiation therapy; HIGRT, hypofractionated image-guided radiation therapy
Information about participating institutions is shown in Table 1. Patients were treated at 27 institutions; 18 (66.7%) were considered academic, and 9 (33.3%) community. Most (n=19, 70.4%) enrolled one patient, while the remaining 8 (29.6%) enrolled two or more patients. The majority of centers (59.3%, n=16) passed phantom irradiation after only one attempt, while the remaining centers (40.7%, n=11) required more than one attempt at passing the phantom irradiation test to become credentialed to treat patients on this study. Most centers (n=23, 85.2%) were approved for moving lung/liver irradiation, while 4 (14.8%) were previously approved for IMRT and thus passed the static head and neck phantom test only. Fourteen centers (51.9%) were credentialed for up to three years prior to study enrollment, while 13 (48.1%) were credentialed for four or more years.
On pretreatment review, five cases (12.8%) required revision and resubmission. Two cases (5.1%) were returned for replanning because they were planned with an outdated version of the trial protocol. Significant changes with respect to guidance on fiducials, target volumes, and dose constraints were made to the outdated protocol versions, thus these cases required replanning. Both cases were replanned with the current (Update 03) protocol version and found to be acceptable after re-review. One case was resubmitted because it was originally planned for SBRT, but insurance coverage denied SBRT and the case was replanned using HIGRT. One case was submitted for replanning because OAR constraints were not met. The reason for resubmission of the fifth case was not documented. Of note, one case was unintentionally treated without undergoing pretreatment review, and was included in the final analysis. While SBRT was used, only one target volume was treated, and the dose delivered was 33 Gy (instead of 36 Gy). Less than 90% of the submitted target volume was covered by ≥95% of the prescription dose, which was considered an unacceptable deviation. The reason why this case was treated without undergoing the mandatory pretreatment review is unknown.
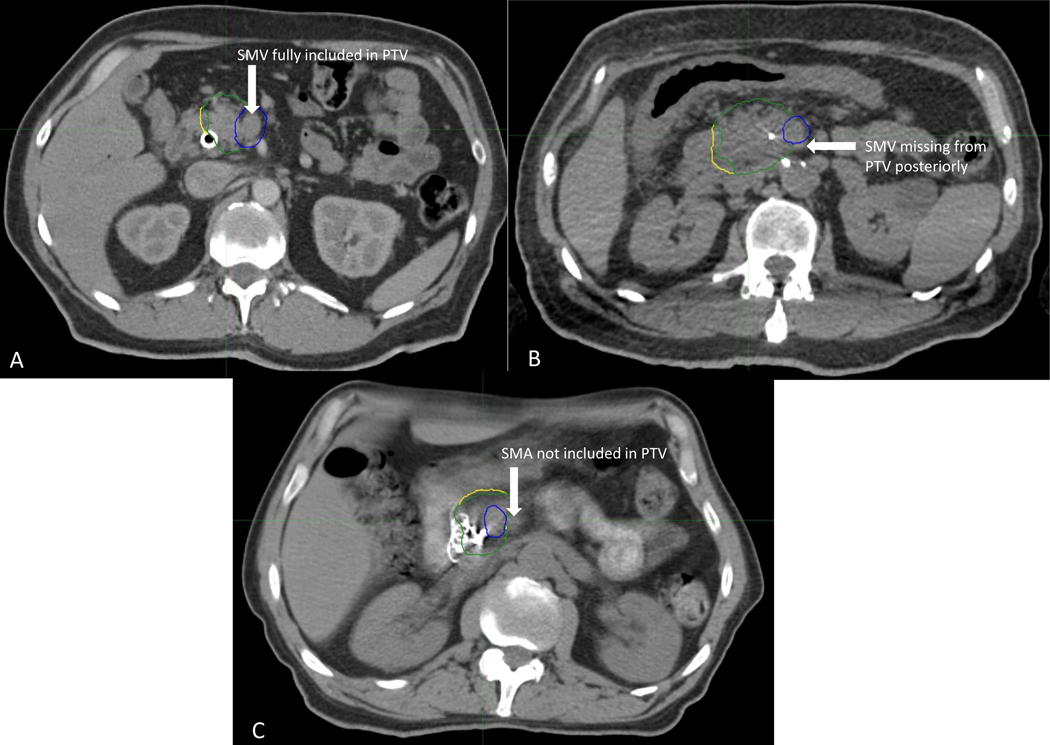
On post hoc review, 16 cases (41%) were assigned a score of 1 (Table 2). Twelve cases (30.8%) were assigned a score of 2, and 11 cases (28.2%) were assigned a score of 3. The reasons for a score of 2 or 3 are shown in Table 2. Most cases were scored a 2 due to a suboptimal target contour (N=9, 75%). Cases were scored a 3 due to suboptimal target contour in 5 cases (45.5%) and due to both suboptimal target and OAR contour in 5 cases (45.5%). . Figure 2 depicts examples of target volumes by score: Figure 2A depicts target contours scored a 1, Figure 2B depicts target contours scored a 2, and Figure 2C depicts target contours scored a 3, with the reason for each score indicated. The one case that was not included in the pretreatment review was scored a 3 due to inappropriate target volume contour, inappropriate dose, and not meeting dose coverage requirements.
Table 2:
Results of post-hoc case review
| Score (N=39) | Number (%) |
|---|---|
| 1 | 16 (41.0) |
| 2 | 12 (30.8) |
| 3 | 11 (28.2) |
| Reason for Deviation (Score=2) (N=12) | Number (%) |
| Suboptimal Target Contour | 9 (75.0) |
| Suboptimal OAR Contour | 1 (8.3) |
| Suboptimal Target and OAR Contour | 1 (8.3) |
| Suboptimal Target Contour and Dose Coverage | 1 (8.3) |
| Reason for Deviation (Score=3) (N=11) | Number (%) |
| Suboptimal Target Contour | 5 (45.5) |
| Suboptimal OAR Contour | 0 (0.0) |
| Suboptimal Target and OAR Contour | 5 (45.5) |
| Suboptimal Target Contour and Dose Coverage | 1 (9.1) |
Abbreviations: N, number; OAR, organ at risk
Figure 2:
Example of each case score for target contours. Panel 2A depicts a case scored as 1 due to appropriate inclusion of the SMV within the tumor-vessel interface contour. Panel 2B depicts a case scored as 2 due to partial omission of the SMV posteriorly. Panel 2C depicts a case scored as 3 due to exclusion of the SMA from the TVI despite abutment of the SMA by gross disease. Blue contour: PTV 36 Gy (iTVI plus 3mm); Green contour: 33 Gy PTV (25 Gy PTV minus the PRVgi); Yellow contour: 25 Gy PTV [(iGTV + iTVI) plus 3mm]. Abbreviations: iGTV, gross target volume accounting for motion; SMV, superior mesenteric vein; SMA, superior mesenteric artery; iTVI, tumor-vessel interface accounting for motion; PTV, planning target volume; PRVgi, gastrointestinal planning risk volume.
Surgical outcomes by treatment technique and case score are shown in Table 3. Of the 39 patients, 28 (71.8%) proceeded to the operating room, although only 19/39 (48.7%) underwent the planned surgical resection with 14 of these 19 (73.6%) achieving an R0 resection. There was no difference in R0 resection rate based on SBRT versus HIGRT or post hoc review score (P=0.15 and P=0.30, respectively). Patterns of failure by treatment technique and case score are shown in Table 4. Twenty-four patients (61.5%) experienced cancer recurrence; 17 (70.8%) had a distant failure only, 4 (16.7%) had local failure only, 2 (8.3%) had both distant and local treatment failure, and 1 (4.2%) had locoregional and distant failure. There was no difference in failure patterns based on RT technique or case score (P=1.0 and P=0.72, respectively). Twenty-eight patients who received RT died; 23 were treated with SBRT and 5 with HIGRT. Of these 28 patients, 19 were assigned a score of 1 or 2 on post-hoc analysis and 9 received a score of 3. There was no difference in overall survival based on RT technique or case score (P=0.61 and P=1.0, respectively) as shown in the Supplementary Figures.
Table 3:
Surgical outcomes by treatment technique and post-hoc review case score.
| Treatment Technique | SBRT (n=32) N (%) | HIGRT (n=7) N (%) | P-value | |
|---|---|---|---|---|
| Patient taken to the operating room * | 23 (71.9) | 5 (71.4) | 1.0001 | |
| Surgery performed (resection completed as planned) | 16 (50) | 3 (42.9) | 1.0001 | |
| R0 | 13 (40.6) | 1 (14.3) | 0.1548 | |
| R1 | 3 (9.4) | 2 (28.6) | - | |
| R2 | 0 (0.0) | 0 (0.0) | - | |
| Case Score | 1 (n=26) N (%) | 2 (n=12) N (%) | 3 (n=11) N (%) | P-Value |
| Patient taken to the operating room * | 12 (75.0) | 6 (50.0) | 10 (90.9) | 0.22831 |
| Surgery performed (resection completed as planned) | 7 (43.8) | 5 (41.7) | 7 (63.6) | 0.68041 |
| R0 | 6 (37.5) | 4 (33.3) | 4 (36.4) | 0.55751 |
| R1 | 1 (6.3) | 1 (8.3) | 3 (27.3) | - |
| R2 | 0 (0.0) | 0 (0.0) | 0 (0.0) | - |
Denotes that a patient was taken to the operating room, but surgery was not necessarily performed.
Abbreviations: SBRT, stereotactic body radiation therapy; HIGRT, hypofractionated image-guided radiation therapy; N, number; R0, margin negative resection; R1, microscopically positive margin; R2, grossly positive resection margin.
Fisher Exact P-value
Table 4:
Patterns of failure by treatment type and case score
| Treatment Technique | SBRT (n=32) N (%) | HIGRT (n=7) N (%) | P-value | |
|---|---|---|---|---|
| Distant Only | 14 (43.8) | 3 (42.9) | 1.00001 | |
| Local Only | 3 (9.4) | 1 (14.3) | - | |
| Local + Distant | 2 (6.3) | 0 (0.0) | ||
| Local + Regional + Distant | 1 (3.1) | 0 (0.0) | ||
| None | 12 (37.5) | 3 (42.9) | ||
| Case Score | 1 (n=16) N (%) | 2 (n=12) N (%) | 3 (n=11) N (%) | P-Value |
| Distant Only | 4 (25.0) | 8 (66.7) | 5 (45.5) | 0.5061 |
| Local Only | 2 (12.5) | 1 (8.3) | 1 (9.1) | - |
| Local + Distant | 2 (12.5) | 0 (0.0) | 0 (0.0) | - |
| Local + Regional + Distant | 1 (6.3) | 0 (0.0) | 0 (0.0) | - |
| None | 7 (43.8) | 3 (25.0) | 5 (45.5) | - |
Abbreviations: SBRT, stereotactic body radiation therapy; HIGRT, hypofractionated image-guided radiation therapy
Fisher Exact P-value
Discussion
Alliance A021501 was the first randomized clinical trial investigating the role of hypofractionated RT in the neoadjuvant setting for patients with BR PDAC. Analyzing the quality of RT delivered on this study is critical to understanding the outcomes of this trial and in guiding the design of future clinical trials in this space. We encountered significant variability with respect to simulation and motion management, and nearly 20% of cases were not treated with the intended RT dose. Further, despite undergoing pretreatment review by an experienced radiation oncologist, post hoc analysis by 3 additional radiation oncologists revealed significant variability in RT quality, with one-third of cases having either suboptimal contours and/or target coverage. The numbers were too small to allow for a meaningful statistical analysis to determine if clinical outcomes were impacted. Despite a concerted effort to mandate rigorous RT QA, the variability in quality seen on Alliance A021501 indicates that future trial design in this space could benefit from increased standardization to further optimize RT consistency and quality.
PDAC is a leading cause of cancer-related death and has increased in incidence over the past several decades, with over 64,000 cases expected to be diagnosed in the U.S. in 2023. The treatment of patients with PDAC is complex and involves multimodality care by experienced practitioners.10 Fewer than 10% of cancer patients participate in clinical trials due to a number of factors that have been identified as barriers to accrual.11–13 The relatively low although rising incidence of PDAC, coupled with these barriers to accrual, can create challenges to accrual onto clinical trials involving complex radiation treatment in the PDAC space. To achieve accrual goals, trials need to be opened at numerous centers across the country, resulting in variability in treatment delivery. RT QA serves to standardize the treatment delivered on clinical trials and minimize this variability to adequately address the study hypothesis.
The importance of strict adherence to protocol specifications when treating patients with pancreatic cancer using RT has been previously demonstrated in prospective PDAC studies. Abrams et al. reported that OS outcomes were superior for patients with resected PDAC of the pancreatic head treated with adjuvant RT per protocol versus those treated outside of protocol (median OS 1.74 years versus 1.46 years, p<0.001).4 On multivariate analysis, treatment per protocol was correlated more strongly with median survival than was assigned treatment arm (p=0.014, p=NS, respectively), highlighting the importance of adhering to protocol specifications when assessing the impact of RT on clinical trial outcomes.5
Resultant from the findings discussed above, PDAC clinical trials, including Alliance A021501 have employed rigorous RT QA methods to optimize treatment efficacy and safety. Treating facilities underwent strict credentialing prior to enrollment. Educational materials, including an online tutorial and practice cases for contouring, were provided. Target volume definitions and plan coverage were described in detail in the protocol. Each case was reviewed prior to treatment by an experienced RO. Cases that were identified to have protocol deviations required revision until compliance criteria was met.
Delivery of high-quality RT for patients with PDAC is challenging. Pancreatic tumors are subject to both intrafraction and interfraction motion and are in proximity to the stomach and bowel, which are especially radiosensitive. As a result, advanced treatment techniques are required for safe and effective RT delivery, including motion management and high-quality real-time imaging.14–16 These techniques are not universally available and can limit treatment options at centers that are not equipped for safe RT delivery. As a result, Alliance A021501 permitted treatment utilizing HIGRT instead of the intended SBRT in selected circumstances as an alternative option. The heterogeneous dose/fractionation schemes permitted in Alliance A201501 highlight the tradeoff between allowing for maximal trial accrual and delivering the intended radiation treatment. Future clinical trials may benefit from the inclusion only of patients who can receive the intended treatment to maximize therapeutic outcome.
Even with high-quality image guidance and improved motion management techniques, tumor excursion and anatomical shifts can occur resulting in compromised tumor coverage or poor OAR sparing.17,18 While some ongoing randomized pancreatic cancer trials such as LAP-ABLATE (NCT05585554) and GRECO-2 (NCT04698915) require review of on-treatment imaging, this process is not yet a standard part of the clinical trial design. Future clinical trials in pancreas cancer should include verification of on-treatment imaging as part of the QA process.
On post hoc analysis, we identified that more than half of cases treated with radiation had suboptimal plans due either to contouring or coverage deficiencies. One-third of cases had inadequate contours or plan coverage that could have potentially impacted treatment quality and patient outcomes. This is in keeping with data reported from other trials.19 RT QA analysis of NRG/RTOG 9704 showed that unacceptable deviations from the protocol occurred in 42% of cases and these were mostly attributable to incorrect GTV contouring or field design/coverage.4 RT QA analysis of NRG/RTOG 0848 showed that 33% of cases required resubmission due to protocol deviations and the most common reason for resubmission was inappropriate GTV contouring. Detailed educational materials were provided along with contouring guidelines for pancreas cancer,20 but, contouring pancreatic cancer target volumes is challenging. Further, ensuring target coverage while protecting adjacent OARs is similarly challenging. Future trials in the pancreatic cancer space would benefit from more rigorous pretreatment review. This can be accomplished either by having more than one peer reviewer or by having the study principal investigator(s) responsible for the trial design participate in the peer review process. While this may be challenging for larger trials, it is feasible as demonstrated by RTOG 0848 which had four times as many patients enrolled on the RT arm as compared to the Alliance trial.21 The policy at Alliance is for the RT review to be done by a single reviewer so this was not possible on the present trial.
Both the technique and also the optimal target volumes for neoadjuvant radiation therapy for PDAC remain to be defined.2,3,22,23 Alliance A021501 was the first randomized trial using dose-escalated radiation in this setting and targeting only the gross disease with a small margin. There was no elective volume treated on the study and the PTV was created using volumetric expansions of the GTV and TVI. Thus, cases involving a more expansive TVI would necessarily have resulted in more unanticipated “elective” nodal coverage. Recent studies have identified the “triangle” volume as a region associated with the majority of locoregional failures.22,24 Given the variability in RT fields on Alliance A021501, it is possible that some cases included coverage of this region while others did not. While we did not find the treatment technique or target contouring/coverage to have impacted treatment outcomes on this trial, patient numbers were too small for adequate comparison. As we have seen, available technology may limit what can be delivered as far as dose and target coverage, and thus future clinical trial design may benefit from mandating the technology used to treat the patients on trial so that only the intended treatment is delivered. While this would limit the number of sites able to open the trial, more meaningful results may be extracted from clinical trials with a very restrictive design.
The impact of protocol deviations on clinical trial outcomes is not limited to treatment of PDAC. A seminal paper published by Ohri et al. in 2013 demonstrated that across multiple disease sites, RT protocol deviations are associated with increased risk of treatment failure.5 In 2014, the NCI established the IROC service to provide a uniform QA program for all National Clinical Trial Network (NCTN) trials.25 The present study shows that although a uniform QA program exists, there remains room for improvement in the design of future clinical trials to further improve RT quality.
There are several limitations to the present analysis. First, IGRT scans were not available for this QA study, and thus we could not determine if treatment was accurately delivered to the target. Second, assessment of target volume and OAR contours is subjective, both during the real-time pretreatment review and on post hoc analysis, and can be challenging to quantify. We attempted to address this limitation by having 3 radiation oncologists conduct the post hoc review and required majority consensus before a score was assigned. Third, the number of patients was small, which did not allow for meaningful statistical comparisons between subgroups. Therefore, the analyses performed had limited statistical power due to the small sample sizes.
In conclusion, lessons learned from the present analysis can be used to inform future clinical trial designs for BR PDAC utilizing dose-escalated neoadjuvant radiation. Every effort should be made to standardize simulation, immobilization, and on-board imaging procedures, including specifications as far as use of contrast, target localization, and motion management. Improvements in on-board imaging (magnetic resonance imaging and cone-beam CT) may further enhance accuracy allowing for dose escalation and RT delivery and decreasing risk of toxicity. Heterogeneity in dose prescription and target coverage should be minimized. Technologies used to treat patients should be standardized as this can impact the dose delivered and target coverage. An NCI pancreatic cancer working group has been convened to address these concerns and to establish a set of working principles/guidelines pertaining to if and how radiation therapy should be incorporated into future NCTN pancreatic cancer trials.
Supplementary Material
Support:
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Numbers U10CA180821 and U10CA180882 (to the Alliance for Clinical Trials in Oncology), UG1CA189850, UG1CA232760, UG1CA233180, UG1CA233290, UG1CA233329; MC, TJF, and JL 1U24CA180803 (IROC); and U10CA180868 (NRG Oncology). https://acknowledgments.alliancefound.org.The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest:
MC has received grant funding from ViewRay and StrataPharma (to institution), consulting fees from ViewRay, payment/honoraria from ViewRay, University of Toronto, IBA, and Sirtex, and travel support from ViewRay; he participates on an advisory board for ViewRay, and serves on the Board of Directors of the Proton Collaborative Group. KS received funding for the current project through Imaging and Radiation Oncology Core (IROC) grant. EO received consulting fees from Boehringer Ingelheim, BioNTech, Ipsen, Merck, Novartis, AstraZeneca, BioSapien, Astellas, Thetis, Autem, Neogene, BMS, Tempus, Fibrogen, Merus, Agios (spouse), Genentech-Roche (spouse), Eisai (spouse) and research finding to her institution from Genentech/Roche, BioNTech, AstraZeneca, Arcus, Boehringer Ingelheim Pharmaceuticals, Inc, Regeneron Pharmaceuticals, Inc., Hoosier Cancer Research Network, Kronos Bio, and Mirati Therapeutics Inc; Honorarium/speaker role from Chugai Pharmaceutical Co., Ltd (to myself), research funds from Celgene/BMS, Roche/Genentech, Janssen, Novartis (to institution). JM is on the advisory board and has received consulting fees from Merck Pharmaceutical. The remaining authors have nothing to disclose. JH received grant funding from the Canopy Cancer Collective (institution), royalties from Springer for a textbook (self), consulting fees from Histosonics and Boston Scientific (self), and has stock in Histosonics.
Footnotes
Clinical trial information: Alliance A021501, NCT02839343
Data sharing statement:
De-identified patient data may be requested from Alliance for Clinical Trials in Oncology via concepts@alliancenctn.org if data are not publicly available. A formal review process includes verifying the availability of data, conducting a review of any existing agreements that may have implications for the project, and ensuring that any transfer is in compliance with the IRB. The investigator will be required to sign a data release form prior to transfer.
References
- 1.Ghaneh P, Palmer D, Cicconi S, et al. Immediate surgery compared with short-course neoadjuvant gemcitabine plus capecitabine, FOLFIRINOX, or chemoradiotherapy in patients with borderline resectable pancreatic cancer (ESPAC5): a four-arm, multicentre, randomised, phase 2 trial. The Lancet Gastroenterology & Hepatology. doi: 10.1016/S2468-1253(22)00348-X [DOI] [PubMed] [Google Scholar]
- 2.Jang JY, Han Y, Lee H, et al. Oncological Benefits of Neoadjuvant Chemoradiation With Gemcitabine Versus Upfront Surgery in Patients With Borderline Resectable Pancreatic Cancer: A Prospective, Randomized, Open-label, Multicenter Phase 2/3 Trial. Ann Surg. Aug 2018;268(2):215–222. doi: 10.1097/sla.0000000000002705 [DOI] [PubMed] [Google Scholar]
- 3.Versteijne E, van Dam JL, Suker M, et al. Neoadjuvant Chemoradiotherapy Versus Upfront Surgery for Resectable and Borderline Resectable Pancreatic Cancer: Long-Term Results of the Dutch Randomized PREOPANC Trial. J Clin Oncol. Jan 27 2022:Jco2102233. doi: 10.1200/jco.21.02233 [DOI] [PubMed] [Google Scholar]
- 4.Abrams RA, Winter KA, Regine WF, et al. Failure to Adhere to Protocol Specified Radiation Therapy Guidelines Was Associated With Decreased Survival in RTOG 9704—A Phase III Trial of Adjuvant Chemotherapy and Chemoradiotherapy for Patients With Resected Adenocarcinoma of the Pancreas. International Journal of Radiation Oncology*Biology*Physics. 2012/02/01/ 2012;82(2):809–816. doi: 10.1016/j.ijrobp.2010.11.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ohri N, Shen X, Dicker AP, Doyle LA, Harrison AS, Showalter TN. Radiotherapy protocol deviations and clinical outcomes: a meta-analysis of cooperative group clinical trials. J Natl Cancer Inst. 2013;105(6):387–393. doi: 10.1093/jnci/djt001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katz MHG, Shi Q, Meyers J, et al. Efficacy of Preoperative mFOLFIRINOX vs mFOLFIRINOX Plus Hypofractionated Radiotherapy for Borderline Resectable Adenocarcinoma of the Pancreas: The A021501 Phase 2 Randomized Clinical Trial. JAMA oncology. 2022;8(9):1263–1270. doi: 10.1001/jamaoncol.2022.2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ibbott GS. QA in Radiation Therapy: The RPC Perspective. Journal of Physics: Conference Series. 2010/11/01 2010;250(1):012001. doi: 10.1088/1742-6596/250/1/012001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edward SS, Alvarez PE, Taylor PA, et al. Differences in the Patterns of Failure Between IROC Lung and Spine Phantom Irradiations. Practical Radiation Oncology. 2020/09/01/ 2020;10(5):372–381. doi: 10.1016/j.prro.2020.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Katz MHG, Shi Q, Meyers J, et al. Efficacy of Preoperative mFOLFIRINOX vs mFOLFIRINOX Plus Hypofractionated Radiotherapy for Borderline Resectable Adenocarcinoma of the Pancreas: The A021501 Phase 2 Randomized Clinical Trial. JAMA Oncol. Sep 1 2022;8(9):1263–1270. doi: 10.1001/jamaoncol.2022.2319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NCCN Clinical Practice Guideline in Oncology: Pancreatic Adenocarcinoma. Version 2.2022. Accessed April 5, 2023, 2023. https://www.nccn.org/professionals/physician_gls/pdf/pancreatic.pdf [Google Scholar]
- 11.Hauck CL, Kelechi TJ, Cartmell KB, Mueller M. Trial-level factors affecting accrual and completion of oncology clinical trials: A systematic review. Contemporary Clinical Trials Communications. 2021/12/01/ 2021;24:100843. doi: 10.1016/j.conctc.2021.100843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Unger JM, Fleury M. Nationally representative estimates of the participation of cancer patients in clinical research studies according to the commission on cancer. Journal of Clinical Oncology. 2021;39(28_suppl):74–74. doi: 10.1200/JCO.2020.39.28_suppl.74 [DOI] [Google Scholar]
- 13.Zaorsky NG, Zhang Y, Walter V, Tchelebi LT, Chinchilli VM, Gusani NJ. Clinical Trial Accrual at Initial Course of Therapy for Cancer and Its Impact on Survival. 2019;17(11):1309. doi: 10.6004/jnccn.2019.7321 [DOI] [PubMed] [Google Scholar]
- 14.Koay EJ, Hall W, Park PC, Erickson B, Herman JM. The role of imaging in the clinical practice of radiation oncology for pancreatic cancer. Abdom Radiol (NY). Feb 2018;43(2):393–403. doi: 10.1007/s00261-017-1373-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill CS, Han-Oh S, Cheng Z, et al. Fiducial-based image-guided SBRT for pancreatic adenocarcinoma: Does inter-and intra-fraction treatment variation warrant adaptive therapy? Radiation Oncology. 2021/03/19 2021;16(1):53. doi: 10.1186/s13014-021-01782-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chuong MD, Bryant JM, Herrera R, et al. Dose-Escalated Magnetic Resonance Image-Guided Abdominopelvic Reirradiation With Continuous Intrafraction Visualization, Soft Tissue Tracking, and Automatic Beam Gating. Adv Radiat Oncol. Mar-Apr 2022;7(2):100840. doi: 10.1016/j.adro.2021.100840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sharma M, Nano TF, Akkati M, Milano MT, Morin O, Feng M. A systematic review and meta-analysis of liver tumor position variability during SBRT using various motion management and IGRT strategies. Radiother Oncol. Jan 2022;166:195–202. doi: 10.1016/j.radonc.2021.11.022 [DOI] [PubMed] [Google Scholar]
- 18.Cusumano D, Dhont J, Boldrini L, et al. Predicting tumour motion during the whole radiotherapy treatment: a systematic approach for thoracic and abdominal lesions based on real time MR. Radiotherapy and Oncology. 2018/12/01/ 2018;129(3):456–462. doi: 10.1016/j.radonc.2018.07.025 [DOI] [PubMed] [Google Scholar]
- 19.Willett CG, Moughan J, O’Meara E, et al. Compliance with therapeutic guidelines in Radiation Therapy Oncology Group prospective gastrointestinal clinical trials. Radiother Oncol. 2012;105(1):9–13. doi: 10.1016/j.radonc.2012.09.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goodman KA, Regine WF, Dawson LA, et al. Radiation Therapy Oncology Group consensus panel guidelines for the delineation of the clinical target volume in the postoperative treatment of pancreatic head cancer. International journal of radiation oncology, biology, physics. 2012;83(3):901–908. doi: 10.1016/j.ijrobp.2012.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tchelebi LT, Winter KA, Abrams RA, et al. Analysis of radiation therapy quality assurance in NRG Oncology RTOG 0848. International Journal of Radiation Oncology*Biology*Physics. 2023/08/19/ 2023:104910. doi: 10.1016/j.ijrobp.2023.08.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill CS, Fu W, Hu C, et al. Location, Location, Location: What Should be Targeted Beyond Gross Disease for Localized Pancreatic Ductal Adenocarcinoma? Proposal of a Standardized Clinical Tumor Volume for Pancreatic Ductal Adenocarcinoma of the Head: The “Triangle Volume”. Pract Radiat Oncol. May-Jun 2022;12(3):215–225. doi: 10.1016/j.prro.2022.01.005 [DOI] [PubMed] [Google Scholar]
- 23.Chuong MD, Kharofa J, Sanford NN. Elective Target Coverage for Pancreatic Cancer: When Less Does Not Clearly Achieve More. Int J Radiat Oncol Biol Phys. Jan 1 2022;112(1):143–145. doi: 10.1016/j.ijrobp.2021.08.024 [DOI] [PubMed] [Google Scholar]
- 24.Hackert T, Strobel O, Michalski CW, et al. The TRIANGLE operation – radical surgery after neoadjuvant treatment for advanced pancreatic cancer: a single arm observational study. HPB. 2017/11/01/ 2017;19(11):1001–1007. doi: 10.1016/j.hpb.2017.07.007 [DOI] [PubMed] [Google Scholar]
- 25.Followill D, Knopp M, Galvin J, et al. WE-G-141–01: The Imaging and Radiation Oncology Core (IROC) Group: A Proposed New Clinical Trial Quality Assurance Organization. Medical Physics. 2013;40(6Part30):507–507. doi: 10.1118/1.4815652 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified patient data may be requested from Alliance for Clinical Trials in Oncology via concepts@alliancenctn.org if data are not publicly available. A formal review process includes verifying the availability of data, conducting a review of any existing agreements that may have implications for the project, and ensuring that any transfer is in compliance with the IRB. The investigator will be required to sign a data release form prior to transfer.