Abstract
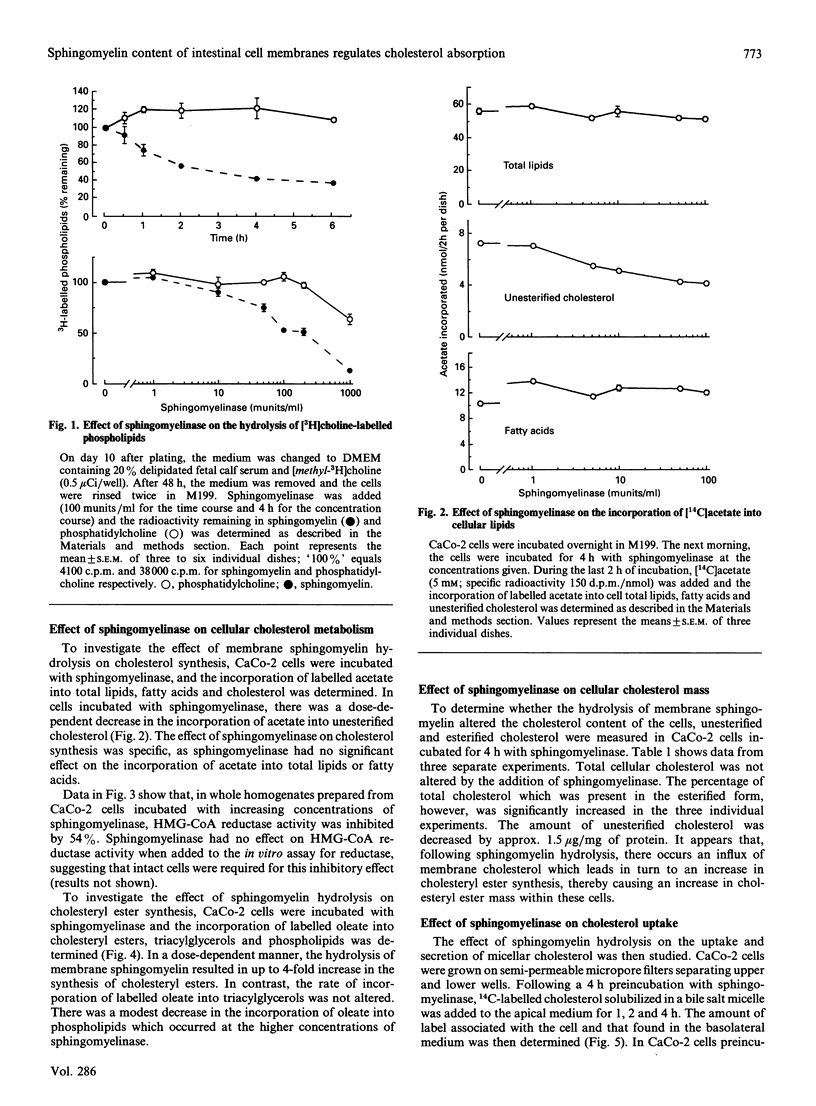
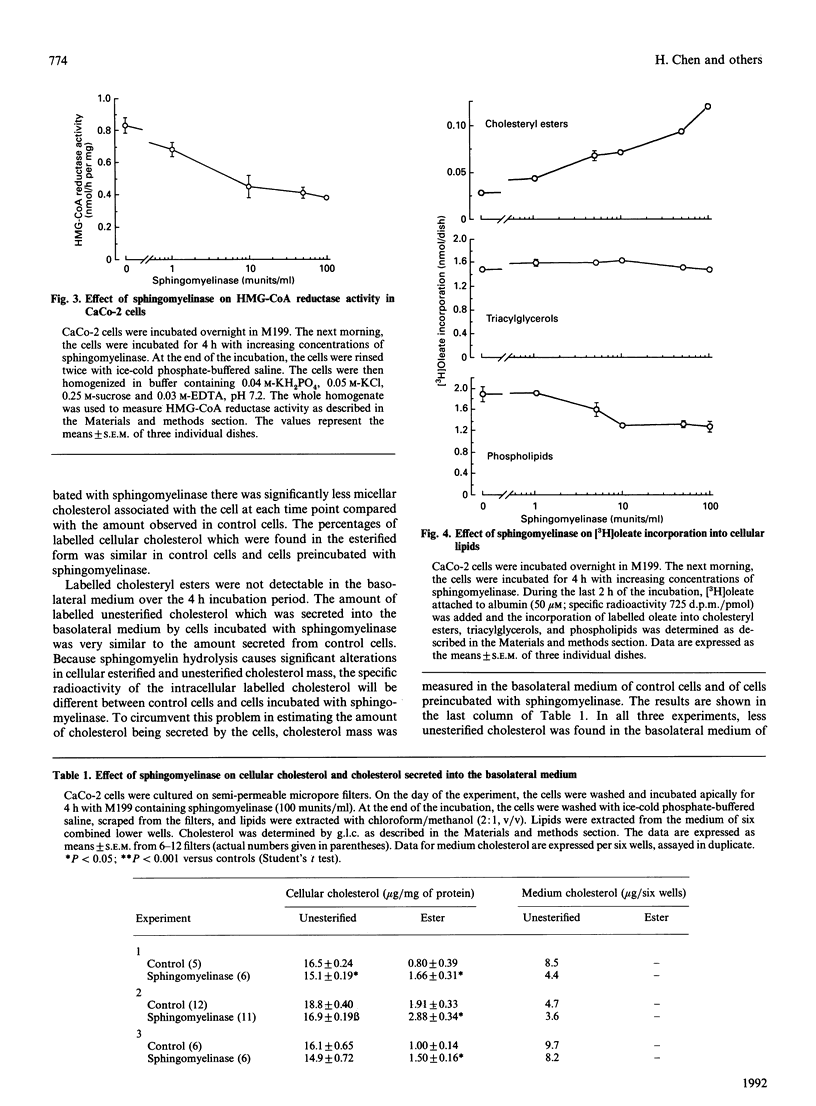
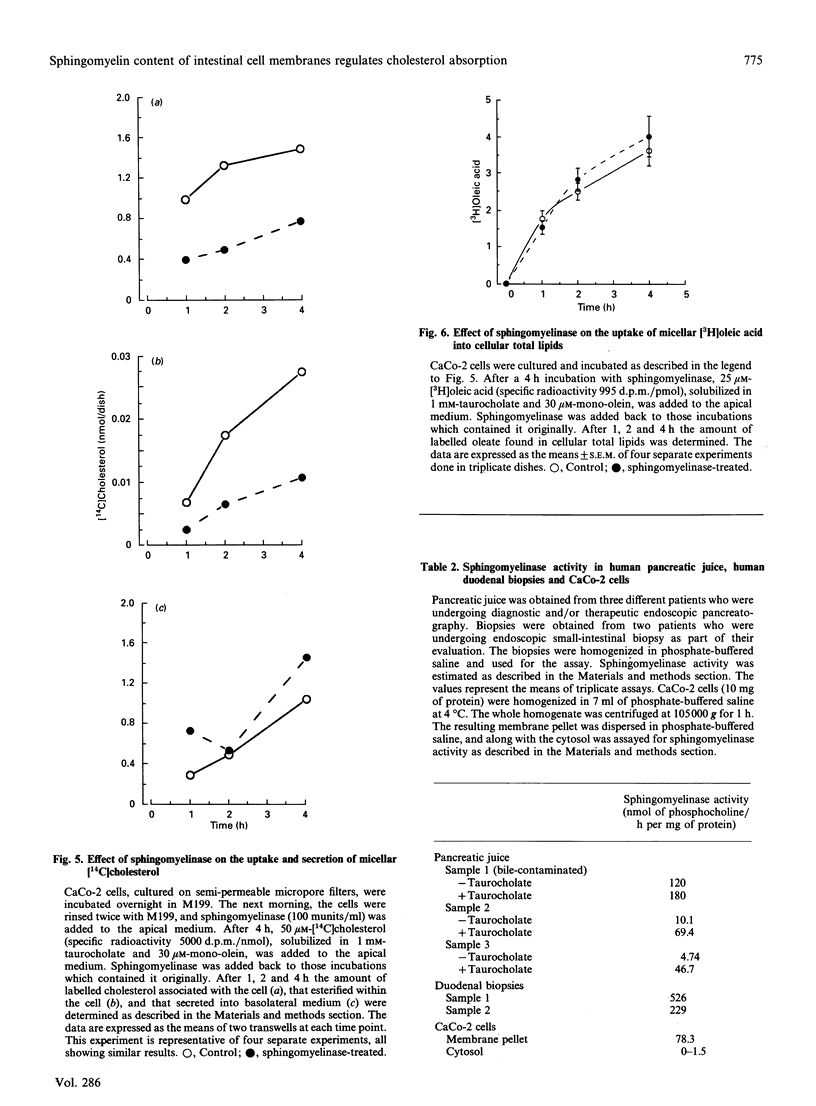
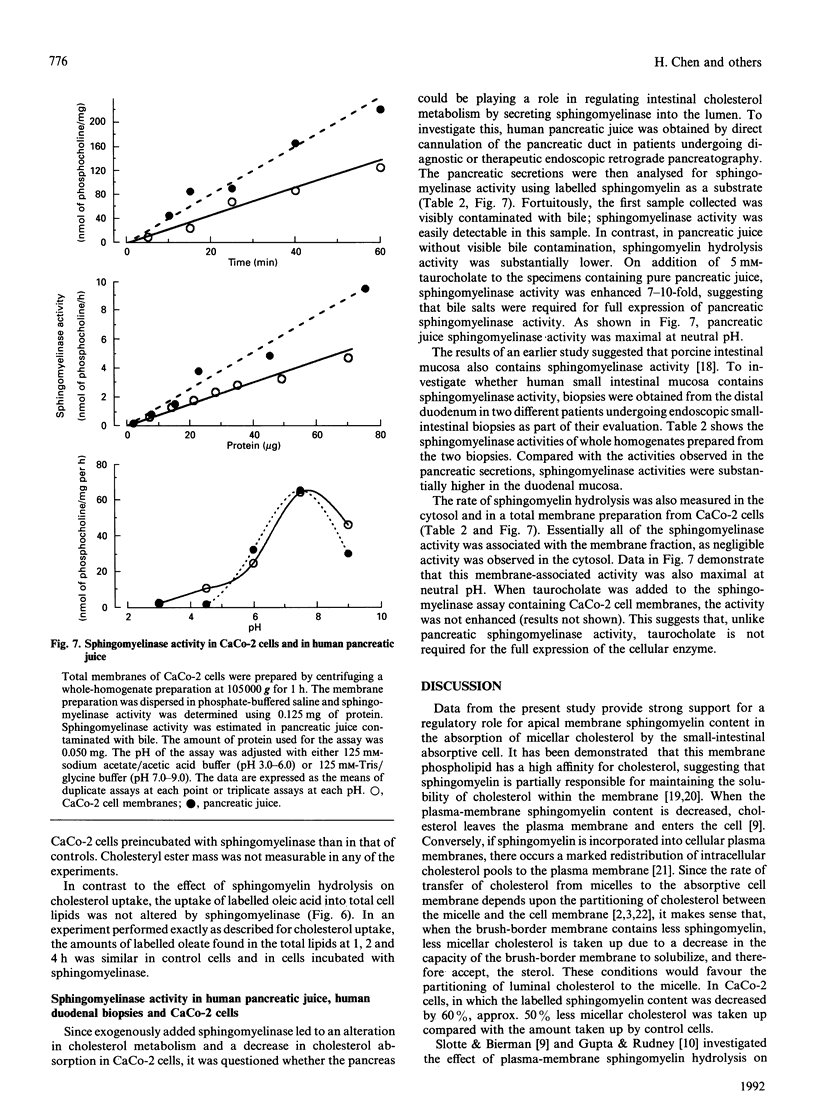
Micellar cholesterol uptake and secretion were investigated in the human intestinal cell line CaCo-2 following depletion of apical membrane sphingomyelin. The addition of exogenous sphingomyelinase, which hydrolysed 60% of prelabelled sphingomyelin, resulted in a 50% decrease in the uptake of cholesterol from bile salt micelles. The flux of membrane cholesterol into the cell by the hydrolysis of membrane sphingomyelin decreased the rate of cholesterol synthesis by 43% and inhibited hydroxymethylglutaryl-CoA reductase activity by 54%. Moreover, the rate of cholesterol esterification was increased 4-fold. Total cellular cholesterol mass was unchanged by the addition of sphingomyelinase; however, cholesteryl esters increased by 50% and the amount of unesterified cholesterol decreased significantly. The basolateral secretion of cholesterol mass was also decreased following sphingomyelin hydrolysis. Human pancreatic juice was found to contain neutral sphingomyelinase activity which required taurocholate for full expression. The presence of neutral sphingomyelinase activity was also documented in membranes prepared from CaCo-2 cells and in whole homogenates from human duodenal biopsies. The data suggest that the amount of sphingomyelin present in the apical membrane of the intestinal absorptive cell regulates cholesterol uptake from bile salt micelles. Sphingomyelinase activity within intestinal cells and in pancreatic juice could alter the sphingomyelin content of brush-border membranes of small intestinal absorptive cells and thus regulate the amount of cholesterol absorbed by the gut.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bosner M. S., Gulick T., Riley D. J., Spilburg C. A., Lange L. G., 3rd Receptor-like function of heparin in the binding and uptake of neutral lipids. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7438–7442. doi: 10.1073/pnas.85.20.7438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Ghosh N. Neutral sphingomyelinase from human urine. Purification and preparation of monospecific antibodies. J Biol Chem. 1989 Jul 25;264(21):12554–12561. [PubMed] [Google Scholar]
- Field F. J., Albright E., Mathur S. N. Regulation of cholesterol esterification by micellar cholesterol in CaCo-2 cells. J Lipid Res. 1987 Sep;28(9):1057–1066. [PubMed] [Google Scholar]
- Field F. J., Albright E., Mathur S. Inhibition of acylcoenzyme A: cholesterol acyltransferase activity by PD128O42: effect on cholesterol metabolism and secretion in CaCo-2 cells. Lipids. 1991 Jan;26(1):1–8. doi: 10.1007/BF02544016. [DOI] [PubMed] [Google Scholar]
- Field F. J. Intestinal cholesterol esterase: intracellular enzyme or contamination of cytosol by pancreatic enzymes? J Lipid Res. 1984 Apr;25(4):389–399. [PubMed] [Google Scholar]
- Field F. J., Kam N. T., Mathur S. N. Regulation of cholesterol metabolism in the intestine. Gastroenterology. 1990 Aug;99(2):539–551. doi: 10.1016/0016-5085(90)91040-d. [DOI] [PubMed] [Google Scholar]
- Field F. J., Mathur S. N. Regulation of acyl CoA:cholesterol acyltransferase by 25-hydroxycholesterol in rabbit intestinal microsomes and absorptive cells. J Lipid Res. 1983 Aug;24(8):1049–1059. [PubMed] [Google Scholar]
- Field F. J., Shreves T., Fujiwara D., Murthy S., Albright E., Mathur S. N. Regulation of gene expression and synthesis and degradation of 3-hydroxy-3-methylglutaryl coenzyme A reductase by micellar cholesterolin CaCo-2 cells. J Lipid Res. 1991 Nov;32(11):1811–1821. [PubMed] [Google Scholar]
- Gatt S., Bierman E. L. Sphingomyelin suppresses the binding and utilization of low density lipoproteins by skin fibroblasts. J Biol Chem. 1980 Apr 25;255(8):3371–3376. [PubMed] [Google Scholar]
- Gupta A. K., Rudney H. Plasma membrane sphingomyelin and the regulation of HMG-CoA reductase activity and cholesterol biosynthesis in cell cultures. J Lipid Res. 1991 Jan;32(1):125–136. [PubMed] [Google Scholar]
- Hyun J., Kothari H., Herm E., Mortenson J., Treadwell C. R., Vahouny G. V. Purification and properties of pancreatic juice cholesterol esterase. J Biol Chem. 1969 Apr 10;244(7):1937–1945. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lange Y., Swaisgood M. H., Ramos B. V., Steck T. L. Plasma membranes contain half the phospholipid and 90% of the cholesterol and sphingomyelin in cultured human fibroblasts. J Biol Chem. 1989 Mar 5;264(7):3786–3793. [PubMed] [Google Scholar]
- Murthy S., Albright E., Mathur S. N., Field F. J. Modification of CaCo-2 cell membrane fatty acid composition by eicosapentaenoic acid and palmitic acid: effect on cholesterol metabolism. J Lipid Res. 1988 Jun;29(6):773–780. [PubMed] [Google Scholar]
- Nilsson A. The presence of spingomyelin- and ceramide-cleaving enzymes in the small intestinal tract. Biochim Biophys Acta. 1969 Mar 4;176(2):339–347. doi: 10.1016/0005-2760(69)90192-1. [DOI] [PubMed] [Google Scholar]
- Slotte J. P., Bierman E. L. Depletion of plasma-membrane sphingomyelin rapidly alters the distribution of cholesterol between plasma membranes and intracellular cholesterol pools in cultured fibroblasts. Biochem J. 1988 Mar 15;250(3):653–658. doi: 10.1042/bj2500653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson A. B., Keelan M., Garg M. L., Clandinin M. T. Intestinal aspects of lipid absorption: in review. Can J Physiol Pharmacol. 1989 Mar;67(3):179–191. doi: 10.1139/y89-031. [DOI] [PubMed] [Google Scholar]
- Thurnhofer H., Hauser H. The uptake of phosphatidylcholine by small intestinal brush border membrane is protein-mediated. Biochim Biophys Acta. 1990 May 24;1024(2):249–262. doi: 10.1016/0005-2736(90)90351-n. [DOI] [PubMed] [Google Scholar]
- Thurnhofer H., Hauser H. Uptake of cholesterol by small intestinal brush border membrane is protein-mediated. Biochemistry. 1990 Feb 27;29(8):2142–2148. doi: 10.1021/bi00460a026. [DOI] [PubMed] [Google Scholar]
- Thurnhofer H., Schnabel J., Betz M., Lipka G., Pidgeon C., Hauser H. Cholesterol-transfer protein located in the intestinal brush-border membrane. Partial purification and characterization. Biochim Biophys Acta. 1991 May 7;1064(2):275–286. doi: 10.1016/0005-2736(91)90312-v. [DOI] [PubMed] [Google Scholar]
- Wattenberg B. W., Silbert D. F. Sterol partitioning among intracellular membranes. Testing a model for cellular sterol distribution. J Biol Chem. 1983 Feb 25;258(4):2284–2289. [PubMed] [Google Scholar]
- van Blitterswijk W. J., van der Meer B. W., Hilkmann H. Quantitative contributions of cholesterol and the individual classes of phospholipids and their degree of fatty acyl (un)saturation to membrane fluidity measured by fluorescence polarization. Biochemistry. 1987 Mar 24;26(6):1746–1756. doi: 10.1021/bi00380a038. [DOI] [PubMed] [Google Scholar]


