Abstract
A study of the catecholase activity of a latent plant polyphenol oxidase, extracted and purified from the chloroplast membranes of grapes (Vitis vinifera cv. Airen), revealed for the first time a lag phase above pH 5.0, whereas a steady-state rate was reached immediately when pH values were lower, thus suggesting the hysteretic nature of the enzyme. During steady state, the enzyme showed negative co-operativity concomitant with the presence of the lag period, and followed classical Michaelis-Menten kinetics under more acid pH conditions. Statistical analysis of these data showed a minimal value for the extreme Hill coefficient of 0.54 at pH 6.0. This kinetic behaviour of polyphenol oxidase has been interpreted in terms of the pH-induced 'slow' transition mechanism reported by Ricard, Noat & Nari [(1984) Eur. J. Biochem. 145, 311-317] in which the conformational change does not affect the active site of the enzyme.
Full text
PDF
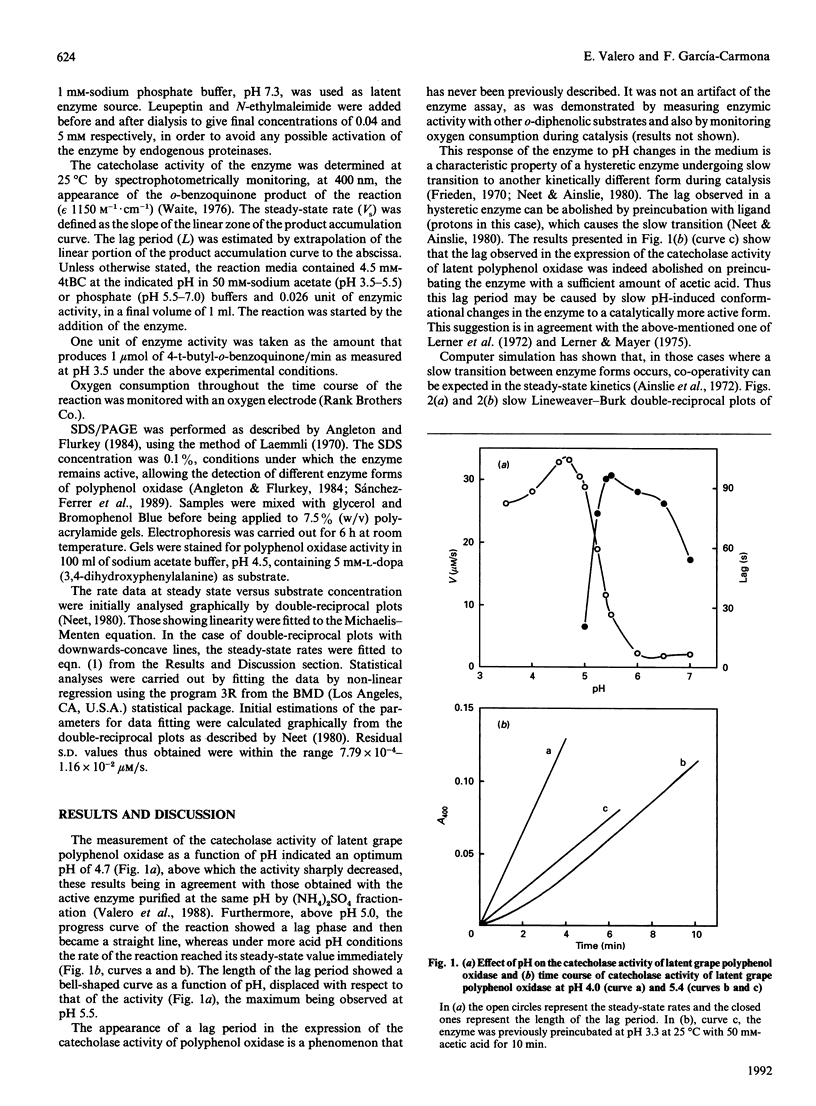
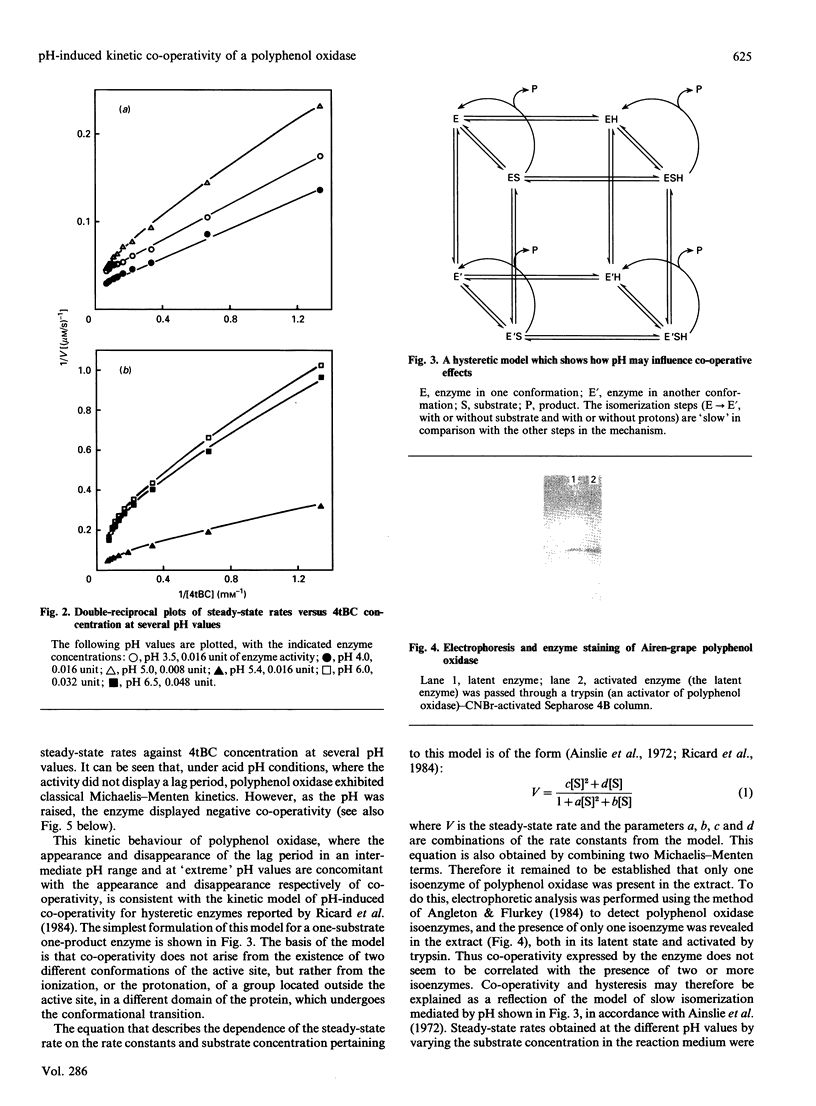
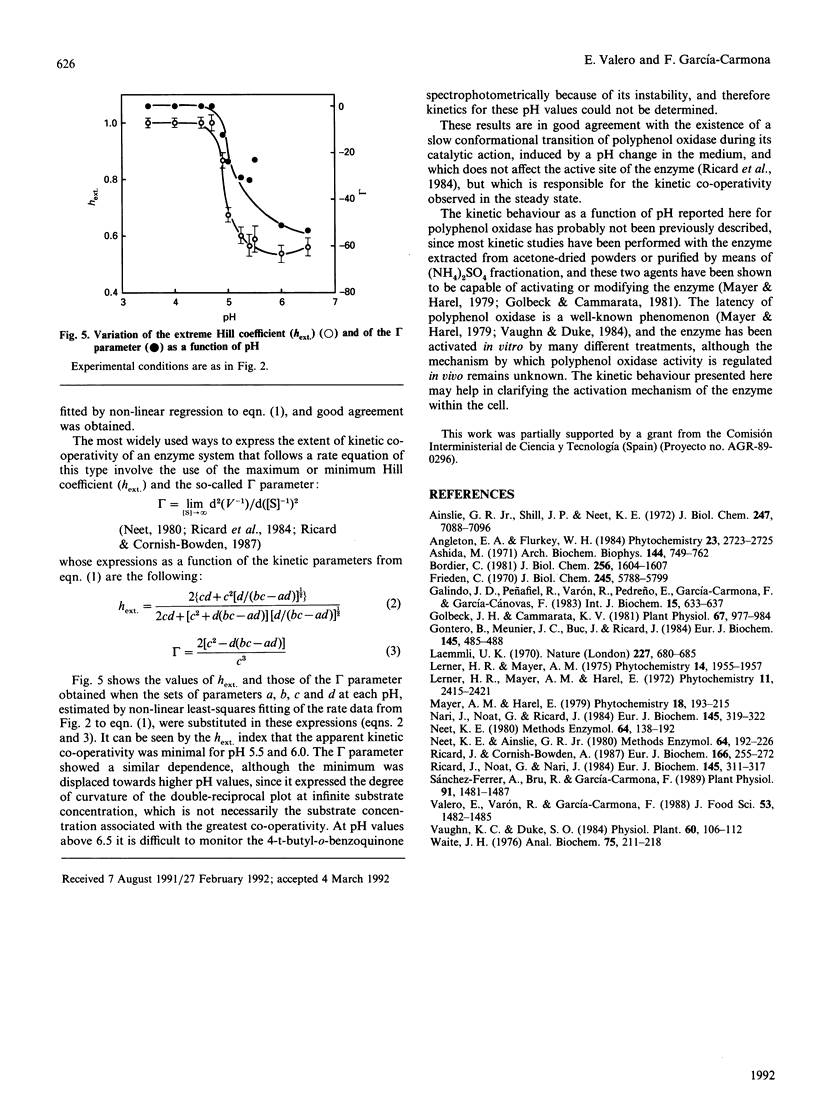
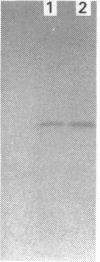
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ainslie G. R., Jr, Shill J. P., Neet K. E. Transients and cooperativity. A slow transition model for relating transients and cooperative kinetics of enzymes. J Biol Chem. 1972 Nov 10;247(21):7088–7096. [PubMed] [Google Scholar]
- Ashida M. Purification and characterization of pre-phenoloxidase from hemolymph of the silkworm Bombyx mori. Arch Biochem Biophys. 1971 Jun;144(2):749–762. doi: 10.1016/0003-9861(71)90383-3. [DOI] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Frieden C. Kinetic aspects of regulation of metabolic processes. The hysteretic enzyme concept. J Biol Chem. 1970 Nov 10;245(21):5788–5799. [PubMed] [Google Scholar]
- Galindo J. D., Peñafiel R., Varon R., Pedreño E., Garcia-Carmona F., García-Cánovas F. Kinetic study of the activation process of frog epidermis pro-tyrosinase by trypsin. Int J Biochem. 1983;15(5):633–637. doi: 10.1016/0020-711x(83)90187-8. [DOI] [PubMed] [Google Scholar]
- Golbeck J. H., Cammarata K. V. Spinach Thylakoid Polyphenol Oxidase : ISOLATION, ACTIVATION, AND PROPERTIES OF THE NATIVE CHLOROPLAST ENZYME. Plant Physiol. 1981 May;67(5):977–984. doi: 10.1104/pp.67.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gontero B., Meunier J. C., Buc J., Ricard J. The 'slow' pH-induced conformational transition of chloroplast fructose 1,6-bisphosphatase and the control of the Calvin cycle. Eur J Biochem. 1984 Dec 17;145(3):485–488. doi: 10.1111/j.1432-1033.1984.tb08582.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Nari J., Noat G., Ricard J. pH-induced co-operative effects in hysteretic enzymes. 2. pH-induced co-operative effects in a cell-wall beta-glucosyltransferase. Eur J Biochem. 1984 Dec 3;145(2):319–322. doi: 10.1111/j.1432-1033.1984.tb08555.x. [DOI] [PubMed] [Google Scholar]
- Neet K. E., Ainslie G. R., Jr Hysteretic enzymes. Methods Enzymol. 1980;64:192–226. doi: 10.1016/s0076-6879(80)64010-5. [DOI] [PubMed] [Google Scholar]
- Ricard J., Cornish-Bowden A. Co-operative and allosteric enzymes: 20 years on. Eur J Biochem. 1987 Jul 15;166(2):255–272. doi: 10.1111/j.1432-1033.1987.tb13510.x. [DOI] [PubMed] [Google Scholar]
- Ricard J., Noat G., Nari J. pH-induced co-operative effects in hysteretic enzymes. 1. A theoretical model of a new type of co-operative behaviour controlled by pH. Eur J Biochem. 1984 Dec 3;145(2):311–317. doi: 10.1111/j.1432-1033.1984.tb08554.x. [DOI] [PubMed] [Google Scholar]
- Sánchez-Ferrer A., Bru R., Garcia-Carmona F. Novel procedure for extraction of a latent grape polyphenoloxidase using temperature-induced phase separation in triton x-114. Plant Physiol. 1989 Dec;91(4):1481–1487. doi: 10.1104/pp.91.4.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite J. H. Calculating extinction coefficients for enzymatically produced o-quinones. Anal Biochem. 1976 Sep;75(1):211–218. doi: 10.1016/0003-2697(76)90072-5. [DOI] [PubMed] [Google Scholar]