Abstract
Central neuropathic pain arises from a lesion or disease of the central somatosensory nervous system such as brain injury, spinal cord injury, stroke, multiple sclerosis or related neuroinflammatory conditions. The incidence of central neuropathic pain differs based on its underlying cause. Individuals with spinal cord injury are at the highest risk; however, central post-stroke pain is the most prevalent form of central neuropathic pain worldwide. The mechanisms that underlie central neuropathic pain are not fully understood, but the pathophysiology likely involves intricate interactions and maladaptive plasticity within spinal circuits and brain circuits associated with nociception and antinociception coupled with neuronal hyperexcitability. Modulation of neuronal activity, neuron–glia and neuro-immune interactions and targeting pain-related alterations in brain connectivity, represent potential therapeutic approaches. Current evidence-based pharmacological treatments include antidepressants and gabapentinoids as first-line options. Non-pharmacological pain management options include self-management strategies, exercise and neuromodulation. A comprehensive pain history and clinical examination form the foundation of central neuropathic pain classification, identification of potential risk factors and stratification of patients for clinical trials. Advanced neurophysiological and neuroimaging techniques hold promise to improve the understanding of mechanisms that underlie central neuropathic pain and as predictive biomarkers of treatment outcome.
Introduction
Central neuropathic pain (CNP) is pain caused by a lesion or disease of the central somatosensory nervous system1,2 (Box 1). Lesions of various aetiologies (for example, traumatic, vascular, inflammatory) leading to brain injury or spinal cord injury (SCI), stroke, multiple sclerosis and related neuroinflammatory conditions can cause CNP2,3. CNP is characterized by spontaneous and ongoing pain or intermittent pain4 and may be accompanied by evoked pain, such as allodynia or hyperalgesia to thermal and mechanical stimuli. In rare cases, evoked pain is present without spontaneous pain. Pain is typically felt in areas with altered sensation, particularly to thermal stimuli. A common clinical phenomenon in patients with CNP is a gradient of pain intensity from proximal to distal body regions, with greater pain intensity distally5,6 (Fig. 1). CNP must be distinguished from other sources of pain present among individuals with neurological conditions2,3. For example, although indirectly related to central neurological damage, spasticity-related pain that is driven by nociceptor activation in muscles and joints as a consequence of involuntary muscle contractions is a form of nociceptive pain1. CNP comprises several pain conditions that arise from primary pathologies of the central somatosensory system. CNP is distinct from secondary central neuroplastic changes observed across many pain conditions7. For example, the sensitivity of the nociceptive system may change as a result of sustained afferent input, namely, central sensitization. Although such adaptive and maladaptive changes involve the central somatosensory nervous system and may contribute to the maintenance of CNP, these alterations constitute mechanisms that are not exclusive to CNP8.
Box 1. Key terms.
Paina
An unpleasant sensory and emotional experience associated with, or resembling that associated with, actual or potential tissue damage240.
Central neuropathic paina
Pain caused by a lesion or disease of the central somatosensory nervous systema.
The term central neuropathic pain replaces previous terms: thalamic pain syndrome, Dejerine–Roussy syndrome, deafferentation syndrome, dysaesthetic pain, anaesthesia dolorosa.
Allodyniaa
Pain due to a stimulus that does not normally provoke pain.
Central sensitizationa
Increased responsiveness of nociceptive neurons in the central nervous system to their normal or subthreshold afferent input.
Dysaesthesiaa
An unpleasant abnormal sensation, whether spontaneous or evoked.
Hyperalgesiaa
Increased pain from a stimulus that normally provokes pain.
Hyperpathiaa
A painful syndrome characterized by an abnormally painful reaction to a stimulus, especially a repetitive stimulus, as well as an increased threshold.
Neuronal hyperexcitability
An umbrella term for a broad range of alterations in the response properties of neurons that can arise when the membrane resting potential is lowered, including spontaneous discharges and after-discharges after stimulation, often with irregular firing patterns (sometimes referred to as ectopic activity), and increased responsiveness to both noxious and non-noxious input. These changes can result from various pathophysiological mechanisms but can also be induced from natural or experimental conditions that lead to short-term changes.
Nociceptiona
The neural process of encoding noxious stimuli.
Nociceptive paina
Pain that arises from actual or threatened damage to non-neural tissue and is caused by the activation of nociceptors.
Paraesthesiaa
An abnormal sensation, whether spontaneous or evoked.
Somatosensory nervous system
The somatosensory nervous system lacks a current universally accepted definition. As a working definition, in the context of neuropathic pain, we define it to encompass the ascending, sensory-discriminative neural pathways related to tactile, thermal, proprioceptive, visceral and nociceptive encoding and processing.
Spontaneous pain
Pain that is experienced as ongoing or intermittent without any apparent overt stimulus or trigger. This type of pain can fluctuate over time, and pain exacerbations may occur with stress, illness or environmental factors (for example, weather). Mechanistically, spontaneous pain may be generated autonomously by spontaneous neuronal activity or maintained dynamically by interoceptive or exteroceptive inputs.
a Official terminology of the International Association for the Study of Pain241.
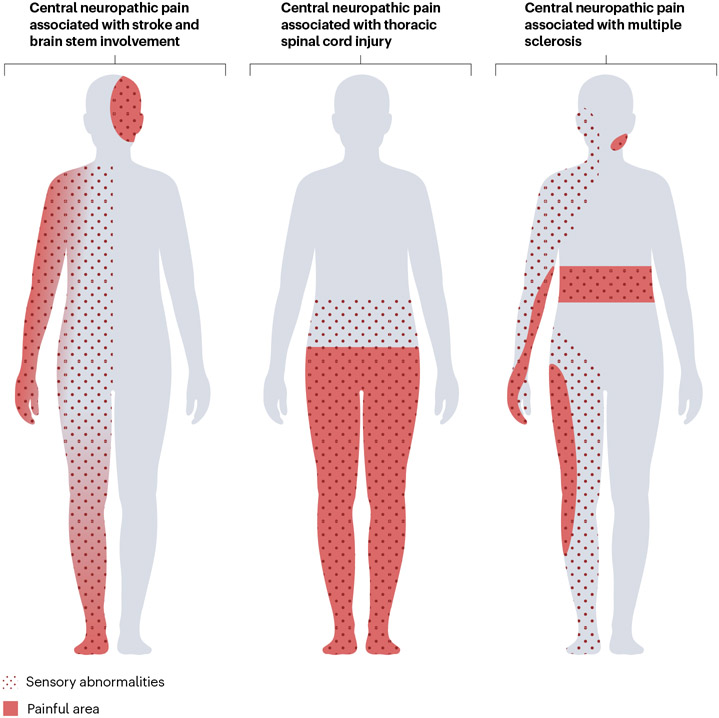
Fig. 1 ∣. Distribution of sensory abnormalities and central neuropathic pain.
In a ventral view of the human body, the distribution of pain and sensory abnormalities associated with central neuropathic pain conditions are presented. Sensory abnormalities and pain are present contralateral to the lesion in stroke but may affect the ipsilateral face in the case of brainstem lesions. After spinal cord injury, the location of pain and sensory abnormalities can be at or below the lesion level. In multiple sclerosis, the location varies depending on the site of the central nervous system lesions, for example, facial pain in trigeminal neuralgia or segmental belt-like pain in the case of spinal cord involvement. Sensory abnormalities (dotted area) often extend beyond the painful area (red). The locations of pain and sensory abnormalities can vary substantially between individuals, with the possibility of uni- or bilateral distribution. Thus, these illustrations aim to depict examples of neuroanatomically plausible locations rather than typical presentations. The proximal to-distal gradient in pain intensity as demonstrated in the left body chart, wherein pain intensity gradually increases towards the distal extremity, is observed in some patients.
CNP has a profound and negative impact on quality of life, sleep and mood, which is largely attributable to the severity of CNP symptoms, their effect on activities of daily living and general refractoriness to interventions4. Challenges in developing effective treatment options — pharmacological or non-pharmacological — is owed in part to a limited understanding of complex pathophysiological mechanisms3.
In this Primer, we provide an overview of the epidemiology, clinical presentation, diagnostic approaches and pathophysiological mechanisms of common CNP conditions. Although acknowledging pain as a multidimensional experience with complex interactions across motivational-affective, cognitive-evaluative and sensory-discriminative dimensions, we focus on sensory-discriminative mechanisms and pathways. Furthermore, we discuss the current pharmacological and non-pharmacological treatments as well as highlight the importance of advancing diagnostic and treatment strategies to address the unmet clinical needs in this field.
Epidemiology
The Global Burden of Disease study does not include specific data for CNP and, therefore, the global prevalence of CNP is unknown. Owing to the sheer number of new stroke cases reported each year (for example, ~12 million cases worldwide in 2019)9, central post-stroke pain (CPSP) is the most common form of CNP worldwide10. The pooled prevalence of CPSP in individuals with stroke is estimated to be 11% (95% CI 7–18%), affecting up to 50% of patients after medullary, thalamic and operculo-insular strokes11,12. In the case of SCI, a meta-analysis of eight studies yielded an overall prevalence estimate of 53% (95% CI 39–67%) for neuropathic pain, which in most cases is CNP but may be peripheral neuropathic pain in some owing to nerve root lesions13. Currently, data on CNP in individuals after traumatic brain injury (TBI) are limited14,15. Among patients with multiple sclerosis, estimates from a meta-analysis indicate that the prevalence of different CNP types, such as neuropathic extremity pain, Lhermitte sign (electric shock-like sensation that originates in the neck and extends down the spine, occasionally reaching the arms and legs) and trigeminal neuralgia is 26% (five studies, 95% CI 7–53%), 16% (six studies, 95% CI 10–25%) and 3.8% (seven studies, 95% CI 2–6%), respectively16. The association between multiple sclerosis lesion location in the brain and spinal cord and the presence and type of CNP is currently unknown17,18. CNP seems to be more frequent in individuals with neuromyelitis optica spectrum disorders (NMOSDs; up to 80% of cases) than in patients with multiple sclerosis, but larger epidemiological studies are warranted to confirm these numbers19. At present, no studies have determined the prevalence or the type of brain injury that is most commonly associated with CNP.
The temporal progression of CNP after stroke and SCI has been extensively characterized20-24. Based on longitudinal studies, CNP may be present at onset or can develop over the course of weeks to months, with signs and symptoms often persisting with varying degrees of severity for the remainder of life21,22,25. Very little is understood regarding the progression of CNP in multiple sclerosis and related neuroinflammatory conditions in comparison with the development of CNP after SCI and stroke26. In addition, data on the duration of pain persistence after TBI are also scarce.
Risk factors
Risk factors associated with CNP are poorly understood. In particular, no studies have investigated genetic risk factors linked to CNP. Thermal sensory loss and damage to the spinothalamic tract or its projections are considered requisite but insufficient for development of CNP27,28. This finding implies that not every patient with a spinothalamic tract lesion and corresponding sensory loss develops CNP. Age and sex, as well as the severity of damage in the central nervous system (CNS) seem to have little to no consistent association with the development of CNP29-31. As in nearly all chronic pain conditions, CNP is strongly associated with depression and anxiety29,30. However, whether psychiatric conditions worsen CNP or CNP exacerbates psychological functioning remains to be elucidated. A limited number of studies, typically in a small number of patients, have examined objective biomarkers to predict the future onset of CNP, yielding mixed outcomes32-35. Thoracic spinal lesion in neuroinflammatory conditions such as NMOSD have been linked to high CNP scores (Brief Pain Inventory’s (BPI) Pain Severity Index)19,36. However, to date, the only well-established predictor of future CNP is initial hypersensitivity to mechanical and thermal stimulation22,37,38.
Mechanisms/pathophysiology
Despite advances in our understanding of CNP mechanisms, why some patients with CNS lesions develop pain whereas others do not is yet unknown. Mechanisms (and treatment) of neuropathic pain are hypothesized to largely overlap across different CNP conditions and between CNP and peripheral neuropathic pain. Nevertheless, mechanisms could possibly differ if the underlying pathophysiology is due to different initiating events (for example, inflammation versus an injury). Besides, the exact sequences involved in the development and maintenance of CNP are incompletely understood. Studies have suggested that mechanisms are linked to different pain phenotypes instead of being linked to underlying neurological condition39-41. Despite this understanding, a thorough knowledge regarding the link between pain phenotypes and mechanisms is still lacking.
Damage to the spinothalamic–thalamocortical projections leads to a decrease in pain perception and temperature perception and the paradoxical development of CNP27,35. Although damage to the ascending nociceptive pathway aligns with the clinical hallmark of sensory loss, the resulting CNP phenotype is associated with additional complex changes along the nociceptive neuroaxis42. Consequently, such damage is widely regarded as requisite, albeit not sufficient, to result in CNP42. Beyond frank neurological damage, a myriad of mechanisms, including deafferentation, ion channel dysfunction, neuro-immune interactions and inflammation, imbalance of inhibitory and excitatory tone, and glial cell activation, have been shown to be part of this complex pathophysiological cascade of CNP8,42-44. These mechanisms are believed to converge and contribute to the development of neuronal hyperexcitability — the electrophysiological correlate of the clinical signs and symptoms observed in spontaneous and evoked CNP in humans45 (Fig. 2).
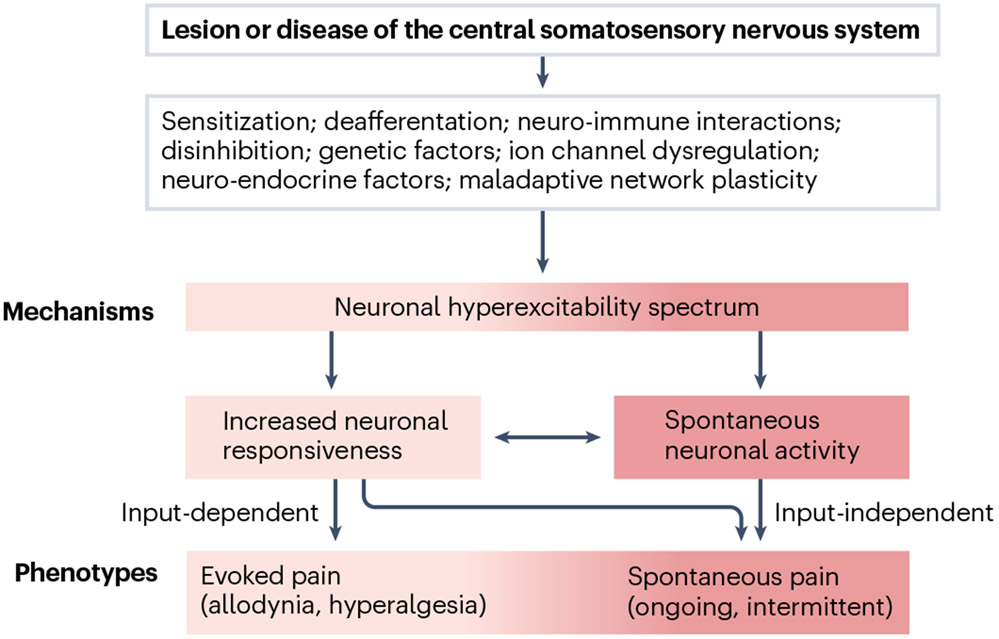
Fig. 2 ∣. From sensory-discriminative mechanisms to the clinical pain phenotype.
A lesion or disease of the central somatosensory nervous system initiates a pathophysiological cascade that involves various mechanisms potentially dependent on injury type and with largely unknown interactions. These mechanisms may, to some extent, converge to modify neuronal excitability. Dynamic changes in the response properties and excitability of central somatosensory neurons can result in two types of pain — spontaneous pain and evoked pain (allodynia, hyperalgesia) and/or spontaneous or evoked dysaesthesia (not shown). Evoked pain depends on preserved afferent input (input-dependent). Spontaneous pain may arise independently of afferent input or may be (partially) maintained by continuous exteroceptive and/or interoceptive input, which may otherwise not be consciously perceived by the patient.
Our understanding of pathophysiological mechanisms largely comes from animal studies that involve both peripheral neuropathic pain and CNP. Insights from these studies are briefly discussed here and they have been extensively covered elsewhere42,45,46.
Changes in neuronal properties
Spontaneous and evoked CNP involve a spectrum of changes in neuronal excitability that result from a primary lesion or disease of the CNS, in addition to induced changes in neuronal responses and CNS plasticity8 (Fig. 2). Spontaneous ectopic discharges within central nociceptive pathways, owing to a direct lesion or secondary to deafferentation, can be sufficient to generate spontaneous pain in the absence of any overt external stimulus45. In support of this hypothesis, one study demonstrated spontaneous and evoked neuronal activity several spinal levels above a SCI, with ablation of the dorsal root entry zone in these segments resulting in pain relief47. Ectopic activity originating from deafferented thalamic neurons or supraspinal neurons may also contribute to CNP48,49, although the role of bursting activity in pain pathophysiology is unclear50. A notable example of such a ‘central pattern generating process’51 can be observed in painful epileptic seizures, in which abnormal neuronal activity in a specific site within the CNS, namely, the operculo-insular cortex, is sufficient to cause pain52. Moreover, studies have reported that tumours within the operculo-insular cortex can cause CNP53. Additionally, direct electrical stimulation of the posterior insula in humans was shown to elicit pain during stimulation, supporting that pain can be generated by activity in these brain areas54. Overall, CNP tends to be more prevalent when the injury involves specific regions salient to nociceptive processing than in other brain regions. Brain regions involved in nociceptive processing include the ventral and posterior anterior pulvinar of the thalamic complex, the posterior insula and medial parietal operculum and the connecting brain areas55,56.
Increased neuronal responsiveness along the nociceptive neuroaxis to preserved afferent input is believed to underlie evoked pain, including phenomena such as allodynia and hyperalgesia57 (Fig. 2). Notably, increased neuronal responsiveness could also explain spontaneous pain in some patients. Abnormally increased responses in central nociceptive networks to continuous (physiological) peripheral afferent input from cutaneous and deep-tissue thermosensory or mechanoreceptive afferents may be the underlying mechanism58. In support of this concept, blockade of peripheral input with peripheral anaesthetic nerve blocks led to complete pain relief in some patients with CPSP59. Moreover, longitudinal clinical data in patients with SCi suggest a potential spectrum of neuronal hyperexcitability, that is, a putatively increased responsiveness and/or spontaneous activity of nociceptive neurons. This process, in turn, translates into the clinical pain phenotype of evoked and spontaneous pain. Early sensory hypersensitivity, for example, evoked pain or dysaesthesia (abnormal unpleasant sensation such as burning or pricking sensations) might be the clinical correlate of increased neuronal responsiveness (Fig. 2). Over time, potentially as a consequence of central neuroplastic changes, the neuronal excitability increases or spontaneous neuronal activity develops, which then manifests clinically as spontaneous ongoing pain38,60 (Fig. 2).
Alterations in neuronal signalling.
Neuronal hyperexcitability can be the consequence of loss of inhibition or processes that increase neuronal responsiveness. For instance, owing to post-translational modifications, enhanced signalling through glutamate receptors, particularly N-methyl-d-aspartate (NMDA) and/or α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, at synapses in the ascending nociceptive pathways61 as well as altered activity of voltage-gated sodium62,63, potassium or calcium channels64, can lead to increased neuronal responsiveness8,42,45 (Fig. 3). Excitotoxicity, resulting from reduced uptake or reduced buffering of the excitatory neurotransmitter glutamate in astrocytes is another mechanism that can contribute to neuronal hyperexcitability65.
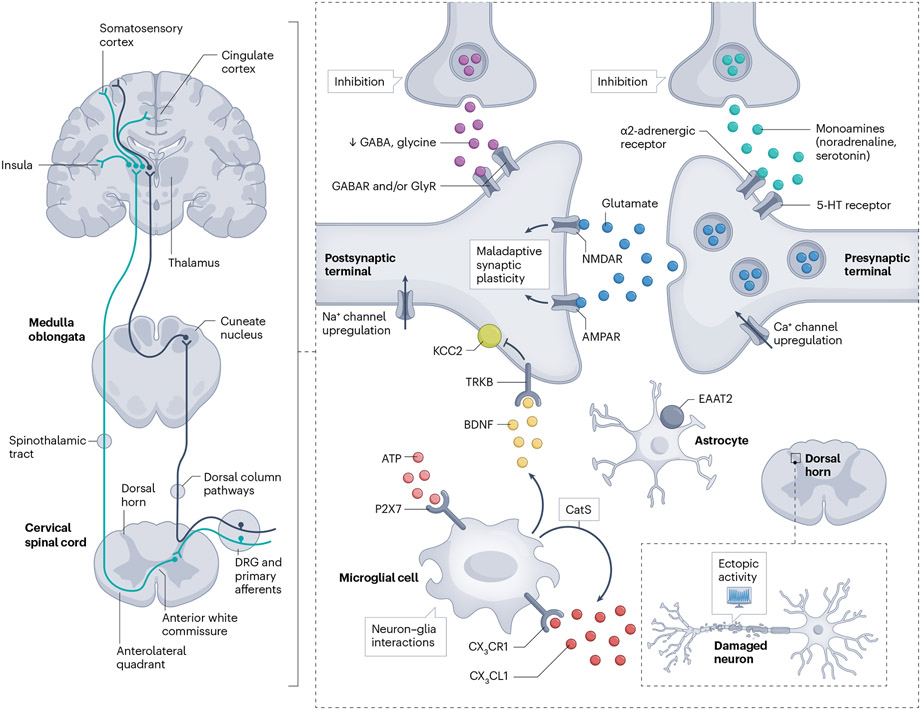
Fig. 3 ∣. Molecular mechanisms in spinal and supraspinal neuronal circuitry.
A lesion or disease in the central nervous system (CNS) causes both direct and secondary changes in the integrity and plasticity of spinal or supraspinal circuitry. On the left, in a limited view of the nociceptive system, two main pathways of the ascending somatosensory nervous system related to sensory-discriminative aspects of pain are displayed. The figure on the right highlights important molecular mechanisms that shape inhibition and excitation within spinal and supraspinal circuits related to the sensory-discriminative aspects of central neuropathic pain (CNP). Dysfunction of local inhibition (γ-aminobutyric acid (GABA) and glycine) and descending inhibition owing to damage can lead to loss of inhibitory control (disinhibition). Altered synaptic plasticity, notably N-methyl-d-aspartate (NMDA)-dependent long-term potentiation, may increase the sensitivity of the nociceptive system to subthreshold input. Such activity-dependent plasticity may be triggered or facilitated by spontaneous discharges arising from neuronal damage or altered inhibitory tone. Non-neuronal cells, including astrocytes and microglia, have important roles — impaired astrocytic glutamate buffering may result in excitotoxicity, whereas microglial activation via messengers such as ATP can affect neuronal function, potentially mediated by brain-derived neurotrophic factor (BDNF)-induced downregulation of the potassium chloride co-transporter KCC2. AMPAR, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor; CatS, cathepsin S; CX3CR/L1, C-X3-C motif chemokine receptor/ligand 1; DRG, dorsal root ganglion; EAAT2, excitatory amino acid transporter 2; GlyR, glycine receptor; NMDAR, N-methyl-d-aspartate receptor; TRKB, tyrosine receptor kinase B.
Disinhibition and its contribution to CNP.
Loss of inhibition at the intra-spinal and/or cortical level or loss of descending inhibition is postulated to be a key mechanism contributing to the development of CNP66. For example, in rodents and human post-mortem spinal cords, the release of brain-derived neurotrophic factor (BDNF) after injury leads to a downregulation of the potassium chloride co-transporter, KCC2, which plays a crucial part in setting the chloride equilibrium potential in neurons of the superficial dorsal horn and spinal projection neurons67. This downregulation, in turn, decreases the effectiveness of γ-aminobutyric acid (GABA)-mediated inhibitory postsynaptic currents, yet again resulting in reduced inhibitory tone (Fig. 3).
In rodents, studies have shown that decrease in the expression of voltage-gated ion channels or decrease in inhibitory neurotransmitter (such as GABA or glycine) release in spinal circuits can contribute to the reduction of inhibitory tone and, consequently, lead to the development of CNP67,68 (Fig. 3). However, inhibition not only regulates neuronal excitability but also shapes neuronal networks by functionally separating nociceptive and non-nociceptive pathways. The recruitment of non-noxious sensory information into the nociceptive system (through disinhibition) provides an additional framework for understanding evoked pain phenotypes, such as allodynia66. The concept that somatosensory modalities are separated in so-called labelled lines, whereby a modality is conveyed by a dedicated neural circuit or pathway from the periphery to the brain has been supplemented by notions of cross-modality interactions and population coding, whereby multiple modalities are conveyed to the brain by shared circuitry. The modalities are distinguished by using different neural coding properties such as intensity, which can be further explored using computational methods69.
Understanding interactions between modalities and the mechanisms involved in the loss of specificity of nociceptive pathways as well as plasticity of polymodal, convergent neurons, for example, wide dynamic range neurons, may potentially close important knowledge gaps in our understanding of spinal microcircuitry69. Population coding provides a framework to understand complex somatosensory processing such as the integration of temperature sensation. In this regard, the thermal grill illusion provides a compelling example of these interactions and a potential human surrogate model of CNP mechanisms (Fig. 4). Paradoxical heat sensations, that is, a warm sensation in response to a cold stimulus, may also shed light onto thermosensory integration. Such sensations have been shown for both central nerve lesions70 and peripheral nerve lesions71,72, but as for the thermal grill illusion, the exact mechanisms and implications for CNP warrant further investigation73.
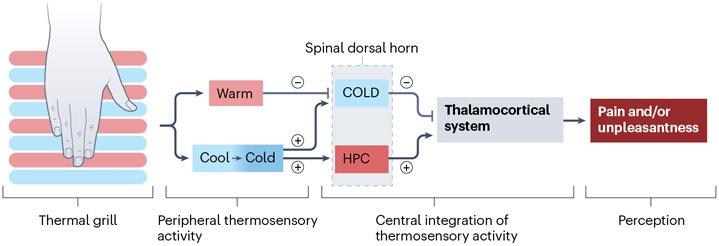
Fig. 4 ∣. The thermal grill illusion — a model for central neuropathic pain?
One hypothesis to explain thermal grill illusions suggests that the integration of simultaneous innocuous warm and cool input to thermoreceptive spinothalamic neurons (COLD), which show selective activation by Aδ-fibres mediating cooling, results in diminished COLD neuron activity. Consequently, this reduction induces a central disinhibition, or ‘unmasking’ of polymodal nociceptive neurons that respond to nociceptive heat, pinch and cold (HPC)235,236. Conceptually, a lesion or disease that affects COLD and HPC neurons differentially could also generate an imbalance, leading to the release of HPC pathway activity236,237. According to this thermal grill illusion model, this release manifests as a burning, painful or unpleasant sensation, which resembles the spontaneous neuropathic pain phenotype of patients with central neuropathic pain237. The thermal grill illusion may facilitate our understanding of the pathophysiology of central neuropathic pain (CNP)237 and has been explored in CNP after multiple sclerosis28 and non-neuropathic conditions238,239.
Role of non-neuronal cells.
Bidirectional interactions between neurons and glial cells (such as astrocytes and microglia)74 or reciprocal interactions between neurons and immune cells such as T cells and macrophages are implicated in the pathogenesis of CNP75,76. Furthermore, chemokines and cytokines such as IL-1β, IL-6 and TNF, released by activated microglia and infiltrating immune cells such as macrophages and T cells, are key mediators involved in the maintenance of CNP65,74 (Fig. 3). In human CNP, NMOSD is an example whereby pro-nociceptive cytokines and neuron–glia interactions contribute to disease pathogenesis65. In rodent models, the chemokine CX3CL1 (also known as fractalkine), released by dorsal horn neurons, physiologically and after injury binds to the receptor CX3CR1, which is upregulated by microglia after neuropathic injury77. Activation of CX3CR1 stimulates the activation of the p38 MAPK signalling pathway, which, in turn, leads to the release of IL-1β and ATP-mediated activation of P2X purinoceptor 7 (ref. 78), causing the release of cathepsin S, which then cleaves additional CX3CL179. Perturbances to this pathway at various stages, for example, by blocking CX3CR1 or inhibiting its downstream effects, may reduce pain-related behaviour in rodent models79,80 (Fig. 3).
In the context of such preclinical studies, the validity of animal models of CNP should be critically appreciated. As a subjective description cannot be obtained, animal studies often rely on surrogate expressions of pain and associated outcomes, such as measures of evoked nocifensive behaviours (for example, withdrawal reflexes to innocuous and noxious stimuli, thereby measuring allodynia and hyperalgesia or spasms)81. Complex behavioural assays such as conditioned place preference or aversion approaches are used as surrogate indicators of spontaneous or ongoing pain82. Moreover, advanced machine learning methods hold the potential to measure surrogate expressions of pain or nociception from pose estimation and facial grimace83,84.
Spinal and brain circuitry changes
Neuroimaging investigations in patients with stroke, SCI or multiple sclerosis have provided important insights into brain mechanisms that underlie CNP. Changes in opioid receptor-binding capacity85 and glial activation have been associated with CNP86. Studies also point towards biochemical changes in the human thalamus, which indicate an alteration in the balance between excitatory and inhibitory mechanisms. In SCI-induced CNP, studies have shown a decrease in N-acetylaspartate, GABA content and blood flow in the thalamic reticular nucleus, along with structural changes in the ventral posterior thalamus87-89. This reduction in inhibitory output may result in disruption of normal thalamocortical rhythm, which may subsequently result in the chronification of SCI-induced CNP.
Progressive damage in the spinothalamic tract has been proposed as a time-dependent mechanism that underlies the development of CNP after SCI32. In addition, studies have reported alterations in conditioned pain modulation in patients with CNP, suggestive of impaired descending inhibitory control34,90,91. Furthermore, electroencephalogram (EEG) and magnetoencephalography (MEG) studies have identified changes in resting oscillatory brain activity92, particularly in the α- and θ-bands and possibly in the high β-band93, which might be potential targets for neuromodulation94. Intriguingly, thalamic deafferentation changes the firing properties of thalamic neurons, which in turn, alters thalamocortical oscillations95-97, often subsumed under the umbrella term of thalamocortical dysrhythmia98-102. Human in vivo electrophysiological recordings shape our current understanding of lesion-induced maladaptive plasticity. Microstimulation of the sensory thalamus provides compelling evidence of changes in pain pathways after an insult to the spinothalamic pathways103. Such changes include induced pain sensations after thalamic microstimulation104-106. The findings of aberrant neuronal activity and stimulation-evoked pain raise several potential mechanisms106. Thalamic hyperexcitability as a result of deafferentation not only may become a generator of constant spontaneous pain signals relayed into cortical networks, but could also simultaneously facilitate residual afferent input107.
A central concept of the brain mechanisms of pain is that no dedicated and specific ‘pain spot’ in the brain exists and that nociceptive neurons are located amongst non-nociceptive neurons. Neuroimaging findings must take this key concept into consideration when interpreting results108,109 to avoid the reverse inference problem110.
A small number of studies have reported pain experiences perceived by patients during neurosurgical procedures in which stimulation of areas of the insular cortex evoked pain, unpleasant or aversive sensations111,112.
In seminal applications of functional MRI in patients with syringomyelia and CNP changes in brain activity to thermal and mechanical stimulation were observed113. Similar to other chronic pain conditions, studies have reported alterations in brain structure, such as changes in grey matter volume and diffusion tensor imaging-derived indices, assessed from quantitative anatomical MRI (for example, voxel-based morphometry, cortical thickness, diffusion tensor imaging) in brain areas related to pain perception in individuals with CNP88,114,115. The role of maladaptive cortical plasticity in CNP has been investigated with task-evoked functional MRI116,117. Moreover, monitoring of resting state brain activity through functional MRI has revealed various changes in brain function associated with CNP, including alterations in connectivity118,119.
Pain is thought to arise from complex interactions of activity over space and time that can engage nociceptive neurons and coordinated activity and interactions within and between central ascending (sensing) pathways, descending (modulation) pathways and brain networks involved in attention, salience, cognition and other functions that shape the experience. A current model that emphasizes the temporal dynamics of this system is known as the dynamic pain connectome120,121.
Diagnosis, screening and prevention
Clinical presentation and phenotypes
CNP is characterized by spontaneous, ongoing pain often described by patients as burning, pricking, squeezing, freezing or electrical shock-like sensations, or may be paroxysmal pain as is observed in trigeminal neuralgia or in Lhermitte sign in multiple sclerosis8,22,25,38,42,122. Patients may express the characteristics of their pain using personal language and terms, for example, “I feel like I have tinfoil under my skin”, implying a paradoxical mixture of hot and cold sensation123. These features may be accompanied by evoked pain such as cold-evoked pain and/or touch-evoked pain. Sensory loss typically encompasses a broader area than the painful region, that is, pain is nested within larger regions of somatosensory deficit. In addition to pain, patients with CNS lesions often describe non-painful dysaesthesia and paraesthesia21-23,38,124. CNP resulting from SCI may manifest as segmental pain or pain below the lesion125 (Fig. 1). CNP resulting from damage at supraspinal levels results in a pain distribution contralateral to the lesion site. Patients with brainstem lesions may exhibit a more complex phenotype, with ‘crossed signs’: damage to the ascending spinothalamic tract results in contralateral hemi-body pain, whereas involvement of brainstem nuclei may cause ipsilateral facial pain126 (Fig. 1). Trigeminal neuralgia secondary to brainstem lesions, for example, in multiple sclerosis, may sometimes cause bilateral facial pain122.
Diagnosis
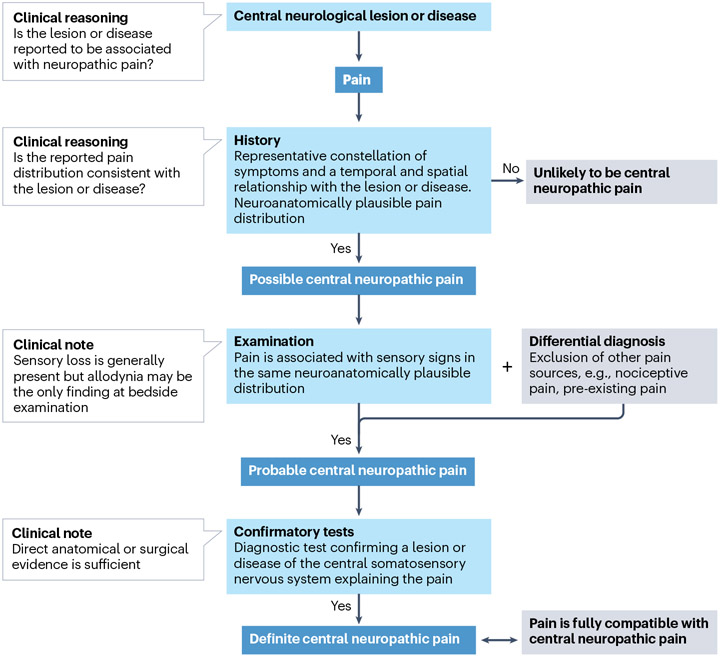
Neuropathic pain (peripheral and central) is diagnosed according to three levels of certainty, which are hierarchically graded127 (Fig. 5). For the diagnosis of CNP, the CNS lesion is, however, often established when the patient presents with pain. Hence, exclusion of other types of pain becomes more important than establishing a CNS lesion. ‘Possible’ CNP is based on a history (symptoms) of a relevant CNS disease or lesion, along with a pain distribution that is plausible neuroanatomically, hence located in body areas exhibiting classic patterns of sensory abnormalities owing to CNS disease (Fig. 1). Symptoms may be systematically assessed using validated screening questionnaires128. In combination with pain drawings, such questionnaires may help to determine whether the pain localization is ‘neuroanatomically plausible’128,129. ‘Probable’ CNP is based on a physical examination that reveals sensory signs in a neuroanatomically plausible distribution. In most cases, sensory signs will include deficits in perception of mechanical and thermal stimuli or signs of hypersensitivity such as allodynia to touch or to non-painful cold temperatures130,131. Other symptoms such as hyperalgesia, paraesthesia or dysaesthesia and hyperpathia may be present to varying degrees132. Although initiation of pain treatment involves several considerations, in terms of diagnostic certainty, the level of ‘probable’ CNP is generally regarded as sufficient to initiate specific therapy for neuropathic pain127. ‘Definite’ CNP is based on positivity of confirmatory examinations that confirm the presence of a lesion or a disease of the central somatosensory nervous system that can explain the spatial distribution of the pain. In addition, other causes of pain such as spasticity or secondary joint degeneration should be excluded or considered unlikely upon clinical judgement (see below).
Fig. 5 ∣. Grading system for central neuropathic pain.
A grading system is used to attain different levels of diagnostic certainty for central neuropathic pain (CNP)127. If reported symptoms are temporally associated with the lesion or disease and align with the anticipated neuroanatomical pain pattern, the presence of neuropathic pain is possible. To advance to ‘probable’, a neurological examination needs to detect somatosensory abnormalities such as loss of sensation or hypersensitivity. Importantly, differential diagnoses such as musculoskeletal pain must be ruled out at this stage. Once a lesion within the central somatosensory nervous system is confirmed and other types of pain excluded, the definite CNP level is reached.
Neuroimaging and neurophysiology.
Imaging studies such as structural MRI and CT scans are mainstream for lesion detection in patients with CNP127. CT scans can reveal scars (gliosis) and subacute strokes, as well as brain neoplasms and structural abnormalities. CT scans are widely available, relatively inexpensive, fast to perform and to interpret results. MRI does not use radiation and is very sensitive to white matter lesions, and is, therefore, the gold standard for imaging the spinal cord114. Findings from MRI are central to the assessment of many CNS diseases associated with CNP such as multiple sclerosis, stroke55 and SCI22,133.
Following the stimulation of cutaneous A-delta and C-fibres, pain-related cortical evoked potentials are phase-locked responses recorded using EEG. Stimulation modalities include radiant heat by laser, electrical stimulation with concentric planar electrodes or contact heat stimuli applied by thermodes with steep ascending temperature ramps leading to synchronized afferent volleys and a good signal-to-noise ratio in EEG recordings133-136. Evoked potentials seek to probe the functional integrity of the nociceptive pathways from peripheral tissues to the higher order neurons in the cortex, mainly located in the posterior insula and parietal operculum137. Changes in amplitude (reduction or absence) or prolonged latencies of pain-related evoked potentials indicate the presence of a lesion at some point along the peripheral nociceptive system or central nociceptive system. Although electrophysiology may reveal the functional significance of a lesion133, neuroimaging remains the gold standard for determining lesion location. Topographical diagnosis, for example, peripheral versus central somatosensory system, is then based on the clinical, neurophysiological and neuroimaging findings.
CNS diseases lead to several pain types besides CNP. In fact, musculoskeletal pain is the most frequent pain after stroke, SCI and other common neurological diseases21,138-142. In up to a third of patients with stroke, a clear non-neuropathic aetiology of pain (such as spasticity or musculoskeletal biomechanical problems) is present and located within the same body area in which sensory deficits are experienced. Hence, consideration of other types of pain within areas of sensory loss as potential differential diagnoses is crucial as other causes of pain after a CNS injury (such as headaches, joint abnormalities, spasticity and pain types unrelated to the injury), are managed differently from CNP143.
Screening
Screening tools and pain descriptors are important to help distinguish neuropathic pain from other pain types. Individuals with neuropathic pain tend to use a relatively specific group of descriptors such as burning, pricking, squeezing, freezing or electric shock-like, to describe their pains144. Many of these descriptors (for example, burning and squeezing pain) are also frequent in non-neuropathic pain conditions. However, as a group, people with neuropathic pain do tend to use ‘neuropathic pain descriptors’ more frequently than individuals with other types of pain, which is the basis for the use of descriptor-based questionnaires to screen for neuropathic pain144,145. Several tools are available for the diagnostic screening of neuropathic pain, some of which are validated for CNP128,146. Some tools include items related to a brief sensory examination, for example, the presence of hypoaesthesia, mechanical allodynia or mechanical hypoalgesia147. After a CNS lesion, screening should be carried out during rehabilitation or during follow-up visits. Items of descriptor-based screening questionnaires for neuropathic pain must concern and be directed to a specific body area at a time, for example, the region where the most severe pain is located148. Of note, screening tools have variable sensitivity (36–95%) and specificity (46–100%) depending on the disease and the questionnaire used, with optimal sensitivity and specificity for Douleur Neuropathique en 4 Questions (DN4), which also included CNP in its validation process128,149.
Prevention
On the basis that CNP often develops over time, several studies have tried to explore a potential window of opportunity for prophylactic intervention before pain develops by using animal models of CNP150. Interestingly, clinical studies that aim to prevent CNP in humans are very limited in number and offer little evidence to suggest that prevention can be achieved151,152. In a randomized, double-blinded, placebo-controlled trial involving 39 individuals with acute thalamic stroke, no significant difference in 1-year incidence of CNP was observed between those administered amitriptyline (a tricyclic antidepressant) and those given placebo (17% versus 21%)151. In a similarly designed study in individuals with traumatic SCI, a month-long administration of carbamazepine (a sodium channel blocker) reduced the development of CNP at 1 month but not at later time points (that is, 3 months and 6 months) compared with placebo152. Lack of understanding of factors that increase the risk of developing CNP serves as a major barrier to translation. Studies have identified early hypersensitivity and dysaesthesia as predictors of spontaneous CNP22,38,60. Predictors are useful to inform clinical trial design and limit the inclusion of individuals unlikely to develop CNP. Given the paucity of data and the lack of effectiveness reported so far, no intervention exists that can be reasonably recommended to prevent CNP.
Management
The management of neuropathic pain is in most instances symptomatic, targeted to relieve pain, rather than causal, targeted to treat the underlying aetiology, and it follows a multimodal approach. The first step is to discuss self-management strategies and patient expectations before making a treatment plan153,154. Given the few and often small controlled studies in CNP, recommendations and treatment guidelines also rely on studies conducted in peripheral neuropathic pain and chronic pain conditions in general.
Non-pharmacological therapy
For individuals with CNP, non-pharmacological treatments include psychological therapy, physical therapy, acupuncture, self-hypnosis, neuro-feedback and virtual reality.
Psychological therapy.
Cognitive behavioural therapy (CBT) is the most frequently used psychological therapy. CBT focuses on changing the way individuals think and behave in response to pain. A Cochrane review of psychological therapies for chronic pain that included 59 studies found only small or very small beneficial effects for CBT in alleviating pain, with insufficient evidence to assess adverse events155. Only one randomized controlled trial in the review targeted people with CNP and, therefore, the effectiveness of CBT in reducing CNP is uncertain. Nevertheless, people with CNP often have comorbid mental health disorders, and CBT, an evidence-based treatment for mental health disorders, can be used to treat these conditions and improve health-related quality of life (HRQoL)156.
Physical therapy.
Insufficient evidence also exists to support the effectiveness of physical therapy in treating CNP. A systematic review and meta-analysis found that exercise had a significant effect in reducing pain in individuals with multiple sclerosis; however, no differentiation was made between CNP and musculoskeletal pain157. Physical therapy is commonly used for neuromuscular rehabilitation in people with CNS injuries. Typically, physical therapy frequently encompasses pain science education and involves exercises that aim to strengthen muscles and reduce musculoskeletal pain158. Despite evidence that pain science education has a small to moderate effect in reducing musculoskeletal pain159, currently no adequate evidence exists to support its use in patients with CNP. Evidence is insufficient to support or decline the use of self-hypnosis and acupuncture in treating CNP160,161.
Neuro-feedback and virtual reality.
Two promising non-invasive treatments are available for CNP. EEG neuro-feedback is a technique that helps individuals to consciously regulate their brain rhythms in a way that may reduce their pain. EEG neuro-feedback protocols include reinforcing α-rhythms (~8–12 Hz) or sensorimotor rhythms (~12–15 Hz) and suppressing θ-rhythms (~4–7 Hz) and/or high β-rhythms (~20–30 Hz). A systematic review reported that EEG neuro-feedback was effective in reducing CNP in individuals with SCI and TBI across six small single-arm studies162. Although these results are promising, high-quality randomized controlled trials with large sample sizes and adequate adverse event reporting are needed to provide high-quality evidence for the use of EEG neuro-feedback for CNP.
Although virtual reality interventions are commonly used to deliver sensorimotor rehabilitation in individuals with CNS injuries, evidence suggests that it could be beneficial in decreasing CNP following SCI. A systematic review found that virtual walking or lower limb movement imagery decreased SCI-associated CNP in eight of nine small single-arm studies163. A non-randomized clinical trial reported that fully immersive, interactive virtual reality walking was more effective in reducing SCI-associated CNP than passive virtual reality walking (that is, observing an avatar walking from a first-person perspective)164. In the interactive condition, pain intensity decreased from a baseline mean of 5.9 (s.d. 3.0) to 3.9 (s.d. 3.1) after treatment on a 0–10 numerical rating scale whereas the pain score increased from 4.8 (s.d. 2.5) to 5.5 (s.d. 2.5) in the control group. This finding provides support for the value of interactivity, immersion and volition in virtual reality walking interventions for SCI-associated CNP. Nevertheless, high-quality randomized controlled trials with adequate sample size and adverse event reporting are warranted to obtain evidence on the effectiveness of virtual reality interventions for CNP.
Pharmacological therapy
First-line pharmacological treatments for neuropathic pain include the tricyclic antidepressants, serotonin and noradrenaline reuptake inhibitors (SNRIs), gabapentin and pregabalin165. The effect on pain is low (on average 20% and with a need for four to eight treated patients to obtain one patient with moderate to good pain relief over placebo). Most trials that inform treatment recommendations were performed in peripheral neuropathic pain, but large studies exist for SCI-associated pain; effect sizes are similar across peripheral neuropathic pain and CNP166. However, patients with CNP seem to be more prone to adverse effects, especially somnolence and dizziness than patients with peripheral neuropathic pain, possibly attributed to additive effects of concomitant drugs, for example, anti-spasticity agents167,168.
Gabapentin and pregabalin and new formulations such as mirogabalin169 are ligands for the α2δ subunit of voltage-gated calcium channels. These ligands bind to calcium channels and reduce the release of excitatory neurotransmitters at spinal levels and supraspinal levels170, thereby causing an analgesic effect. They also act on synaptogenesis and NMDA receptors and the neuro-immune system, which may contribute to an analgesic effect170. Large studies have confirmed the analgesic effect of pregabalin in CNP167,168,171. In light of this finding, the EMA has approved pregabalin for the treatment of CNP and the FDA has approved pregabalin for SCI-associated neuropathic pain.
Antidepressants may act partly by increasing the activity of descending modulation through the inhibition of presynaptic serotonin and noradrenaline reuptake although if and where this happens are unclear. Antidepressants could possibly also bring about analgesia by actions on the opioid system, sodium channels and α-adrenergic receptors170. Studies have demonstrated the effectiveness of antidepressants in various CNP conditions172-176, with only one study failing to reach statistical significance177.
Although evidence of efficacy of lamotrigine in CNP is inconclusive, it is still often used to manage CPSP based on a single positive trial178. In SCI-associated pain, CNP in a subgroup of patients with incomplete lesions and evoked pain was reduced, although this outcome has not been replicated179. Carbamazepine and oxcarbazepine are the mainstay of treatment for trigeminal neuralgia, but evidence is sparse and is not investigated in trigeminal neuralgia associated with multiple sclerosis165. Opioids are generally not recommended for chronic non-cancer pain; however, tramadol, which has shown efficacy in SCI-induced pain180 may be considered for treatment of episodic exacerbations of severe pain180. Most studies find that cannabis-based medicine, for example, δ-9-tetrahydrocannabinol (THC) or cannabidiol (CBD), is ineffective for the treatment of CNP165,181.
General treatment principles.
Despite continued emphasis on mechanism-based treatments, evidence is still limited for an individualized treatment approach. Pregabalin may be considered first-line pharmacological treatment in patients with anxiety167 and antidepressants in patients with concomitant depressive symptoms173. In patients with severe spasticity, gabapentin, which may reduce spasticity, is a better choice than tricyclic antidepressants173.
Pharmacological treatments may be associated with severe adverse effects (Table 1). Patients should be advised to seek medical advice if signs of suicidal behaviour emerge. The FDA and EMA recommend that patients taking pregabalin and gabapentin are monitored for symptoms of misuse or abuse, such as dose escalation and drug-seeking behaviour. In addition, the FDA warns against the use of gabapentin and/or pregabalin in combination with CNS depressants owing to the risk of respiratory depression.
Table 1 ∣.
First-line pharmacological treatments for central neuropathic pain
| Agent | Common adverse effectsa | Contraindications and precautionsa | Mode of action |
|---|---|---|---|
| Tricyclic antidepressants | Somnolence, dizziness, headache, drowsiness, dysarthria, tremor, confusion, orthostatic hypotension, tachycardia, dry mouth, constipation, nausea, weight gain and urinary retention | Cardiac disease, QT interval prolongation (ECG before treatment) High doses should be avoided in elderly patients | Possibly modulation of the descending control systems by inhibiting the reuptake of serotonin and noradrenaline; actions on sodium channels and opioid receptors |
| Duloxetine | Nausea, abdominal pain, constipation, dizziness, fatigue, blurred vision, falls, tinnitus, blood pressure increase, weight changes, sweating, erectile dysfunction, urinary retention | Liver disease, severe renal impairment, hypertension and cardiac disease | Possibly modulation of the descending control systems by inhibiting the reuptake of serotonin and noradrenaline |
| Venlafaxine | Dizziness, insomnia, sedation, fatigue, confusion, headache, hypertension, dry mouth, constipation, nausea, sweating, serum cholesterol increase, weight loss and erectile dysfunction | Hypertension and cardiac disease | Possibly modulation of the descending control systems by inhibiting the reuptake of serotonin and noradrenaline |
| Pregabalin | Dizziness, somnolence, headache, confusion, fatigue, ataxia, weight gain, blurred vision, erectile dysfunction, peripheral oedema, nasopharyngitis | Dose reduction in patients with renal impairment | Attenuation of neuronal hyperexcitability through modulation of synaptic transmission by acting on presynaptic calcium channels (α2δ subunit) |
| Gabapentin | Dizziness, somnolence, confusion, fatigue, ataxia, weight gain, constipation, peripheral oedema, viral infection, leukopenia, dry mouth, impotence and urinary incontinence | Dose reduction in patients with renal impairment | Attenuation of neuronal hyperexcitability through modulation of synaptic transmission by acting on presynaptic calcium channels (α2δ subunit) |
ECG, electrocardiogram. aThis is not a full list of adverse effects or precautions. Please consult ‘Summary of product characteristics’.
The dose should be gradually increased and treatment effect must be reassessed regularly. When discontinuing treatment, the dose should be gradually decreased to reduce the risk of withdrawal symptoms. If partial pain relief is achieved with one drug in maximum tolerated dose, adding another drug with complementary action may be considered. Antidepressants and tramadol should not be combined because of the risk of serotonin syndrome, a potentially life-threatening condition resulting from elevated serotonin levels182. Symptoms of serotonin syndrome include cognitive impairments such as confusion and hallucinations, autonomic dysregulation with tachycardia and nausea, and motor dysfunction including muscle twitching and tremor.
Neuromodulation
Neuromodulation is defined as transient or long-lasting modifications in spontaneous neural activity, plasticity or information processing in the CNS or peripheral nervous system. Neuromodulation can be achieved via various approaches, including the use of physical agents, such as electrical current or magnetic current, for therapeutic purposes183. Many locally applied neuromodulation techniques engage diffuse and widespread responses within the neuroaxis, not merely restricted to the stimulated target184 (Fig. 6). Non-invasive neuromodulation refers to techniques in which electrical currents are delivered to or induced in the spinal cord or brain though coils or electrodes placed on the skin. The two most used approaches are transcranial direct current stimulation and repetitive transcranial magnetic stimulation185. Studies have extensively investigated the effect of stimulation of the primary motor cortex (M1), which induced substantial metabolic and excitability changes in remote extra-motor brain regions. This finding aligns with results from studies that indicate highly connected regions within M1 associated with executive control, visceromotor function and interoceptive brain areas186. Additionally, animal experiments and human studies provide evidence that the analgesic effects of repetitive transcranial magnetic stimulation of M1 are contingent on the presence of μ-opioid receptors, NMDA receptors and the ability to restore impaired intracortical inhibition observed in neuropathic pain94,187,188. Other less frequently explored techniques include trans-spinal direct current stimulation and trans-spinal magnetic stimulation, which are analogous to transcranial direct current stimulation and transcranial magnetic stimulation, but in which the spinal cord is targeted by placing electrode or coils on the posterior neck or dorsum instead of the scalp189.
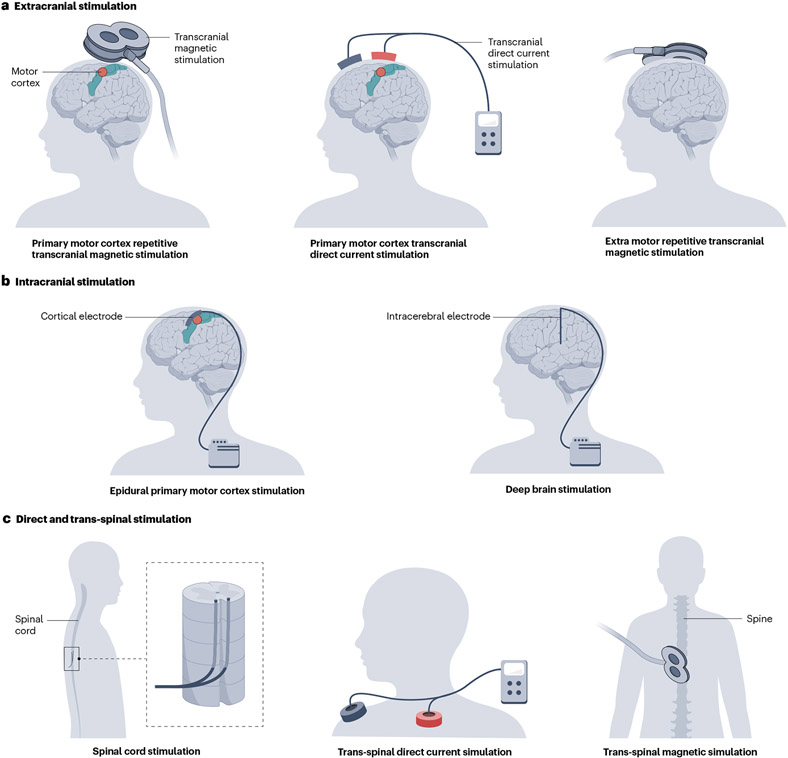
Fig. 6 ∣. Neuromodulatory techniques for central neuropathic pain.
Various modulation techniques using extracranial stimulation (panel a), intracranial stimulation (panel b) and direct and trans-spinal stimulation (panel c). The schematic shows neuromodulation of primary motor cortex (left figures, panels a and b) and deep brain regions (right figures, panels a and b).
Non-invasive neuromodulation techniques are delivered in sessions lasting 15–25 min each. Treatment protocols for non-invasive neuromodulation include induction periods composed of daily stimulation sessions, followed by maintenance periods, when treatment is delivered weekly, fortnightly or monthly190. invasive neuromodulation techniques involve surgery to implant stimulating electrodes epidurally (over the spinal cord or over the primary motor cortex191) or directly into the brain parenchyma (that is, deep brain stimulation — mostly targeting the periventricular or periaqueductal grey, the sensory thalamus and the anterior cingulate cortex)192. In all instances, electrodes are connected to implanted pulse generators placed subcutaneously, which are controlled by the physician or the patient by telemetry.
Overall, repetitive transcranial magnetic stimulation and (surgically implanted) spinal cord stimulation are increasingly being explored for neuropathic pain control and have been recommended for patients refractory to pharmacological therapy, although these techniques have not been approved by regulatory authorities for the treatment of CNP193,194. However, neuromodulation techniques were less frequently explored in CNP. For example, sham-controlled studies have found pain relief with high-frequency repetitive transcranial magnetic stimulation and invasive epidural stimulation of the primary motor cortex in patients with CNP185, but in most instances, people with CNP were mixed with those with peripheral neuropathic pain195. By contrast, no pain relief was achieved when non-motor targets, such as the dorsolateral prefrontal196, the anterior cingulate or posterior insular cortices were targeted197. However, one study showed that repetitive transcranial magnetic stimulation of the primary motor cortex (M1) proved effective in pain management for several weeks in patients with CNP198. Results from small, controlled studies are inconclusive with respect to the effect of transcranial direct current stimulation in people with SCI-associated CNP199,200. Transcranial direct current stimulation was also tested as an add-on strategy to other rehabilitation approaches such as visual illusion therapy in individuals with SCI, with positive analgesic results in a small study201. Although neuromodulation is being increasingly investigated in CNP and could have a role in patient management, larger studies specifically focusing on these patients are needed, along with cost-efficacy analyses, proper blinding and controls202.
Quality of life
CNP is associated with a wide range of physical and emotional symptoms, which markedly impact an individual’s HRQoL. For example, people with CNP often experience anxiety, depression, social isolation, sleep disturbances, loss of physical function and impaired cognitive function, which makes performing everyday tasks difficult203-205. HRQoL questionnaires are typically generic measures that assess an individual’s self-reported health status, physical and emotional functioning. The Nottingham Health Profile (NHP), the EuroQol five dimensions (EQ-5D), the Patient-Reported Outcomes Measurement Information System (PROMIS) and the 36-item Short Form Health Survey (SF-36) are used to measure HRQoL in individuals with CNP206,207. The SF-36 questionnaire has been modified for people with SCI to improve content validity responsiveness by replacing ‘walk’ with ‘wheel’ for three of the physical function questions (SF-36 walk–wheel)208.
Evidence shows that CNP leads to worse HRQoL in people with neurological conditions such as SCI, brain injury, stroke and multiple sclerosis. For example, a population-based study involving 1,549 individuals with SCI found that those experiencing moderate to severe chronic pain reported lower HRQoL than individuals with mild or no chronic pain (P < 0.001)203. Several studies have demonstrated a strong association between higher pain intensity levels and lower HRQoL scores in people with SCI203,204; however, only a limited number of studies differentiate between neuropathic pain and nociceptive pain after SCI. Interestingly, evidence shows that the effect of pain intensity on HRQoL in SCI is mediated by both participation satisfaction and participation restriction in everyday life203. Furthermore, the available longitudinal data indicate that pain intensity is a major predictor of HRQoL in individuals with SCI. Consistent with the findings on SCI-associated CNP, studies have demonstrated that pain after TBI also has an adverse effect on HRQoL205.
Studies have shown that CPSP is associated with low HRQoL209,210. In addition, longitudinal studies demonstrated that higher intensity of chronic pain predicted lower HRQoL scores up to a decade after initial assessment211. Evidence also shows that pain severity correlates significantly with HRQoL in individuals with multiple sclerosis212,213, although most studies do not differentiate between neuropathic pain and non-neuropathic pain.
Importantly, effective pain management can lead to an improvement in HRQoL. For example, transcranial direct current stimulation resulted in a significant reduction in pain accompanied by an improvement in HRQoL in individuals with multiple sclerosis-associated CNP214. In addition, pregabalin treatment resulted in a substantial improvement in HRQoL scores, measured by the EQ-5D questionnaire in patients with CNP caused by brain injury or SCI171.
Outlook
Considerable gaps remain in our understanding of the pathophysiology, risk factors and progression of CNP. These gaps present obstacles to effective pain management, development of new analgesic drugs and the design of clinical trials. By tailoring interventions to individual patients based on their clinical pain phenotype and putative pain mechanisms, including genetic variations39, precision medicine has the potential to improve treatment outcomes and minimize adverse effects.
To this end, a refined understanding of the various pain phenotypes in patients with central neurological lesions is fundamental. Disentangling neuropathic pain and nociceptive pain is crucial, and classification systems should emphasize differential diagnoses of CNP. Another important area for future research is to examine pain mechanisms in central neurological disorders, particularly those characterized by diffuse patterns of impairment that lack focal lesions of ascending somatosensory pathways. For example, studies should aim to determine whether and how CNS changes in conditions such as post-traumatic stress disorder and neurodegenerative diseases such as Alzheimer disease, Parkinson disease and amyotrophic lateral sclerosis can lead to chronic pain. Currently, evidence of involvement of the somatosensory system following these conditions is inconclusive, which precludes the diagnosis of CNP.
Risk factors and prevention
Risk factors underlying the development of CNP need to be elucidated. Longitudinal studies that can help to detect signs and symptoms of CNP in the early stages of disease and monitor pain progression up to chronic time points (for example, 1 year) are warranted. Larger neurophysiological studies, which can help to diagnose lesions along the nociceptive pathway, are needed to assess the various pain phenotypes that naturally develop over time. Objective markers of increased neuronal responsiveness may be derived from evoked potentials215 or pain-autonomic markers134,216, which may provide insights into maladaptive neuroplastic changes before they manifest clinically as sensory hypersensitivity or spontaneous CNP.
With this knowledge in hand, preventive trials can be informed to reduce the burden of intervention to only those at risk of CNP (Fig. 7). One factor that should be considered in future preventive clinical trials, regardless of risk factors, is the length of treatment, which should ideally match the known time course progression. An important aspect in this regard lies in identifying and understanding the spatiotemporal patterns of neuronal hyperexcitability that accompany the development of CNP.
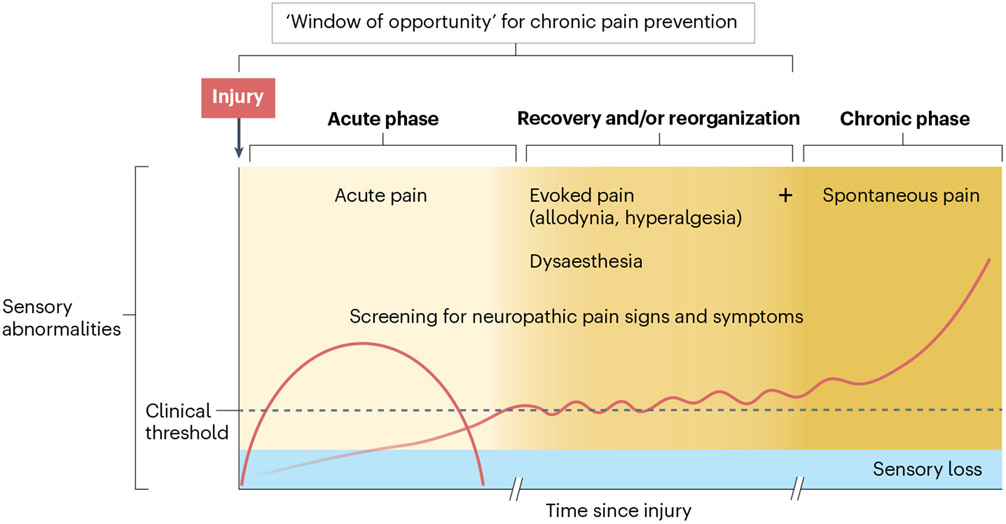
Fig. 7 ∣. Progression and monitoring of central neuropathic pain.
In the immediate aftermath of a central nervous system lesion, acute pain can occur owing to changes induced by the trauma or disease. Neurological function is compromised, leading to sensory impairments as the primary somatosensory manifestation. As time progresses, individuals may experience heightened sensitivity to sensory stimuli, which can progress to the emergence of spontaneous central neuropathic pain (CNP), along with evoked pain. This phase is particularly important for implementing preventive treatments and clinical monitoring, emphasizing the need for screening for abnormal sensory signs. Once spontaneous CNP has developed (with possible concurrent evoked pain), the focus shifts to achieving an optimal (differential) diagnosis and implementing effective pain management strategies. Note that this is only one example for a patient trajectory; not all patients experience evoked pain, and many other trajectories exist.
Mechanisms
In the field of basic pain research, technological advances have allowed ground-breaking experiments that may uncover CNP-related changes in spinal circuits and supraspinal microcircuitry and identify novel therapeutic targets69. Advanced methods of optical imaging of the spinal cord may reveal patterns of neuronal activity linked to pain-related behaviour217. Cell-targeted expression of optogenetic receptors or chemogenetic receptors, which confer temporal and/or spatial control of neural activity, allow for precise dissection of the functional role of specific cell populations in different aspects of nociception and/or pain218,219. Single-cell sequencing methods are generating precisely defined, cross-species atlases of neuronal and non-neuronal cell types under normal and pathological conditions, including humans, which is crucial for the identification and validation of molecular and cellular therapeutic targets220. Advancements in genetic engineering of large animal models, such as non-human primates221,222, will improve our understanding of pain circuitry and mechanisms in humans, thereby enabling the development of more effective treatments. Mechanism-based treatments are sought after to manage peripheral neuropathic pain and CNP. Delivering pharmacological and non-pharmacological treatment to specific sites of abnormal neuronal activity is a key endeavour.
Modern neuroimaging and neurophysiological approaches play a crucial part in pinpointing specific CNP-related changes in the human CNS. Studying CNP using neuroimaging is particularly challenging owing to the mixture of negative (sensory loss) and positive (pain and hypersensitivity) signs and symptoms, co-morbidities and the lesion itself109,223-225. Thus, unravelling data pertaining to regions and pathways related to pain versus sensory loss is warranted. Neuronal activity causing CNP must be distinguished from activity arising secondary to CNP and other pain, aberrant neuronal activity, motor or emotional consequences. To accomplish this, we require a deeper understanding of general principles of nociceptive processes and brain regions involved in pain processing and somatosensation, related to neural signatures of the dynamic pain connectome120 and the basis and limitations of neuroimaging tools and analyses (Box 2).
Box 2. Challenges in understanding the mechanisms of central neuropathic pain.
Unanswered questions
What initiates and maintains central neuropathic pain (CNP)?
Is there a specific neural code of CNP?
Which central nervous system (CNS) regions and networks are primary drivers as opposed to secondary consequences or epiphenomena of CNP?
Does CNS reorganization and plasticity drive or reflect CNP?
Can clinical assessment, electrophysiology, structural or functional imaging identify potential therapeutic targets tailored to individuals?
Challenges in relating phenotypes to potential underlying mechanisms
Discriminating single-cell, ensemble and network processes related to inhibitory versus excitatory mechanisms
Distinguishing modes and features of neural activity (for example, aberrant discharge patterns, steady or normal patterns, bursting, etc.)
Distinguishing correlation from causation in the context of neural activity and associated phenotypes
Differentiating general pain from CNP-specific mechanisms
The impact of transient state and long-lasting trait-like contributions
In addition, whether maladaptive plasticity linked to various CNP conditions is a driver of CNP or simply reflects decreased use of the limb and sensory perceptions or secondary changes to pain is not clear yet. In this regard, longitudinal studies and treatment effect studies are attempting to disentangle causation from correlation and to identify predictive markers of pain chronicity and treatment outcome92, 224,226,227. Compelling examples of distinct treatment effects may be derived from disease-modifying therapies applied to neuroinflammatory conditions. Modern therapies targeting molecular cascades that are implicated in CNP pathophysiology65, for example, tocilizumab (to target IL-6)228, may offer unprecedented insights into pain mechanism by dissecting the clinical pain phenotype in response to a specific treatment.
New therapies
In general, lesion-induced upstream and downstream plasticity need to be taken into account when designing therapeutic studies and clinical trials aimed to restore the equilibrium between inhibition and excitation229. For example, alterations in ion channel expression and structural changes within the peripheral nervous system after CNS lesions may hold potential as therapeutic targets in the future. However, at present the relationship between peripheral changes and adaptations to CNP has not been systematically explored43. Novel therapeutic strategies that aim to restore function after a central neurological lesion might not only promote neuroplasticity to enable motor function, but also induce maladaptive changes that may predispose to pain230. In this regard, clinical trials to improve motor function should simultaneously track changes in neurological status and specific pain-related abnormalities, such as the development of sensory hypersensitivities.
Advances in neuromodulation such as closed-loop strategies allow real-time physiological readouts to guide or to dose therapy231,232. Machine learning algorithms have shown promising findings with regard to prediction of pain based on individual patterns of neural activity233. In this context, the investigation and understanding of neural connectivity, synchrony of activity, firing patterns (such as bursting, synchronous versus asynchronous and so forth) and frequency are fundamental as they can serve as potential parameters in neuromodulation techniques (Box 3). Similarly, structural imaging, including connectivity, may identify targets for specific therapeutic strategies such as focused ultrasound.
Box 3. Future directions for understanding central neuropathic pain.
- A multimodal approach (clinical examination, psychophysics, neurophysiology and neuroimaging) to deconstruct the central neuropathic pain (CNP) phenotype
- Distinguish manifestations of pain and sensory loss or deafferentation from pain mechanisms
- Identify neural structures and functions specifically associated with CNP versus those that reflect nociceptive activity
- Use advanced neuroimaging paradigms to expand our understanding of CNP on a group level
- Disentangle findings that may point towards a root cause or maintaining factor of CNP (potential treatment target) versus secondary findings that merely reflect symptoms or state characteristics (unlikely to be a treatment target)
- Leverage resting state functional connectivity and electroencephalogram (EEG)–magnetoencephalography (MEG) functional coupling approaches to identify aberrant localized activity and inter-regional synchrony
Investigate measures of neuroinflammation based on diffusion-weighed MRI and PET
- Investigate clusters of sensory signs (assessed via psychophysics) and symptoms (assessed through questionnaires) and establish connections with specific features of the underlying condition, for example, patterns of modality-specific sparing using a multimodal approach involving evoked potentials. Identify risk factors, specifically structural and functional determinants of CNP occurrence and progression
- Use structural imaging to map lesion characteristics and complement the clinical examination with objective neurophysiological measures
Improve the diagnosis and classification in central neurological disorders to determine whether pain related to neurodegenerative diseases such as Alzheimer disease, Parkinson disease and amyotrophic lateral sclerosis are related to lesions or diseases of the central somatosensory system
Advance multimodal neurophysiology and imaging approaches to identify correlates of structural or functional reorganization and connectivity, including neuronal patterns (bursting, dysrhythmias), to inform neuromodulation therapies to reset away from an aberrant state
- Establish reliable diagnostic, prognostic, predictive and response biomarkers to improve clinical practice and trial design
- Move from empirical therapies to precision medicine including mechanism-based treatments, disease-modifying therapies, preventive treatments and individualized neuromodulation, for example, closed-loop deep brain stimulation
Explore sex-specific mechanisms and clinical presentations in CNP
In addition, large trials with rigorous study and appropriate controls are warranted to improve the management of CNP192. Stratified clinical trials and personalized treatment trials need large numbers of patients, which is only possible through multicentre clinical trials and standardized diagnostic criteria and outcome measures234.
Acknowledgements
J.R. is supported by a postdoctoral fellowship from the International Foundation for Research in Paraplegia (IRP, no. P191F) and the Clinical Research Priority Program (CRPP) “Pain” of the University of Zurich. D.C.A. is working at the Center for Neuroplasticity and Pain (CNAP), which is supported by the Danish National Research Foundation (DNRF121). D.C.A. is supported by a Novo Nordisk grant NNF21OC0072828 and ERC Horizon Europe Consolidator grant PersoNINpain 101087925. K.D.D. is the Canada Research Chair in Acute and Chronic Pain Research and is supported by funds from the Canadian Institutes of Health Research and the Mayday Fund. S.M.G. acknowledges support from the Rebecca L. Cooper Medical Research Foundation. R.P.S. is supported by NS 107364. N.B.F.’s research is supported by the Lundbeck Foundation R359-2020-2620.
Footnotes
Competing interests
J.R. and J.L.K.K. receive consultancy fees from AXONIS Therapeutics. N.B.F. has received consultancy fees from Vertex, Novartis Pharma, NeuroPN, Nanobiotix, Neurvati and Samiona and has undertaken consultancy work for Aarhus University with remunerated work for Biogen, Merz and Confo Therapeutics. She has received grants from IMI2PainCare an EU IMI 2 (Innovative Medicines Initiative) public–private consortium and the companies
References
- 1.Jensen TS et al. A new definition of neuropathic pain. Pain 152, 2204–2205 (2011). [DOI] [PubMed] [Google Scholar]
- 2. Scholz J. et al. The IASP classification of chronic pain for ICD-11: chronic neuropathic pain. Pain 160, 53–59 (2019). This paper is an overview of conditions included in the International Classification of Diseases 11th Revision classification of chronic neuropathic pain, including CNP.
- 3.Borsook D. Neurological diseases and pain. Brain 135, 320–344 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Widerström-Noga E, Loeser JD, Jensen TS & Finnerup NB AAPT diagnostic criteria for central neuropathic pain. J. Pain 18, 1417–1426 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Widerström-Noga E, Felix ER, Adcock JP, Escalona M & Tibbett J Multidimensional neuropathic pain phenotypes after spinal cord injury. J. Neurotrauma 33, 482–492 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Klit H, Finnerup NB, Andersen G & Jensen TS Central poststroke pain: a population-based study. Pain 152, 818–824 (2011). [DOI] [PubMed] [Google Scholar]
- 7.Treede RD, Hoheisel U, Wang D & Magerl W Central sensitization: clinical utility of a physiological concept for the International Statistical Classification of Diseases and Related Health Problems and for nociplastic pain. Pain 163, S99–s107 (2022). [DOI] [PubMed] [Google Scholar]
- 8. Colloca L. et al. Neuropathic pain. Nat. Rev. Dis. Primers 3, 17002 (2017). This paper is an overview of neuropathic pain, including mechanisms, diagnosis and management.
- 9.GBD 2019 Stroke Collaborators. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 20, 795–820 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Watson JC & Sandroni P Central neuropathic pain syndromes. Mayo Clin. Proc 91, 372–385 (2016). [DOI] [PubMed] [Google Scholar]
- 11.Liampas A. et al. Prevalence and management challenges in central post-stroke neuropathic pain: a systematic review and meta-analysis. Adv. Ther 37, 3278–3291 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vartiainen N. et al. Thalamic pain: anatomical and physiological indices of prediction. Brain 139, 708–722 (2016). [DOI] [PubMed] [Google Scholar]
- 13.Burke D, Fullen BM, Stokes D & Lennon O Neuropathic pain prevalence following spinal cord injury: a systematic review and meta-analysis. Eur. J. Pain 21, 29–44 (2017). [DOI] [PubMed] [Google Scholar]
- 14.Robayo LE et al. Multidimensional pain phenotypes after traumatic brain injury. Front. Pain. Res 3, 947562 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ofek H & Defrin R The characteristics of chronic central pain after traumatic brain injury. Pain 131, 330–340 (2007). [DOI] [PubMed] [Google Scholar]
- 16.Foley PL et al. Prevalence and natural history of pain in adults with multiple sclerosis: systematic review and meta-analysis. Pain 154, 632–642 (2013). [DOI] [PubMed] [Google Scholar]
- 17.Seixas D. et al. Pain in multiple sclerosis: a systematic review of neuroimaging studies. Neuroimage Clin. 5, 322–331 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Svendsen KB, Sørensen L, Jensen TS, Hansen HJ & Bach FW MRI of the central nervous system in MS patients with and without pain. Eur. J. Pain 15, 395–401 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Asseyer S, Cooper G & Paul F Pain in NMOSD and MOGAD: a systematic literature review of pathophysiology, symptoms, and current treatment strategies. Front. Neurol 11, 778 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersen G, Vestergaard K, Ingeman-Nielsen M & Jensen TS Incidence of central post-stroke pain. Pain 61, 187–193 (1995). [DOI] [PubMed] [Google Scholar]
- 21.Siddall PJ, McClelland JM, Rutkowski SB & Cousins MJ A longitudinal study of the prevalence and characteristics of pain in the first 5 years following spinal cord injury. Pain 103, 249–257 (2003). [DOI] [PubMed] [Google Scholar]
- 22.Finnerup NB et al. Phenotypes and predictors of pain following traumatic spinal cord injury: a prospective study. J. Pain 15, 40–48 (2014). [DOI] [PubMed] [Google Scholar]
- 23.Rosner J. et al. Characterization of hyperacute neuropathic pain after spinal cord injury: a prospective study. J. Pain 23, 89–97 (2022). [DOI] [PubMed] [Google Scholar]
- 24.Warner FM et al. Progression of neuropathic pain after acute spinal cord injury: a meta-analysis and framework for clinical trials. J. Neurotrauma 36, 1461–1468 (2019). [DOI] [PubMed] [Google Scholar]
- 25.Klit H, Finnerup NB & Jensen TS Central post-stroke pain: clinical characteristics, pathophysiology, and management. Lancet Neurol. 8, 857–868 (2009). [DOI] [PubMed] [Google Scholar]
- 26.Heitmann H. et al. Prevalence of neuropathic pain in early multiple sclerosis. Mult. Scler 22, 1224–1230 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Finnerup NB, Johannesen IL, Fuglsang-Frederiksen A, Bach FW & Jensen TS Sensory function in spinal cord injury patients with and without central pain. Brain 126, 57–70 (2003). [DOI] [PubMed] [Google Scholar]
- 28.Rivel M. et al. Central neuropathic pain in multiple sclerosis is associated with impaired innocuous thermal pathways and neuronal hyperexcitability. Pain. Med 22, 2311–2323 (2021). [DOI] [PubMed] [Google Scholar]
- 29.Klit H, Finnerup NB, Overvad K, Andersen G & Jensen TS Pain following stroke: a population-based follow-up study. PLoS One 6, e27607 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Donnell MJ et al. Chronic pain syndromes after ischemic stroke: PRoFESS trial. Stroke 44, 1238–1243 (2013). [DOI] [PubMed] [Google Scholar]
- 31.Harno H. et al. Central poststroke pain in young ischemic stroke survivors in the Helsinki Young Stroke Registry. Neurology 83, 1147–1154 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Gruener H. et al. Biomarkers for predicting central neuropathic pain occurrence and severity after spinal cord injury: results of a long-term longitudinal study. Pain 161, 545–556 (2020). [DOI] [PubMed] [Google Scholar]
- 33.Scheuren PS et al. Tracking changes in neuropathic pain after acute spinal cord injury. Front. Neurol 10, 90 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gagné M. et al. Conditioned pain modulation decreases over time in patients with neuropathic pain following a spinal cord injury. Neurorehabil. Neural Repair 34, 997–1008 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Defrin R. et al. From acute to long-term alterations in pain processing and modulation after spinal cord injury: mechanisms related to chronification of central neuropathic pain. Pain 163, e94–e105 (2022). [DOI] [PubMed] [Google Scholar]
- 36.Tackley G. et al. Chronic neuropathic pain severity is determined by lesion level in aquaporin 4-antibody-positive myelitis. J. Neurol. Neurosurg. Psychiatry 88, 165–169 (2017). [DOI] [PubMed] [Google Scholar]
- 37.Defrin R, Ohry A, Blumen N & Urca G Sensory determinants of thermal pain. Brain 125, 501–510 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Klit H, Hansen AP, Marcussen NS, Finnerup NB & Jensen TS Early evoked pain or dysesthesia is a predictor of central poststroke pain. Pain 155, 2699–2706 (2014). [DOI] [PubMed] [Google Scholar]
- 39.Baron R, Dickenson AH, Calvo M, Dib-Hajj SD & Bennett DL Maximizing treatment efficacy through patient stratification in neuropathic pain trials. Nat. Rev. Neurol 19, 53–64 (2023). [DOI] [PubMed] [Google Scholar]
- 40.Attal N & Bouhassira D Translational neuropathic pain research. Pain 160, S23–s28 (2019). [DOI] [PubMed] [Google Scholar]
- 41.Themistocleous AC, Crombez G, Baskozos G & Bennett DL Using stratified medicine to understand, diagnose, and treat neuropathic pain. Pain 159, S31–s42 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finnerup NB, Kuner R & Jensen TS Neuropathic pain: from mechanisms to treatment. Physiol. Rev 101, 259–301 (2021). [DOI] [PubMed] [Google Scholar]
- 43.Gurba KN, Chaudhry R & Haroutounian S Central neuropathic pain syndromes: current and emerging pharmacological strategies. CNS Drugs 36, 483–516 (2022). [DOI] [PubMed] [Google Scholar]
- 44.Costigan M, Scholz J & Woolf CJ Neuropathic pain: a maladaptive response of the nervous system to damage. Annu. Rev. Neurosci 32, 1–32 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gwak YS & Hulsebosch CE Neuronal hyperexcitability: a substrate for central neuropathic pain after spinal cord injury. Curr. Pain Headache Rep 15, 215–222 (2011). [DOI] [PubMed] [Google Scholar]
- 46.Peirs C & Seal RP Neural circuits for pain: recent advances and current views. Science 354, 578–584 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Falci S, Best L, Bayles R, Lammertse D & Starnes C Dorsal root entry zone microcoagulation for spinal cord injury-related central pain: operative intramedullary electrophysiological guidance and clinical outcome. J. Neurosurg 97, 193–200 (2002). [DOI] [PubMed] [Google Scholar]
- 48.Hirayama T, Dostrovsky JO, Gorecki J, Tasker RR & Lenz FA Recordings of abnormal activity in patients with deafferentation and central pain. Stereotact. Funct. Neurosurg 52, 120–126 (1989). [DOI] [PubMed] [Google Scholar]
- 49.Radhakrishnan V. et al. A comparison of the burst activity of lateral thalamic neurons in chronic pain and non-pain patients. Pain 80, 567–575 (1999). [DOI] [PubMed] [Google Scholar]
- 50.Tasker RR, Gorecki J, Lenz FA, Hirayama T & Dostrovsky JO Thalamic microelectrode recording and microstimulation in central and deafferentation pain. Appl. Neurophysiol 50, 414–417 (1987). [DOI] [PubMed] [Google Scholar]
- 51. Melzack R & Loeser JD Phantom body pain in paraplegics: evidence for a central “pattern generating mechanism” for pain. Pain 4, 195–210 (1978). This is a landmark study providing neurophysiological evidence of an ectopic pain generator within the CNS after SCI.
- 52.Garcia-Larrea L. et al. Operculo-insular pain (parasylvian pain): a distinct central pain syndrome. Brain 133, 2528–2539 (2010). [DOI] [PubMed] [Google Scholar]
- 53.Greenspan JD & Winfield JA Reversible pain and tactile deficits associated with a cerebral tumor compressing the posterior insula and parietal operculum. Pain 50, 29–39 (1992). [DOI] [PubMed] [Google Scholar]
- 54.Jobst BC et al. The insula and its epilepsies. Epilepsy Curr. 19, 11–21 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Delboni Lemos M. et al. Dissecting neuropathic from poststroke pain: the white matter within. Pain 163, 765–778 (2022). [DOI] [PubMed] [Google Scholar]
- 56.Dum RP, Levinthal DJ & Strick PL The spinothalamic system targets motor and sensory areas in the cerebral cortex of monkeys. J. Neurosci 29, 14223–14235 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Jensen TS & Finnerup NB Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol. 13, 924–935 (2014). [DOI] [PubMed] [Google Scholar]
- 58.Bennett GJ What is spontaneous pain and who has it? J. Pain 13, 921–929 (2012). [DOI] [PubMed] [Google Scholar]
- 59.Haroutounian S. et al. How central is central poststroke pain? The role of afferent input in poststroke neuropathic pain: a prospective, open-label pilot study. Pain 159, 1317–1324 (2018). [DOI] [PubMed] [Google Scholar]
- 60.Zeilig G, Enosh S, Rubin-Asher D, Lehr B & Defrin R The nature and course of sensory changes following spinal cord injury: predictive properties and implications on the mechanism of central pain. Brain 135, 418–430 (2012). [DOI] [PubMed] [Google Scholar]
- 61.Jiang L, Voulalas P, Ji Y & Masri R Post-translational modification of cortical GluA receptors in rodents following spinal cord lesion. Neuroscience 316, 122–129 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Waxman SG & Hains BC Fire and phantoms after spinal cord injury: Na+ channels and central pain. Trends Neurosci. 29, 207–215 (2006). [DOI] [PubMed] [Google Scholar]
- 63.Black JA, Newcombe J & Waxman SG Astrocytes within multiple sclerosis lesions upregulate sodium channel Nav1.5. Brain 133, 835–846 (2010). [DOI] [PubMed] [Google Scholar]
- 64.Boroujerdi A. et al. Calcium channel alpha-2-delta-1 protein upregulation in dorsal spinal cord mediates spinal cord injury-induced neuropathic pain states. Pain 152, 649–655 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bradl M. et al. Pain in neuromyelitis optica — prevalence, pathogenesis and therapy. Nat. Rev. Neurol 10, 529–536 (2014). [DOI] [PubMed] [Google Scholar]
- 66.Sandkühler J. The organization and function of endogenous antinociceptive systems. Prog. Neurobiol 50, 49–81 (1996). [DOI] [PubMed] [Google Scholar]
- 67.Coull JA et al. BDNF from microglia causes the shift in neuronal anion gradient underlying neuropathic pain. Nature 438, 1017–1021 (2005). [DOI] [PubMed] [Google Scholar]
- 68.Boyle KA et al. Defining a spinal microcircuit that gates myelinated afferent input: implications for tactile allodynia. Cell Rep. 28, 526–540.e526 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Prescott SA, Ma Q & De Koninck Y Normal and abnormal coding of somatosensory stimuli causing pain. Nat. Neurosci 17, 183–191 (2014). This paper is an overview of central somatosensory coding in physiology and related to pain pathophysiology.
- 70.Hansen C, Hopf HC & Treede RD Paradoxical heat sensation in patients with multiple sclerosis. Evidence for a supraspinal integration of temperature sensation. Brain 119, 1729–1736 (1996). [DOI] [PubMed] [Google Scholar]
- 71.Schaldemose EL, Raaschou-Nielsen L, Böhme RA, Finnerup NB & Fardo F It is one or the other: no overlap between healthy individuals perceiving thermal grill illusion or paradoxical heat sensation. Neurosci. Lett 802, 137169 (2023). [DOI] [PubMed] [Google Scholar]
- 72.Vollert J. et al. Paradoxical heat sensation as a manifestation of thermal hypesthesia: a study of 1090 patients with lesions of the somatosensory system. Pain 10.1097/j.pain.0000000000003014 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fardo F, Beck B, Allen M & Finnerup NB Beyond labeled lines: a population coding account of the thermal grill illusion. Neurosci. Biobehav. Rev 108, 472–479 (2020). [DOI] [PubMed] [Google Scholar]
- 74.Hanisch UK Microglia as a source and target of cytokines. Glia 40, 140–155 (2002). [DOI] [PubMed] [Google Scholar]
- 75.Zhou X, He X & Ren Y Function of microglia and macrophages in secondary damage after spinal cord injury. Neural Regen. Res 9, 1787–1795, (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Scholz J & Woolf CJ The neuropathic pain triad: neurons, immune cells and glia. Nat. Neurosci 10, 1361–1368 (2007). [DOI] [PubMed] [Google Scholar]
- 77.Silva R & Malcangio M Fractalkine/CX(3)CR(1) pathway in neuropathic pain: an update. Front. Pain Res 2, 684684 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kuan YH, Shih HC, Tang SC, Jeng JS & Shyu BC Targeting P(2)X(7) receptor for the treatment of central post-stroke pain in a rodent model. Neurobiol. Dis 78, 134–145 (2015). [DOI] [PubMed] [Google Scholar]
- 79.Clark AK et al. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc. Natl Acad. Sci. USA 104, 10655–10660 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Staniland AA et al. Reduced inflammatory and neuropathic pain and decreased spinal microglial response in fractalkine receptor (CX3CR1) knockout mice. J. Neurochem 114, 1143–1157 (2010). [DOI] [PubMed] [Google Scholar]
- 81.Baastrup C, Maersk-Moller CC, Nyengaard JR, Jensen TS & Finnerup NB Spinal-, brainstem- and cerebrally mediated responses at- and below-level of a spinal cord contusion in rats: evaluation of pain-like behavior. Pain 151, 670–679 (2010). [DOI] [PubMed] [Google Scholar]
- 82.Tappe-Theodor A, King T & Morgan MM Pros and cons of clinically relevant methods to assess pain in rodents. Neurosci. Biobehav. Rev 100, 335–343 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Tuttle AH et al. A deep neural network to assess spontaneous pain from mouse facial expressions. Mol. Pain 14, 1744806918763658 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bohic M. et al. Mapping the neuroethological signatures of pain, analgesia, and recovery in mice. Neuron 111, 2811–2830.e8 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jones AK, Watabe H, Cunningham VJ & Jones T Cerebral decreases in opioid receptor binding in patients with central neuropathic pain measured by [11C] diprenorphine binding and PET. Eur. J. Pain 8, 479–485 (2004). [DOI] [PubMed] [Google Scholar]
- 86.Widerström-Noga E. et al. Metabolite concentrations in the anterior cingulate cortex predict high neuropathic pain impact after spinal cord injury. Pain 154, 204–212 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Widerström-Noga E, Cruz-Almeida Y, Felix ER & Pattany PM Somatosensory phenotype is associated with thalamic metabolites and pain intensity after spinal cord injury. Pain 156, 166–174 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gustin SM, Wrigley PJ, Siddall PJ & Henderson LA Brain anatomy changes associated with persistent neuropathic pain following spinal cord injury. Cereb. Cortex 20, 1409–1419 (2010). [DOI] [PubMed] [Google Scholar]
- 89.Gustin SM et al. Thalamic activity and biochemical changes in individuals with neuropathic pain after spinal cord injury. Pain 155, 1027–1036 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lütolf R. et al. Anti- and Pro-Nociceptive mechanisms in neuropathic pain after human spinal cord injury. Eur. J. Pain 26, 2176–2187 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Albu S, Gómez-Soriano J, Avila-Martin G & Taylor J Deficient conditioned pain modulation after spinal cord injury correlates with clinical spontaneous pain measures. Pain 156, 260–272 (2015). [DOI] [PubMed] [Google Scholar]
- 92.Barbosa LM et al. Corticomotor excitability is altered in central neuropathic pain compared with non-neuropathic pain or pain-free patients. Neurophysiol. Clin 53, 102845 (2023). [DOI] [PubMed] [Google Scholar]
- 93.Mussigmann T, Bardel B & Lefaucheur JP Resting-state electroencephalography (EEG) biomarkers of chronic neuropathic pain. A systematic review. Neuroimage 258, 119351 (2022). [DOI] [PubMed] [Google Scholar]
- 94.Lefaucheur JP, Drouot X, Ménard-Lefaucheur I, Keravel Y & Nguyen JP Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic pain. Neurology 67, 1568–1574 (2006). [DOI] [PubMed] [Google Scholar]
- 95.Llinás RR, Ribary U, Jeanmonod D, Kronberg E & Mitra PP Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc. Natl Acad. Sci. USA 96, 15222–15227 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Fauchon C. et al. A hidden Markov model reveals magnetoencephalography spectral frequency-specific abnormalities of brain state power and phase-coupling in neuropathic pain. Commun. Biol 5, 1000 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kim JA & Davis KD Neural oscillations: understanding a neural code of pain. Neuroscientist 27, 544–570 (2021). [DOI] [PubMed] [Google Scholar]
- 98.Fauchon C. et al. Exploring sex differences in alpha brain activity as a potential neuromarker associated with neuropathic pain. Pain 163, 1291–1302 (2022). [DOI] [PubMed] [Google Scholar]
- 99.Kisler LB et al. Abnormal alpha band power in the dynamic pain connectome is a marker of chronic pain with a neuropathic component. Neuroimage Clin. 26, 102241 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kim JA et al. Cross-network coupling of neural oscillations in the dynamic pain connectome reflects chronic neuropathic pain in multiple sclerosis. Neuroimage Clin. 26, 102230 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim JA et al. Neuropathic pain and pain interference are linked to alpha-band slowing and reduced beta-band magnetoencephalography activity within the dynamic pain connectome in patients with multiple sclerosis. Pain 160, 187–197 (2019). [DOI] [PubMed] [Google Scholar]
- 102.Boord P. et al. Electroencephalographic slowing and reduced reactivity in neuropathic pain following spinal cord injury. Spinal Cord 46, 118–123 (2008). [DOI] [PubMed] [Google Scholar]
- 103.Lenz FA, Kwan HC, Dostrovsky JO & Tasker RR Characteristics of the bursting pattern of action potentials that occurs in the thalamus of patients with central pain. Brain Res. 496, 357–360 (1989). [DOI] [PubMed] [Google Scholar]
- 104.Lenz FA et al. Stimulation in the human somatosensory thalamus can reproduce both the affective and sensory dimensions of previously experienced pain. Nat. Med 1, 910–913 (1995). [DOI] [PubMed] [Google Scholar]
- 105.Lenz FA et al. Thermal and pain sensations evoked by microstimulation in the area of human ventrocaudal nucleus. J. Neurophysiol 70, 200–212 (1993). [DOI] [PubMed] [Google Scholar]
- 106.Davis KD, Kiss ZH, Tasker RR & Dostrovsky JO Thalamic stimulation-evoked sensations in chronic pain patients and in nonpain (movement disorder) patients. J. Neurophysiol 75, 1026–1037 (1996). [DOI] [PubMed] [Google Scholar]
- 107.Vierck C. Mechanisms of below-level pain following spinal cord injury (SCI). J. Pain 21, 262–280 (2020). [DOI] [PubMed] [Google Scholar]
- 108.Davis KD Neurophysiological and anatomical considerations in functional imaging of pain. Pain 105, 1–3 (2003). [DOI] [PubMed] [Google Scholar]
- 109. Davis KD et al. Brain imaging tests for chronic pain: medical, legal and ethical issues and recommendations. Nat. Rev. Neurol 13, 624–638 (2017). This paper is an overview of ethical and legal issues for brain imaging pain biomarker development.
- 110.Iannetti GD, Salomons TV, Moayedi M, Mouraux A & Davis KD Beyond metaphor: contrasting mechanisms of social and physical pain. Trends Cogn. Sci 17, 371–378 (2013). [DOI] [PubMed] [Google Scholar]
- 111.Mazzola L, Isnard J, Peyron R & Mauguière F Stimulation of the human cortex and the experience of pain: wilder penfield’s observations revisited. Brain 135, 631–640 (2012). [DOI] [PubMed] [Google Scholar]
- 112.Afif A, Minotti L, Kahane P & Hoffmann D Anatomofunctional organization of the insular cortex: a study using intracerebral electrical stimulation in epileptic patients. Epilepsia 51, 2305–2315 (2010). [DOI] [PubMed] [Google Scholar]
- 113.Ducreux D, Attal N, Parker F & Bouhassira D Mechanisms of central neuropathic pain: a combined psychophysical and fMRI study in syringomyelia. Brain 129, 963–976 (2006). [DOI] [PubMed] [Google Scholar]
- 114.Hatem SM et al. Clinical, functional and structural determinants of central pain in syringomyelia. Brain 133, 3409–3422 (2010). [DOI] [PubMed] [Google Scholar]
- 115.KyathanahaUy SP et al. Microstructural plasticity in nociceptive pathways after spinal cord injury. J. Neurol. Neurosurg. Psychiatry 92, 863–871 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wrigley PJ et al. Neuropathic pain and primary somatosensory cortex reorganization following spinal cord injury. Pain 141, 52–59 (2009). [DOI] [PubMed] [Google Scholar]
- 117.Jutzeler CR, Freund P, Huber E, Curt A & Kramer JLK Neuropathic pain and functional reorganization in the primary sensorimotor cortex after spinal cord injury. J. Pain 16, 1256–1267 (2015). [DOI] [PubMed] [Google Scholar]
- 118.Kowalski JL et al. Resting state functional connectivity differentiation of neuropathic and nociceptive pain in individuals with chronic spinal cord injury. Neuroimage Clin. 38, 103414 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Huynh V. et al. Supraspinal nociceptive networks in neuropathic pain after spinal cord injury. Hum. Brain Mapp 42, 3733–3749 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kucyi A & Davis KD The dynamic pain connectome. Trends Neurosci. 38, 86–95 (2015). [DOI] [PubMed] [Google Scholar]
- 121.Kucyi A & Davis KD The neural code for pain: from single-cell electrophysiology to the dynamic pain connectome. Neuroscientist 23, 397–414 (2017). [DOI] [PubMed] [Google Scholar]
- 122.Cruccu G. et al. Trigeminal neuralgia: new classification and diagnostic grading for practice and research. Neurology 87, 220–228 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.McHenry KW in Spinal Cord Injury Pain (eds. Sang CN & Hulsebosch CE) xxiii–xxx (Academic Press, 2022). [Google Scholar]
- 124.Berić A, Dimitrijević MR & Lindblom U Central dysesthesia syndrome in spinal cord injury patients. Pain 34, 109–116 (1988). [DOI] [PubMed] [Google Scholar]
- 125.Widerström-Noga E. Neuropathic pain and spinal cord injury: management, phenotypes, and biomarkers. Drugs 83, 1001–1025 (2023). [DOI] [PubMed] [Google Scholar]
- 126.Fitzek S. et al. Pain and itch in Wallenberg’s syndrome: anatomical–functional correlations. Suppl. Clin. Neurophysiol 58, 187–194 (2006). [PubMed] [Google Scholar]
- 127. Finnerup NB et al. Neuropathic pain: an updated grading system for research and clinical practice. Pain 157, 1599–1606 (2016). This study is an overview of a diagnostic approach towards neuropathic pain, including various stages of diagnostic certainty, which has important implications for research and clinical practice.
- 128. Truini A. et al. Joint European Academy of Neurology-European Pain Federation-Neuropathic Pain Special Interest Group of the International Association for the Study of Pain guidelines on neuropathic pain assessment. Eur. J. Neurol 30, 2177–2196 (2023). This paper is an overview of multimodal neuropathic pain assesssment.
- 129.Rosner J. et al. Assessment of neuropathic pain after spinal cord injury using quantitative pain drawings. Spinal Cord. 59, 529–537 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Boivie J, Leijon G & Johansson I Central post-stroke pain–a study of the mechanisms through analyses of the sensory abnormalities. Pain 37, 173–185 (1989). [DOI] [PubMed] [Google Scholar]
- 131.Bowsher D. Central pain: clinical and physiological characteristics. J. Neurol. Neurosurg. Psychiatry 61, 62–69 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Leijon G & Bowsher D Somatosensory findings in central post-stroke pain (CPSP) and controls. Pain 41, S468 (1990). [Google Scholar]
- 133.Scheuren PS et al. Combined neurophysiologic and neuroimaging approach to reveal the structure-function paradox in cervical myelopathy. Neurology 97, e1512–e1522 (2021). [DOI] [PubMed] [Google Scholar]
- 134.Lütolf R, Rosner J, Curt A & Hubli M Indicators of central sensitization in chronic neuropathic pain after spinal cord injury. Eur. J. Pain 26, 2162–2175 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Convers P. et al. A hidden mesencephalic variant of central pain. Eur. J. Pain 24, 1393–1399 (2020). [DOI] [PubMed] [Google Scholar]
- 136.Garcia-Larrea L & Hagiwara K Electrophysiology in diagnosis and management of neuropathic pain. Rev. Neurol 175, 26–37 (2019). [DOI] [PubMed] [Google Scholar]
- 137.Garcia-Larrea L & Peyron R Pain matrices and neuropathic pain matrices: a review. Pain 154, S29–s43 (2013). [DOI] [PubMed] [Google Scholar]
- 138.Valerio F. et al. Characterization of pain syndromes in patients with neuromyelitis optica. Eur. J. Pain 24, 1548–1568 (2020). [DOI] [PubMed] [Google Scholar]
- 139.Lopes LCG et al. Beyond weakness: characterization of pain, sensory profile and conditioned pain modulation in patients with motor neuron disease: a controlled study. Eur. J. Pain 22, 72–83 (2018). [DOI] [PubMed] [Google Scholar]
- 140.Cury RG et al. Effects of deep brain stimulation on pain and other nonmotor symptoms in Parkinson disease. Neurology 83, 1403–1409 (2014). [DOI] [PubMed] [Google Scholar]
- 141.Finnerup NB Pain in patients with spinal cord injury. Pain 154, S71–s76 (2013). [DOI] [PubMed] [Google Scholar]
- 142.Finnerup NB et al. A prospective study of pain and psychological functioning following traumatic spinal cord injury. Spinal Cord 54, 816–821 (2016). [DOI] [PubMed] [Google Scholar]
- 143.Siddall PJ & Middleton JW Spinal cord injury-induced pain: mechanisms and treatments. Pain Manag. 5, 493–507 (2015). [DOI] [PubMed] [Google Scholar]
- 144.Bouhassira D & Attal N Diagnosis and assessment of neuropathic pain: the saga of clinical tools. Pain 152, S74–s83 (2011). [DOI] [PubMed] [Google Scholar]
- 145.Jensen MP, Friedman M, Bonzo D & Richards P The validity of the neuropathic pain scale for assessing diabetic neuropathic pain in a clinical trial. Clin. J. Pain 22, 97–103 (2006). [DOI] [PubMed] [Google Scholar]
- 146.Bouhassira D. et al. Comparison of pain syndromes associated with nervous or somatic lesions and development of a new neuropathic pain diagnostic questionnaire (DN4). Pain 114, 29–36 (2005). [DOI] [PubMed] [Google Scholar]
- 147.Bennett MI, Smith BH, Torrance N & Potter J The S-LANSS score for identifying pain of predominantly neuropathic origin: validation for use in clinical and postal research. J. Pain 6, 149–158 (2005). [DOI] [PubMed] [Google Scholar]
- 148.Attal N, Bouhassira D & Baron R Diagnosis and assessment of neuropathic pain through questionnaires. Lancet Neurol. 17, 456–466 (2018). [DOI] [PubMed] [Google Scholar]
- 149.Hallström H & Norrbrink C Screening tools for neuropathic pain: can they be of use in individuals with spinal cord injury? Pain 152, 772–779 (2011). [DOI] [PubMed] [Google Scholar]
- 150.Georgieva M. et al. Fatty acid suppression of glial activation prevents central neuropathic pain after spinal cord injury. Pain 160, 2724–2742 (2019). [DOI] [PubMed] [Google Scholar]
- 151.Lampl C, Yazdi K & Röper C Amitriptyline in the prophylaxis of central poststroke pain. Preliminary results of 39 patients in a placebo-controlled, long-term study. Stroke 33, 3030–3032 (2002). [DOI] [PubMed] [Google Scholar]
- 152.Salinas FA, Lugo LH & García HI Efficacy of early treatment with carbamazepine in prevention of neuropathic pain in patients with spinal cord injury. Am. J. Phys. Med. Rehabil 91, 1020–1027 (2012). [DOI] [PubMed] [Google Scholar]
- 153.Norrbrink C, Sörling K, Hultling C, von Kieseritzky F & Wahman K Challenges and facilitators-navigating in the landscape of spinal cord injury neuropathic pain”-a qualitative study on the use of prescribed drugs. Spinal Cord 59, 215–224 (2021). [DOI] [PubMed] [Google Scholar]
- 154.Henwood P & Ellis JA Chronic neuropathic pain in spinal cord injury: the patient’s perspective. Pain Res. Manag 9, 39–45 (2004). [DOI] [PubMed] [Google Scholar]
- 155.Williams ACC, Fisher E, Hearn L & Eccleston C Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst. Rev 8, CD007407 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fordham B. et al. The evidence for cognitive behavioural therapy in any condition, population or context: a meta-review of systematic reviews and panoramic meta-analysis. Psychol. Med 51, 21–29 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kannan P, Bello UM & Winser SJ Physiotherapy interventions may relieve pain in individuals with central neuropathic pain: a systematic review and meta-analysis of randomised controlled trials. Ther. Adv. Chronic Dis 13, 20406223221078672 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ma X, Chen R, Li W & Huang P A systematic review and meta-analysis of pain neuroscience education for chronic low back pain: short-term outcomes of pain and disability. Physiother. Theory Pract 10.1080/09593985.2023.2232003 (2023). [DOI] [PubMed] [Google Scholar]
- 159.Bülow K, Lindberg K, Vaegter HB & Juhl CB Effectiveness of pain neurophysiology education on musculoskeletal pain: a systematic review and meta-analysis. Pain Med. 22, 891–904 (2021). [DOI] [PubMed] [Google Scholar]
- 160.Ju ZY et al. Acupuncture for neuropathic pain in adults. Cochrane Database Syst. Rev 12, CD012057 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.McKittrick ML, Connors EL & McKernan LC Hypnosis for chronic neuropathic pain: a scoping review. Pain Med. 23, 1015–1026 (2022). [DOI] [PubMed] [Google Scholar]
- 162.Hesam-Shariati N. et al. The analgesic effect of electroencephalographic neurofeedback for people with chronic pain: a systematic review and meta-analysis. Eur. J. Neurol 29, 921–936 (2022). [DOI] [PubMed] [Google Scholar]
- 163.Austin PD & Siddall PJ Virtual reality for the treatment of neuropathic pain in people with spinal cord injuries: a scoping review. J. Spinal Cord Med 44, 8–18 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Trost Z. et al. Immersive interactive virtual walking reduces neuropathic pain in spinal cord injury: findings from a preliminary investigation of feasibility and clinical efficacy. Pain 163, 350–361 (2022). [DOI] [PubMed] [Google Scholar]
- 165.Finnerup NB et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 14, 162–173 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Finnerup NB et al. Neuropathic pain clinical trials: factors associated with decreases in estimated drug efficacy. Pain 159, 2339–2346 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Siddall PJ et al. Pregabalin in central neuropathic pain associated with spinal cord injury: a placebo-controlled trial. Neurology 67, 1792–1800 (2006). [DOI] [PubMed] [Google Scholar]
- 168.Cardenas DD et al. A randomized trial of pregabalin in patients with neuropathic pain due to spinal cord injury. Neurology 80, 533–539 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ushida T. et al. Mirogabalin for central neuropathic pain after spinal cord injury: a randomized, double-blind, placebo-controlled, phase 3 study in Asia. Neurology 100, e1193–e1206 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Kremer M, Salvat E, Muller A, Yalcin I & Barrot M Antidepressants and gabapentinoids in neuropathic pain: mechanistic insights. Neuroscience 338, 183–206 (2016). [DOI] [PubMed] [Google Scholar]
- 171.Vranken JH et al. Pregabalin in patients with central neuropathic pain: a randomized, double-blind, placebo-controlled trial of a flexible-dose regimen. Pain 136, 150–157 (2008). [DOI] [PubMed] [Google Scholar]
- 172.Leijon G & Boivie J Central post-stroke pain–a controlled trial of amitriptyline and carbamazepine. Pain 36, 27–36 (1989). [DOI] [PubMed] [Google Scholar]
- 173.Rintala DH et al. Comparison of the effectiveness of amitriptyline and gabapentin on chronic neuropathic pain in persons with spinal cord injury. Arch. Phys. Med. Rehabil 88, 1547–1560 (2007). [DOI] [PubMed] [Google Scholar]
- 174.Brown TR & Slee A A randomized placebo-controlled trial of duloxetine for central pain in multiple sclerosis. Int. J. MS Care 17, 83–89 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Vollmer TL, Robinson MJ, Risser RC & Malcolm SK A randomized, double-blind, placebo-controlled trial of duloxetine for the treatment of pain in patients with multiple sclerosis. Pain Pract. 14, 732–744 (2014). [DOI] [PubMed] [Google Scholar]
- 176.Mahesh B. et al. Efficacy of duloxetine in patients with central post-stroke pain: a randomized double blind placebo controlled trial. Pain Med. 24, 610–617 (2023). [DOI] [PubMed] [Google Scholar]
- 177.Vranken JH et al. Duloxetine in patients with central neuropathic pain caused by spinal cord injury or stroke: a randomized, double-blind, placebo-controlled trial. Pain 152, 267–273 (2011). [DOI] [PubMed] [Google Scholar]
- 178.Vestergaard K, Andersen G, Gottrup H, Kristensen BT & Jensen TS Lamotrigine for central poststroke pain: a randomized controlled trial. Neurology 56, 184–190 (2001). [DOI] [PubMed] [Google Scholar]
- 179.Finnerup NB, Sindrup SH, Bach FW, Johannesen IL & Jensen TS Lamotrigine in spinal cord injury pain: a randomized controlled trial. Pain 96, 375–383 (2002). [DOI] [PubMed] [Google Scholar]
- 180.Norrbrink C & Lundeberg T Tramadol in neuropathic pain after spinal cord injury: a randomized, double-blind, placebo-controlled trial. Clin. J. Pain 25, 177–184 (2009). [DOI] [PubMed] [Google Scholar]
- 181.Hansen JS et al. Cannabis-based medicine for neuropathic pain and spasticity-a multicenter, randomized, double-blinded, placebo-controlled trial. Pharmaceuticals 16, 1079 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Mikkelsen N, Damkier P & Pedersen SA Serotonin syndrome-a focused review. Basic Clin. Pharmacol. Toxicol 133, 124–129 (2023). [DOI] [PubMed] [Google Scholar]
- 183.Moisset X, de Andrade DC & Bouhassira D From pulses to pain relief: an update on the mechanisms of rTMS-induced analgesic effects. Eur. J. Pain 20, 689–700 (2016). [DOI] [PubMed] [Google Scholar]
- 184.Maarrawi J. et al. Brain opioid receptor density predicts motor cortex stimulation efficacy for chronic pain. Pain 154, 2563–2568 (2013). [DOI] [PubMed] [Google Scholar]
- 185.García-Larrea DCDAAL Beyond trial-and-error: individualizing therapeutic transcranial neuromodulation for chronic pain. Eur. J. Pain 27, 1065–1083 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Gordon EM et al. A somato-cognitive action network alternates with effector regions in motor cortex. Nature 617, 351–359 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fonoff ET et al. Antinociception induced by epidural motor cortex stimulation in naive conscious rats is mediated by the opioid system. Behav. Brain Res. 196, 63–70 (2009). [DOI] [PubMed] [Google Scholar]
- 188.Maarrawi J. et al. Motor cortex stimulation for pain control induces changes in the endogenous opioid system. Neurology 69, 827–834 (2007). [DOI] [PubMed] [Google Scholar]
- 189.Lapa J. et al. Burst transspinal magnetic stimulation alleviates nociceptive pain in parkinson disease-a pilot phase II double-blind, randomized study. Neuromodulation 26, 840–849 (2022). [DOI] [PubMed] [Google Scholar]
- 190.Attal N. et al. Repetitive transcranial magnetic stimulation for neuropathic pain: a randomized multicentre sham-controlled trial. Brain 144, 3328–3339 (2021). [DOI] [PubMed] [Google Scholar]
- 191.Nuti C. et al. Motor cortex stimulation for refractory neuropathic pain: four year outcome and predictors of efficacy. Pain 118, 43–52 (2005). [DOI] [PubMed] [Google Scholar]
- 192.Knotkova H. et al. Neuromodulation for chronic pain. Lancet 397, 2111–2124 (2021). [DOI] [PubMed] [Google Scholar]
- 193.Lefaucheur JP et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014-2018). Clin. Neurophysiol 131, 474–528 (2020). [DOI] [PubMed] [Google Scholar]
- 194.Moisset X. et al. Pharmacological and non-pharmacological treatments for neuropathic pain: systematic review and French recommendations. Rev. Neurol 176, 325–352 (2020). [DOI] [PubMed] [Google Scholar]
- 195.Hamani C. et al. Motor cortex stimulation for chronic neuropathic pain: results of a double-blind randomized study. Brain 144, 2994–3004 (2021). [DOI] [PubMed] [Google Scholar]
- 196.de Oliveira RA et al. Repetitive transcranial magnetic stimulation of the left premotor/ dorsolateral prefrontal cortex does not have analgesic effect on central poststroke pain. J. Pain 15, 1271–1281 (2014). [DOI] [PubMed] [Google Scholar]
- 197.Galhardoni R. et al. Insular and anterior cingulate cortex deep stimulation for central neuropathic pain: disassembling the percept of pain. Neurology 92, e2165–e2175 (2019). [DOI] [PubMed] [Google Scholar]
- 198.Quesada C. et al. New procedure of high-frequency repetitive transcranial magnetic stimulation for central neuropathic pain: a placebo-controlled randomized crossover study. Pain 161, 718–728 (2020). [DOI] [PubMed] [Google Scholar]
- 199.Wrigley PJ et al. Longstanding neuropathic pain after spinal cord injury is refractory to transcranial direct current stimulation: a randomized controlled trial. Pain 154, 2178–2184 (2013). [DOI] [PubMed] [Google Scholar]
- 200.Fregni F. et al. A sham-controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain 122, 197–209 (2006). [DOI] [PubMed] [Google Scholar]
- 201.Soler MD et al. Effectiveness of transcranial direct current stimulation and visual illusion on neuropathic pain in spinal cord injury. Brain 133, 2565–2577 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Matthiesen ST, Lunde SJ, Wohlert Kjær S, Carlino E & Vase L Placebo analgesia effects across central nervous system diseases: what do we know and where do we need to go? Pain Rep. 4, e717 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Müller R. et al. Chronic pain, depression and quality of life in individuals with spinal cord injury: mediating role of participation. J. Rehabil. Med 49, 489–496 (2017). [DOI] [PubMed] [Google Scholar]
- 204.Sturm C. et al. Which factors have an association to the quality of life (QoL) of people with acquired spinal cord injury (SCI)? A cross-sectional explorative observational study. Spinal Cord. 59, 925–932 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Weyer Jamora C, Schroeder SC & Ruff RM Pain and mild traumatic brain injury: the implications of pain severity on emotional and cognitive functioning. Brain Inj. 27, 1134–1140 (2013). [DOI] [PubMed] [Google Scholar]
- 206.Norrbrink Budh C, Kowalski J & Lundeberg T A comprehensive pain management programme comprising educational, cognitive and behavioural interventions for neuropathic pain following spinal cord injury. J. Rehabil. Med 38, 172–180 (2006). [DOI] [PubMed] [Google Scholar]
- 207.Naess H, Beiske AG & Myhr KM Quality of life among young patients with ischaemic stroke compared with patients with multiple sclerosis. Acta Neurol. Scand 117, 181–185 (2008). [DOI] [PubMed] [Google Scholar]
- 208.Lee BB, Simpson JM, King MT, Haran MJ & Marial O The SF-36 walk-wheel: a simple modification of the SF-36 physical domain improves its responsiveness for measuring health status change in spinal cord injury. Spinal Cord 47, 50–55 (2009). [DOI] [PubMed] [Google Scholar]
- 209.Choi-Kwon S, Choi JM, Kwon SU, Kang DW & Kim JS Factors that affect the quality of life at 3 years post-stroke. J. Clin. Neurol 2, 34–41 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Payton H & Soundy A The experience of post-stroke pain and the impact on quality of life: an integrative review. Behav. Sci 10, 128 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Young J, Amatya B, Galea MP & Khan F Chronic pain in multiple sclerosis: a 10-year longitudinal study. Scand. J. Pain 16, 198–203 (2017). [DOI] [PubMed] [Google Scholar]
- 212.Gustavsen S. et al. The association of selected multiple sclerosis symptoms with disability and quality of life: a large Danish self-report survey. BMC Neurol. 21, 317 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhang Y. et al. Feelings of depression, pain and walking difficulties have the largest impact on the quality of life of people with multiple sclerosis, irrespective of clinical phenotype. Mult. Scler 27, 1262–1275 (2021). [DOI] [PubMed] [Google Scholar]
- 214.Mori F. et al. Effects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis. J. Pain 11, 436–442 (2010). [DOI] [PubMed] [Google Scholar]
- 215.Di Lionardo A. et al. Modulation of the N13 component of the somatosensory evoked potentials in an experimental model of central sensitization in humans. Sci. Rep 11, 20838 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Salameh C, Perchet C, Hagiwara K & Garcia-Larrea L Sympathetic skin response as an objective tool to estimate stimulus-associated arousal in a human model of hyperalgesia. Neurophysiol. Clin 52, 436–445 (2022). [DOI] [PubMed] [Google Scholar]
- 217.Ahanonu B, Crowther A, Kania A, Casillas MR & Basbaum A Long-term optical imaging of the spinal cord in awake, behaving animals. Preprint at bioRxiv 10.1101/2023.05.22.541477 (2023). [DOI] [Google Scholar]
- 218.Sternson SM & Roth BL Chemogenetic tools to interrogate brain functions. Annu. Rev. Neurosci 37, 387–407 (2014). [DOI] [PubMed] [Google Scholar]
- 219.Kim CK, Adhikari A & Deisseroth K Integration of optogenetics with complementary methodologies in systems neuroscience. Nat. Rev. Neurosci 18, 222–235 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Renthal W. et al. Human cells and networks of pain: transforming pain target identification and therapeutic development. Neuron 109, 1426–1429 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chen X. et al. Functional gene delivery to and across brain vasculature of systemic AAVs with endothelial-specific tropism in rodents and broad tropism in primates. Nat. Commun 14, 3345 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Tremblay S. et al. An open resource for non-human primate optogenetics. Neuron 108, 1075–1090.e1076 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Davis KD Neuroimaging of pain: what does it tell us? Curr. Opin. Support. Palliat. Care 5, 116–121 (2011). [DOI] [PubMed] [Google Scholar]
- 224. Davis KD et al. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: challenges and opportunities. Nat. Rev. Neurol 16, 381–400 (2020). This paper is an overview related to biomarkers of pain in the context of pain management.
- 225.Davis KD & Moayedi M Central mechanisms of pain revealed through functional and structural MRI. J. Neuroimmune Pharmacol 8, 518–534 (2013). [DOI] [PubMed] [Google Scholar]
- 226.Davis KD Imaging vs quantitative sensory testing to predict chronic pain treatment outcomes. Pain 160, S59–s65 (2019). [DOI] [PubMed] [Google Scholar]
- 227.Bosma RL et al. Brain dynamics and temporal summation of pain predicts neuropathic pain relief from ketamine infusion. Anesthesiology 129, 1015–1024 (2018). [DOI] [PubMed] [Google Scholar]
- 228.Ringelstein M. et al. Long-term therapy with interleukin 6 receptor blockade in highly active neuromyelitis optica spectrum disorder. JAMA Neurol. 72, 756–763 (2015). [DOI] [PubMed] [Google Scholar]
- 229.Freund P. et al. Embodied neurology: an integrative framework for neurological disorders. Brain 139, 1855–1861 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Hofstetter CP et al. Allodynia limits the usefulness of intraspinal neural stem cell grafts; directed differentiation improves outcome. Nat. Neurosci 8, 346–353 (2005). [DOI] [PubMed] [Google Scholar]
- 231.Shirvalkar P, Veuthey TL, Dawes HE & Chang EF Closed-loop deep brain stimulation for refractory chronic pain. Front. Comput. Neurosci 12, 18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Mekhail N. et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomised, controlled trial. Lancet Neurol. 19, 123–134 (2020). [DOI] [PubMed] [Google Scholar]
- 233.Shirvalkar P. et al. First-in-human prediction of chronic pain state using intracranial neural biomarkers. Nat. Neurosci 26, 1090–1099 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Hohenschurz-Schmidt D. et al. Recommendations for the development, implementation, and reporting of control interventions in efficacy and mechanistic trials of physical, psychological, and self-management therapies: the CoPPS Statement. BMJ 381, e072108 (2023). [DOI] [PubMed] [Google Scholar]
- 235. Craig AD & Bushnell MC The thermal grill illusion: unmasking the burn of cold pain. Science 265, 252–255 (1994). This is a landmark paper on a key putative CNP mechanism, that is, disinhibition of a central pain-signalling pathway.
- 236.Craig AD How do you feel? Interoception: the sense of the physiological condition of the body. Nat. Rev. Neurosci 3, 655–666 (2002). [DOI] [PubMed] [Google Scholar]
- 237.Craig BAD Can the basis for central neuropathic pain be identified by using a thermal grill? Pain 135, 215–216 (2008). [DOI] [PubMed] [Google Scholar]
- 238.Rivel M. et al. Unique features of central neuropathic pain in multiple sclerosis: results of a cluster analysis. Eur. J. Pain 26, 1107–1122 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Adam F. et al. Thermal grill illusion of pain in patients with chronic pain: a clinical marker of central sensitization? Pain 164, 638–644 (2023). [DOI] [PubMed] [Google Scholar]
- 240.Raja SN et al. The revised International Association for the Study of Pain definition of pain: concepts, challenges, and compromises. Pain 161, 1976–1982 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.International Association for the Study of Pain. Pain terms and definitions. International Association for the Study of Pain https://www.iasp-pain.org/resources/terminology/ (2020). [Google Scholar]