Abstract
Immune checkpoint inhibitors (ICIs) have become an important cornerstone of many tumour treatments. However, the toxicity profile of immune-chemotherapy combination treatment approaches among older adult cancer patients is still unclear. Patients with any cancer who received camrelizumab-based immunotherapy were eligible for inclusion. The primary endpoints were adverse events (AEs) and immune-related adverse events (irAEs), which were defined based on Naranjo's algorithm. Patients were stratified by age (≥ 70 years and < 70 years), and comparisons were made based on the type of camrelizumab-based therapy (monotherapy, combined chemotherapy, or combined anti-VEGF therapy). A total of 185 patients were administered camrelizumab-based immunotherapy, 55 (30%) of whom were ≥ 70 years old. A total of 146 (78.9%) patients received camrelizumab-based combination treatment. The incidence of all-grade AEs was 56.8% (105 patients), while that of irAEs was 36.8% (68 patients). There was no difference in the percentage of patients experiencing any grade or grade ≥ 3 AEs between age groups. However, the frequency of irAEs (both any grade and grade ≥ 3) significantly differed by age group (P = 0.001 and 0.009, respectively). The results of multivariable analysis revealed that age ≥ 70 years was the only independent risk factor for irAEs. The results of subgroup analysis revealed that the incidence of irAEs was higher in older patients treated with camrelizumab-chemotherapy, while the incidence rates were similar between age groups in the monotherapy and combination anti-VEGF treatment subgroups. Immune-related diabetes mellitus occurred more frequently among older adults. The spectrum of irAEs showed that combination immunotherapy had more widely effects on the organ system than monotherapy. In this study, older (≥ 70 years) patients had a higher risk of all-grade and high-grade irAEs when receiving camrelizumab chemotherapy combination treatment. Notably, long-term random glucose monitoring should be performed during ICI-based immunotherapy in older cancer patients.
Keywords: Immune checkpoint inhibitors, Ageing, Immunotherapy, Cancer, Toxicity
Subject terms: Cancer, Health care, Medical research, Oncology
Introduction
Immune checkpoint inhibitors (ICIs) have significantly improved treatment outcomes for patients with multiple tumour types and have become an important cornerstone of many tumour treatments. Programmed cell death protein 1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA4), expressed on the surface of activated T-cells, are important immune checkpoints for maintaining immune tolerance during normal T-cell activation1. Activation of effector T lymphocytes by ICI can reverse immune escape, thereby restoring antitumour immunity and leading to tumour cell death2. Meanwhile, the over-activated immune system can cause the development of immune-related adverse events (irAEs), affecting multiple organ systems3,4. However, many patients failed to respond to the monotherapy with ICIs due to primary or acquired resistance5. Combining immunotherapy with chemotherapy6 or antiangiogenic agents7 can improve cancer immunotherapy efficacy.
As individuals age, the functions of multiple organ become impaired, which is known as immunosenescence. Preclinical studies suggested that aging is associated with remodeling of T cell immunity, increased pool of PD-1 + T cells and the expression of PD-L1/PD-L2 on antigen-presenting cells8. Aging affects not only the immune system, but also other multiple organ systems, which might lead to serious irAEs during drug therapy in older adults9. Moreover, ICIs combination therapy, comorbidities, underlying diseases, geriatric impairments and aging are also risk factors for the irAEs10–12.
According to the available clinical studies data, the effect of single-agent immunotherapy among elderly patients seems to be similar to that among younger patients without excess toxicity13–15. In contrast, the true impact of using immune-chemotherapy combinations in the elderly population, especially the safety profile of this approach, remains unclear16. Clinical trials have been conducted to evaluate the efficacy of immunotherapy plus chemotherapy as a first-line treatment for various types of tumours, including PD-1/PD-L1 inhibitors; however, these trials did not provide safety data stratified by patient age17–20. Moreover, clinical trials of combination regimens with ICIs have not assessed toxicity characteristics based on patient age; thus, data in this setting are largely lacking21.
Camrelizumab, an anti-PD-1 antibody22, has been approved for the treatment of classical Hodgkin’s lymphoma, advanced live cancer, advanced oesophageal cancer, recurrent or metastatic nasopharyngeal carcinoma, and advanced non-small-cell lung cancer in China based on its positive efficacy in clinical trials23–28 (see Supplementary Table 1 for detailed indications). The present study aimed to describe the toxicity of camrelizumab in older adults with advanced cancer (patients older than 70 years of age)29,30 in order to complement the safety data of immune-based combination use in older cancer patients.
Materials and methods
Study design and sample
This was a single-centre, retrospective cohort study of adult patients with any cancer who received camrelizumab-based immunotherapy in the standard of care, clinical trial, and off-label settings between January 1, 2019, and June 30, 2021. All patients were treated at the First Hospital of Quanzhou Affiliated to Fujian Medical University, which is a tertiary general hospital. The data were collected from our institutional database and from electronic medical records. Patient demographic data, pathologic information, and clinical characteristics were retrieved. The clinical data included age, sex, Eastern Cooperative Oncology Group performance status (ECOG PS) score, Charlson Comorbidity Index (CCI) score, cancer type, cancer stage, and line of systemic therapy. Detailed information regarding irAEs, including date of diagnosis, attribution, and clinical interventions, was abstracted using information available in clinical documentation by investigators with a background in oncology. The data cut-off date was August 15, 2021. The inclusion criteria were as follows: (1) age ≥ 18 years; (2) cytological or histological confirmation of solid malignancy; (3) locally advanced or metastatic cancer; (4) received camrelizumab-based immunotherapy; and (5) completed at least one cycle of immunotherapy. Patients with missing values or those without available data about AEs were excluded. All prescriptions were submitted electronically.
Toxicities
Immune-related adverse events (irAEs) were defined and graded according to the Society for Immunotherapy of Cancer (SITC) consensus definitions and National Comprehensive Cancer Network Clinical Practice Guidelines31,32, and other adverse drug reactions (ADRs) were defined and graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events, version 533. Naranjo’s causality assessment was used to determine whether camrelizumab was a definite, probable, possible, or doubtful cause of the immune-related toxicities34. The immune-related toxicities were assigned to a probability category from the total score as follows: definite if the overall score was 9 or greater, probable for a score of 5–8, possible for 1–4, and doubtful if the score was 0. The prevalence of toxicities as possible, probable, or definite irAEs was calculated.
Statistical analysis
Statistical analysis was performed using SPSS version 21.0 (SPSS, Inc., Chicago, IL, USA). Baseline demographic, clinical, and toxicity characteristics were summarized and compared between patients aged < 70 years and those aged ≥ 70 years using chi-square or Fisher’s exact tests. Multivariable analyses were performed to determine those variables that significantly contributed to the irAEs. The odds ratios for the independent variables were generated by logistic regression. A two-tailed P < 0.05 was considered to indicate statistical significance in all tests.
Ethical statements
The study was performed in accordance with the guidelines outlined in the Declaration of Helsinki and was approved by the Ethics Committee of the First Hospital of Quanzhou Affiliated to Fujian Medical University. Since the study was retrospective, most of the study subjects have died or been lost to follow-up, and all data were collected anonymously; therefore, the Ethics Committee of the First Hospital of Quanzhou Affiliated to Fujian Medical University agreed to waive the need for informed consent. All methods were performed in accordance with the relevant guidelines and regulations.
Results
Baseline characteristics
We identified 185 patients who received camrelizumab, 55 (30%) of whom were ≥ 70 years old and 78% of whom were male. Among all patients, 97.3% had advanced cancer (stage III/IV), 29.7% had colorectal cancer, 23.2% had lung cancer, and 16.8% had oesophageal cancer. Camrelizumab combination is used off-label for colorectal cancer and gastric cancer. The majority of patients received camrelizumab-based combination treatment (78.9%), including combined chemotherapy (59.4%) and combined anti-VEGF therapy (19.5%). The cohort of patients aged ≥ 70 years versus < 70 years did not differ significantly with respect to sex, ECOG performance status, CCI score, cancer type, cancer stage, or line of systemic therapy. Patient and treatment characteristics are detailed in Table 1.
Table 1.
Patient characteristics (N = 185).
| Patient characteristic | < 70 years (N = 130) | ≥ 70 years (N = 55) | P value | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Sex | 0.605 | ||||
| Male | 97 | 74.6 | 43 | 78.2 | |
| Female | 33 | 25.4 | 12 | 21.8 | |
| ECOG PS | |||||
| 0 | 29 | 22.3 | 11 | 20.0 | 0.075 |
| 1 | 40 | 30.8 | 14 | 25.5 | |
| 2 | 44 | 33.8 | 14 | 25.5 | |
| 3 | 17 | 13.1 | 16 | 29.1 | |
| CCI | |||||
| 0 | 51 | 39.2 | 14 | 25.5 | 0.062 |
| 1 | 42 | 32.3 | 16 | 29.1 | |
| ≥ 2 | 37 | 28.7 | 25 | 45.6 | |
| Cancer type | 0.103 | ||||
| Colorectal | 39 | 30.0 | 16 | 29.1 | |
| Lung | 23 | 17.7 | 20 | 36.4 | |
| Oesophageal | 27 | 20.8 | 4 | 7.3 | |
| Liver | 13 | 10.0 | 4 | 7.3 | |
| Gastric | 13 | 10.0 | 6 | 10.9 | |
| Head and neck | 3 | 2.3 | 1 | 1.8 | |
| Other | 12 | 9.2 | 4 | 7.3 | |
| Cancer stage | 0.216 | ||||
| 3 | 7 | 5.4 | 5 | 9.1 | |
| 4 | 120 | 92.3 | 48 | 87.3 | |
| Other | 2 | 1.5 | 3 | 5.5 | |
| Line of systemic therapy | 0.474 | ||||
| First | 81 | 62.3 | 39 | 70.9 | |
| Second | 27 | 20.8 | 10 | 18.2 | |
| Third or above | 22 | 16.9 | 6 | 10.9 | |
| Immuno-based therapy | 0.755 | ||||
| Monotherapy | 25 | 19.2 | 14 | 25.5 | |
| Combined chemotherapy | 78 | 60.0 | 32 | 58.2 | |
| Combined anti-VEGF therapy | 27 | 20.8 | 9 | 16.4 | |
ECOG PS Eastern Cooperative Oncology Group Performance Status; CCI Charlson Comorbidity Index.
Toxicity
As shown in Table 2, 56.8% of 185 patients had any grade of AEs, while 18.9% had grade ≥ 3 AEs. The frequency of AEs (both any grade and grade ≥ 3) was not significantly different between age groups. A total of 36.8% of such patients had any grade of irAEs, while 13.5% had grade ≥ 3 irAEs. The results of causality between irAEs and camrelizumab using Naranjo’s causality assessment score are shown in Supplementary Table 2. Compared with younger patients, older adults experienced a significantly higher rate of any grade and grade ≥ 3 irAEs (P = 0.001 and 0.009, respectively).
Table 2.
Toxicity summary.
| < 70 years | ≥ 70 years | P value | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Any grade AE | 69 | 53.1 | 36 | 65.5 | 0.12 |
| ≥ Grade 3 AE | 20 | 15.4 | 15 | 27.3 | 0.059 |
| Any grade irAE | 38 | 29.2 | 30 | 54.5 | 0.001 |
| Monotherapy | 7 | 28 | 8 | 57.1 | 0.073 |
| Combined chemotherapy | 23 | 29.5 | 19 | 59.4 | 0.003 |
| Combined anti-VEGF therapy | 8 | 29.6 | 3 | 33.3 | 0.835 |
| ≥ Grade 3 irAE | 12 | 9.2 | 13 | 23.6 | 0.009 |
| Monotherapy | 3 | 12.0 | 4 | 28.6 | 0.196 |
| Combined chemotherapy | 8 | 10.2 | 8 | 25 | 0.046 |
| Combined anti-VEGF therapy | 1 | 3.7 | 1 | 11.1 | 0.401 |
| Fatal irAE | 3 | 2.3 | 1 | 1.8 | 0.834 |
| Any grade irAE type | |||||
| Pneumonitis | 4 | 3.1 | 2 | 3.6 | 0.844 |
| Myocarditis | 5 | 3.8 | 3 | 5.5 | 0.923 |
| Thyroiditis | 17 | 13.1 | 6 | 10.9 | 0.683 |
| Diabetes mellitus | 0 | 0.0 | 4 | 7.3 | 0.011 |
| Hepatitis | 7 | 5.4 | 8 | 14.5 | 0.073 |
| Dermatitis | 4 | 3.1 | 6 | 10.9 | 0.708 |
| RCCEP | 2 | 1.5 | 5 | 9.1 | 0.119 |
| Renal toxicity | 6 | 4.6 | 0 | 0.0 | 0.244 |
| Myasthenia gravis | 0 | 0.0 | 1 | 1.8 | 0.507 |
| Ocular toxicity | 0 | 0.0 | 1 | 1.8 | 0.297 |
| Infusion-related reactions | 4 | 3.1 | 1 | 1.8 | 1 |
| Diarrhea/colitis | 3 | 2.3 | 0 | 0.0 | 0.556 |
| Pancreatitis | 0 | 0.0 | 1 | 1.8 | 0.297 |
| Hematotoxicity | 3 | 2.3 | 5 | 9.1 | 0.093 |
RCCEP, reactive cutaneous capillary endothelial proliferation.
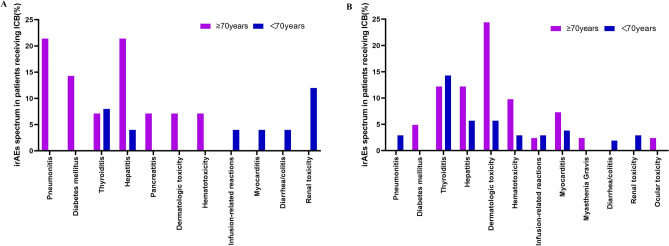
According to the subgroup analysis, the incidence of any grade and grade ≥ 3 irAEs was higher in older patients treated with camrelizumab-chemotherapy (P = 0.003 and 0.046, respectively), while the incidence of monotherapy and combination anti-VEGF treatment were similar. The spectrum of irAEs revealed that combination immunotherapy affected organ systems more widely than monotherapy (Fig. 1). The rates of each toxicity type of any grade irAE were similar except for diabetes mellitus, which had a higher frequency among older adults (P = 0.011). Further analysis of the types of immune-related adverse events was performed, with patients subdivided based on the specific treatments used (Supplementary Table 3). No significant differences were found in the use of immune chemotherapy among older adults.
Figure1.
Spectrum of irAEs for patients with advanced cancer receiving (A) mono- or (B) combination camrelizumab immuno.
Notably, although the percentage of patients with fatal irAEs was not significantly different between age groups (P > 0.05), all 4 deaths (2.2%) occurred in the immune-chemotherapy group, including 3 patients aged < 70 years (2 patients with pneumonitis and 1 patient with severe liver injury) and 1 patient aged ≥ 70 years (myasthenia gravis).
In addition, there was one patient with a mild binocular optic disc lesion aged ≥ 70 years who was treated with camrelizumab monotherapy, which was not described in the drug labelling section. One patient with oesophageal squamous cancer with lung metastasis developed pulmonary tuberculosis during 3 cycles (105 days) of first-line combined chemoimmunotherapy treatment.
Multivariable analysis
The multivariable analysis of associations between study variables and the development of irAEs is shown in Table 3. Age ≥ 70 years was the only independent risk factor for all-grade irAEs (OR 2.66, 95% CI 1.29–5.50, P = 0.008) and grade ≥ 3 irAEs (OR 2.76, 95% CI 1.05–7.26, P = 0.04) after controlling for the covariates (sex, ECOG PS, CCI score, cancer type, cancer stage, line of systemic therapy, or treatment category).
Table 3.
Multivariable analysis for irAE.
| Variable | Any grade irAE | Grade 3 irAE | ||
|---|---|---|---|---|
| OR (95% CI) | P value | OR (95% CI) | P value | |
| Male | 1.06 (0.47–2.41) | 0.892 | 1.30 (0.40–4.26) | 0.665 |
| ECOG PS (0) | ||||
| 1 | 1.25 (0.42–3.69) | 0.685 | 1.00 (0.21–4.74) | 0.996 |
| 2 | 1.01 (0.31–3.26) | 0.984 | 1.15 (0.23–5.81) | 0.869 |
| 3 | 0.97 (0.25–3.74) | 0.962 | 0.66 (0.09–4.42) | 0.664 |
| CCI(0) | ||||
| 1 | 1.14 (0.51–2.55) | 0.748 | 0.99 (0.29–3.40) | 0.992 |
| ≥ 2 | 1.56 (0.71–3.43) | 0.264 | 1.79 (0.60–5.31) | 0.295 |
| Cancer type (Colorectal) | ||||
| Lung | 1.86 (0.62–5.60) | 0.266 | 1.76 (0.38–8.05) | 0.468 |
| Esophageal | 1.32 (0.39–4.47) | 0.659 | 1.04 (1.16–6.57) | 0.968 |
| Liver | 1.02 (0.21–5.06) | 0.978 | 1.78 (0.21–15.03) | 0.598 |
| Gastric | 1.15 (0.32–4.21) | 0.828 | 1.68 (0.28–9.98) | 0.570 |
| Head and neck | 0.76 (0.06–9.54) | 0.829 | NE | 0.999 |
| Other | 2.06 (0.52–8.15) | 0.305 | 0.59 (0.05–6.67) | 0.674 |
| Cancer stage (3) | ||||
| 4 | 1.31 (0.09–18.70 | 0.840 | 0.35 (0.02–8.44) | 0.520 |
| Other | 2.77 (0.27–28.10) | 0.390 | 0.72 (0.06–8.48) | 0.792 |
| Line of systemic therapy (First) | ||||
| Second | 1.06 (0.45–2.50) | 0.894 | 1.07 (0.33–3.51) | 0.910 |
| Third or above | 0.70 (0.23–2.14) | 0.535 | 0.83 (0.15–4.69) | 0.837 |
| Immuno-based therapy (Monotherapy) | ||||
| Combined chemotherapy | 1.07 (0.43–2.71) | 0.881 | 1.02 (0.29–3.60) | 0.972 |
| Combined anti-VEGF therapy | 0.78 (0.27–2.25) | 0.641 | 0.28 (0.05–1.59) | 0.153 |
| Age ≥ 70 years | 2.71 (1.31–5.60) | 0.007 | 2.78 (1.06–7.32) | 0.038 |
Reference values for each variable are listed in parentheses.
Discussion
Immune checkpoint inhibitors are being widely used for treating a range of malignancies. They are effective at treating certain cancers but can be associated with a spectrum of immune-related adverse events. However, to our knowledge, few real-world data are available about irAEs associated with immune-based combinations in older adult patients. This real-world retrospective study evaluated the toxicity of camrelizumab-based immunotherapy in older adults with advanced cancer and showed that treatment with camrelizumab in combination with chemotherapy was associated with increased incidences rates of and risks for irAEs among older patients, whereas treatment with monotherapy and combined targeted therapies had similar effects.
Previous studies on ICIs in older adult patients have focused mostly on PD-(L)1 and/or CTLA4, which used different age cut-offs to define subgroups and demonstrated that the immune-related toxicity of mono-immunotherapy was similar for older patients13–15,35,36. Chemotherapeutic and targeted agents can improve the efficacy of ICIs37 by inducing immunogenic cell death, promoting CD8 + T cell infiltration, or inhibiting immunosuppressive cells in the tumour microenvironment37,38. Currently, several chemo-immunotherapy combinations have been clinically approved to improve patient survival, but this has been accompanied by more serious treatment-related adverse events18,20,24,25. Notably, in these studies, there are no reference to toxicity in the group of older patients.
A real-world study included 299 patients receiving first-line chemo-immunotherapy combination of pembrolizumab with pemetrexed, and platinum for Non-squamous NSCLC39. The rate of severe AEs was higher in the older patients than in younger patients (26% versus 19%) with 75 years cut-off, although the difference was not significant, the AE-related treatment discontinuation was significantly higher in older patients than in younger patients. Johns reported no difference in the percentage of patients with G3 toxicity between younger (< 70 years) and older (≥ 70 years) patients in a retrospective cohort study of 673 patients with advanced cancer, with only 1.2% of patients receiving ICIs combined with chemotherapy40. In our study, although the difference was not significant, the rate of severe AEs was higher in the older patients than in younger patients (27.3% versus 15.4%) using 70 years cut-off with 60% receiving ICIs combined with chemotherapy.
We used Naranjo’s causality assessment to evaluate whether camrelizumab was a definite, probable, possible, or doubtful cause of immune-related toxicity and calculated the prevalence of toxicity as a possible, probable, or definite irAE. The results showed that for combined chemotherapy, the incidence of irAEs was greater for patients aged ≥ 70 years, while the incidence of irAEs for patients treated with combined anti-vascular targeting drugs was not significantly different for patients aged ≥ 70 years. The main reason for this may be that the anti-VEGF drugs involved in the study was mainly apatinib (a VEGFR-2 tyrosine kinase inhibitor), which has been shown to improve the efficacy and decrease the incidence of camrelizumab-specific reactive cutaneous capillary endothelial proliferation41. Thus, although the incidence of irAEs was higher in patients receiving the ICIs plus anti-VEGF regimens, the difference was not significant. The toxicity profile of the camrelizumab-chemo combination was greater in patients aged ≥ 70 years than in younger patients, probably because of impaired multi-organ function in older people9. However, due to the diverse types of irAEs involved in this study and the relatively small number of patients treated with different immunotherapies, no increase in the incidence of irAEs associated with a specific type of ICI combination regimen was found.
Our study revealed that diabetes mellitus occurred more frequently in older patients, which is similar to the Chans’s study42, but different from the results of the previous study40. One possible explanation for the discrepancy between our results and previous studies is that the previous studies had a high percentage of melanoma patients. A systematic review showed that melanoma patients had a higher frequency of skin irAE42. Of the 185 patients, 4 patients with no prior history developed diabetes mellitus with a median duration of 9.67 months, all of whom were aged ≥ 70 years. One 75-year-old patient who was critically ill (with a glucose concentration of 394 mg/dL) was treated with camrelizumab monotherapy for 4.83 months. Subsequently, this patient was diagnosed with diabetic ketoacidosis after an emergency evaluation. The patient’s condition improved after receiving standard treatment.
ICI-associated diabetes mellitus (ICI-DM), an endocrine toxicity of ICIs that possibly mediated through increased proliferation of auto reactive T cells, occurs in slightly less than 1% of patients treated with ICIs and is been increasing in recognition and diagnosis43–45. Chans reported that older patients (≥ 65 years) had a higher risk of diabetes mellitus, with an incidence of 2.9%46.In the real-life setting with different ages, the incidence of ICI-DM was 2.2%, and two cases occurred several months after camrelizumab cessation. Therefore, ICI-DM occurs over a long period of time, and has a better prognosis with symptomatic treatment. Long-term random glucose monitoring should be performed during the use of ICIs in older cancer patients.
Despite these strengths, there are several notable limitations. First, the limitations of this study are attributed to potential confounding bias from the retrospective nature of the study with a small sample size. Second, the process of extracting patient characteristics and toxicity information was limited due to the quality of the available documentation. This limitation was potentially mitigated by the focus on ≥ G3 toxicities, which are more likely to be comprehensively documented in medical records. In addition, the potential impacts of tumour type on combination immunotherapy were not taken into account due to the small sample size. An important future direction related to this limitation would be the analysis of different ICI classes and tumour types in older patients.
In conclusion, all-grade irAEs and high-grade irAEs were more common in older adults with advanced cancer treated with a camrelizumab-chemotherapy combination approach. More attention should be devoted to diabetes mellitus in older adults despite the low prevalence of this condition in this population. Our pharmacovigilance study provides additional safety data on the toxicity profile of immune-based combination treatments among older cancer patients.
Supplementary Information
Author contributions
H.Z.: Conceptualization, Methodology, Software, Writing- Original draft preparation. L.L.C. and F.Y.L Conceptualization, Data. J.J.M: Supervision, Writing- Reviewing and Editing.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-69944-w.
References
- 1.Cook, S. L. et al. Immune checkpoint inhibitors in geriatric oncology. Curr. Oncol. Rep.26(5), 562–572 (2024). 10.1007/s11912-024-01528-3 [DOI] [PubMed] [Google Scholar]
- 2.Martins, F. et al. Adverse effects of immune-checkpoint inhibitors: Epidemiology, management and surveillance. Nat. Rev. Clin. Oncol.16(9), 563–580 (2019). 10.1038/s41571-019-0218-0 [DOI] [PubMed] [Google Scholar]
- 3.Okiyama, N. & Tanaka, R. Immune-related adverse events in various organs caused by immune checkpoint inhibitors. Allergol. Int.71(2), 169–178 (2022). 10.1016/j.alit.2022.01.001 [DOI] [PubMed] [Google Scholar]
- 4.Shankar, B. et al. Multisystem immune-related adverse events associated with immune checkpoint inhibitors for treatment of non-small cell lung cancer. JAMA Oncol.6(12), 1952–1956 (2020). 10.1001/jamaoncol.2020.5012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Passaro, A. et al. Managing resistance to immune checkpoint inhibitors in lung cancer: Treatment and novel strategies. J. Clin. Oncol.40(6), 598–610 (2022). 10.1200/JCO.21.01845 [DOI] [PubMed] [Google Scholar]
- 6.Ogawara, D. et al. Presence of few PD-1-expressing tumor-infiltrating immune cells is a potential predictor of improved response to salvage chemotherapy following nivolumab for non-small cell lung cancer: An exploratory case series. Thorac. Cancer9(10), 1305–1311 (2018). 10.1111/1759-7714.12844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Song, Y. et al. Anti-angiogenic agents in combination with immune checkpoint inhibitors: A promising strategy for cancer treatment. Front. Immunol.11, 1956 (2020). 10.3389/fimmu.2020.01956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Han, S. et al. Age-associated remodeling of T cell immunity and metabolism. Cell Metab.35(1), 36–55 (2023). 10.1016/j.cmet.2022.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikeda, H. & Togashi, Y. Aging, cancer, and antitumor immunity. Int. J. Clin. Oncol.27(2), 316–322 (2022). 10.1007/s10147-021-01913-z [DOI] [PubMed] [Google Scholar]
- 10.Oren, O. et al. Cardiovascular health and outcomes in cancer patients receiving immune checkpoint inhibitors. Am. J. Cardiol.125(12), 1920–1926 (2020). 10.1016/j.amjcard.2020.02.016 [DOI] [PubMed] [Google Scholar]
- 11.Zamami, Y. et al. Factors associated with immune checkpoint inhibitor-related myocarditis. JAMA Oncol.5(11), 1635–1637 (2019). 10.1001/jamaoncol.2019.3113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Özkan, A. et al. Geriatric predictors of response and adverse events in older patients with cancer treated with immune checkpoint inhibitors: A systematic review. Crit. Rev. Oncol. Hematol.194, 104259 (2024). 10.1016/j.critrevonc.2024.104259 [DOI] [PubMed] [Google Scholar]
- 13.Corbaux, P. et al. Older and younger patients treated with immune checkpoint inhibitors have similar outcomes in real-life setting. Eur. J. Cancer121, 192–201 (2019). 10.1016/j.ejca.2019.08.027 [DOI] [PubMed] [Google Scholar]
- 14.Nebhan, C. A. et al. Clinical outcomes and toxic effects of single-agent immune checkpoint inhibitors among patients aged 80 years or older with cancer: A multicenter international cohort study. JAMA Oncol.7(12), 1856–1861 (2021). 10.1001/jamaoncol.2021.4960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Landre, T. et al. Immune checkpoint inhibitors for patients aged ≥ 75 years with advanced cancer in first- and second-line settings: A meta-analysis. Drugs Aging37(10), 747–754 (2020). 10.1007/s40266-020-00788-5 [DOI] [PubMed] [Google Scholar]
- 16.Casaluce, F. & Gridelli, C. Combined chemo-immunotherapy in advanced non-small cell lung cancer: Feasible in the elderly?. Expert Opin. Emerg. Drugs28(2), 121–127 (2023). 10.1080/14728214.2023.2211346 [DOI] [PubMed] [Google Scholar]
- 17.Gandhi, L. et al. Pembrolizumab plus chemotherapy in metastatic non-small-cell lung cancer. N. Engl. J. Med.378(22), 2078–2092 (2018). 10.1056/NEJMoa1801005 [DOI] [PubMed] [Google Scholar]
- 18.Paz-Ares, L. et al. Pembrolizumab plus chemotherapy for squamous non-small-cell lung cancer. N. Engl. J. Med.379(21), 2040–2051 (2018). 10.1056/NEJMoa1810865 [DOI] [PubMed] [Google Scholar]
- 19.West, H. et al. Atezolizumab in combination with carboplatin plus nab-paclitaxel chemotherapy compared with chemotherapy alone as first-line treatment for metastatic non-squamous non-small-cell lung cancer (IMpower130): A multicentre, randomised, open-label, phase 3 trial. Lancet Oncol.20(7), 924–937 (2019). 10.1016/S1470-2045(19)30167-6 [DOI] [PubMed] [Google Scholar]
- 20.Jotte, R. et al. Atezolizumab in combination with carboplatin and nab-paclitaxel in advanced squamous NSCLC (IMpower131): Results from a randomized phase III trial. J. Thorac. Oncol. Off. Publication Int. Assoc. Study Lung Cancer15(8), 1351–1360 (2020). 10.1016/j.jtho.2020.03.028 [DOI] [PubMed] [Google Scholar]
- 21.Tagliamento, M. et al. The use of immunotherapy in older patients with advanced non-small cell lung cancer. Cancer Treat. Rev.106, 102394 (2022). 10.1016/j.ctrv.2022.102394 [DOI] [PubMed] [Google Scholar]
- 22.Song, H. et al. Current status and prospects of camrelizumab, a humanized antibody against programmed cell death receptor 1. Recent Pat. Anticancer Drug Discov.16(3), 312–332 (2021). 10.2174/22123970MTE09MDYg0 [DOI] [PubMed] [Google Scholar]
- 23.Qin, S. et al. Camrelizumab plus rivoceranib versus sorafenib as first-line therapy for unresectable hepatocellular carcinoma (CARES-310): A randomised, open-label, international phase 3 study. Lancet402(10408), 1133–1146 (2023). 10.1016/S0140-6736(23)00961-3 [DOI] [PubMed] [Google Scholar]
- 24.Zhou, C. et al. Camrelizumab plus carboplatin and pemetrexed as first-line treatment for advanced nonsquamous NSCLC: Extended follow-up of CameL phase 3 trial. J. Thorac. Oncol.18(5), 628–639 (2023). 10.1016/j.jtho.2022.12.017 [DOI] [PubMed] [Google Scholar]
- 25.Yang, Y. et al. Camrelizumab versus placebo in combination with gemcitabine and cisplatin as first-line treatment for recurrent or metastatic nasopharyngeal carcinoma (CAPTAIN-1st): A multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol.22(8), 1162–1174 (2021). 10.1016/S1470-2045(21)00302-8 [DOI] [PubMed] [Google Scholar]
- 26.Luo, H. et al. Effect of camrelizumab vs placebo added to chemotherapy on survival and progression-free survival in patients with advanced or metastatic esophageal squamous cell carcinoma: The ESCORT-1st randomized clinical trial. JAMA326(10), 916–925 (2021). 10.1001/jama.2021.12836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang, J. et al. Camrelizumab versus investigator’s choice of chemotherapy as second-line therapy for advanced or metastatic oesophageal squamous cell carcinoma (ESCORT): A multicentre, randomised, open-label, phase 3 study. Lancet Oncol.21(6), 832–842 (2020). 10.1016/S1470-2045(20)30110-8 [DOI] [PubMed] [Google Scholar]
- 28.Ren, S. et al. Camrelizumab plus carboplatin and paclitaxel as first-line treatment for advanced squamous NSCLC (CameL-Sq): A phase 3 trial. J. Thorac. Oncol.17(4), 544–557 (2022). 10.1016/j.jtho.2021.11.018 [DOI] [PubMed] [Google Scholar]
- 29.Wildiers, H. et al. International society of geriatric oncology consensus on geriatric assessment in older patients with cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol.32(24), 2595–2603 (2014). 10.1200/JCO.2013.54.8347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Donovan, A., Mohile, S. G. & Leech, M. Expert consensus panel guidelines on geriatric assessment in oncology. Eur. J. Cancer Care24(4), 574–589 (2015). 10.1111/ecc.12302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Naidoo, J. et al. Society for Immunotherapy of Cancer (SITC) consensus definitions for immune checkpoint inhibitor-associated immune-related adverse events (irAEs) terminology. J. Immunother. Cancer11(3), e006398 (2023). 10.1136/jitc-2022-006398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thompson, J. A. et al. Management of immunotherapy-related toxicities, version 1.2022, NCCN clinical practice guidelines in oncology. J. Natl. Compr. Cancer Netw.20(4), 387–405 (2022). 10.6004/jnccn.2022.0020 [DOI] [PubMed] [Google Scholar]
- 33.Freites-Martinez, A. et al. Using the common terminology criteria for adverse events (CTCAE-version 5.0) to evaluate the severity of adverse events of anticancer therapies. Actas Dermo Sifiliogr.112(1), 90–92 (2021). 10.1016/j.ad.2019.05.009 [DOI] [PubMed] [Google Scholar]
- 34.Naranjo, C. A. et al. A method for estimating the probability of adverse drug reactions. Clin. Pharmacol. Ther.30(2), 239–245 (1981). 10.1038/clpt.1981.154 [DOI] [PubMed] [Google Scholar]
- 35.Samani, A. et al. Impact of age on the toxicity of immune checkpoint inhibition. J. Immunother. Cancer8(2), e000871 (2020). 10.1136/jitc-2020-000871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baldini, C. et al. Impact of aging on immune-related adverse events generated by anti-programmed death (ligand)PD-(L)1 therapies. Eur. J. Cancer129, 71–79 (2020). 10.1016/j.ejca.2020.01.013 [DOI] [PubMed] [Google Scholar]
- 37.Li, J. Y. et al. Chemotherapeutic and targeted agents can modulate the tumor microenvironment and increase the efficacy of immune checkpoint blockades. Mol. Cancer20(1), 27 (2021). 10.1186/s12943-021-01317-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Heinhuis, K. M. et al. Enhancing antitumor response by combining immune checkpoint inhibitors with chemotherapy in solid tumors. Ann. Oncol.30(2), 219–235 (2019). 10.1093/annonc/mdy551 [DOI] [PubMed] [Google Scholar]
- 39.Fujimoto, D. et al. A real-world study on the effectiveness and safety of pembrolizumab plus chemotherapy for nonsquamous NSCLC. JTO Clin. Res. Rep.3(2), 100265 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johns, A. C. et al. Checkpoint inhibitor immunotherapy toxicity and overall survival among older adults with advanced cancer. J. Geriatr. Oncol.12(5), 813–819 (2021). 10.1016/j.jgo.2021.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu, B. & Sun, H. C. Camrelizumab: An investigational agent for hepatocellular carcinoma. Expert Opin. Investig. Drugs31(4), 337–346 (2022). 10.1080/13543784.2022.2022121 [DOI] [PubMed] [Google Scholar]
- 42.Khoja, L. et al. Tumour- and class-specific patterns of immune-related adverse events of immune checkpoint inhibitors: A systematic review. Ann. Oncol.28(10), 2377–2385 (2017). 10.1093/annonc/mdx286 [DOI] [PubMed] [Google Scholar]
- 43.Wright, J. J., Powers, A. C. & Johnson, D. B. Endocrine toxicities of immune checkpoint inhibitors. Nat. Rev. Endocrinol.17(7), 389–399 (2021). 10.1038/s41574-021-00484-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wright, J. J. et al. Increased reporting of immune checkpoint inhibitor-associated diabetes. Diabetes Care41(12), e150–e151 (2018). 10.2337/dc18-1465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martinov, T. et al. PD-1 pathway-mediated regulation of islet-specific CD4(+) T cell subsets in autoimmune diabetes. Immunoendocrinology (Houst)3, e1164 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chan, W. L. et al. Immune-related endocrine dysfunctions in combined modalities of treatment: Real-world data. Cancers (Basel)13(15), 3797 (2021). 10.3390/cancers13153797 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.