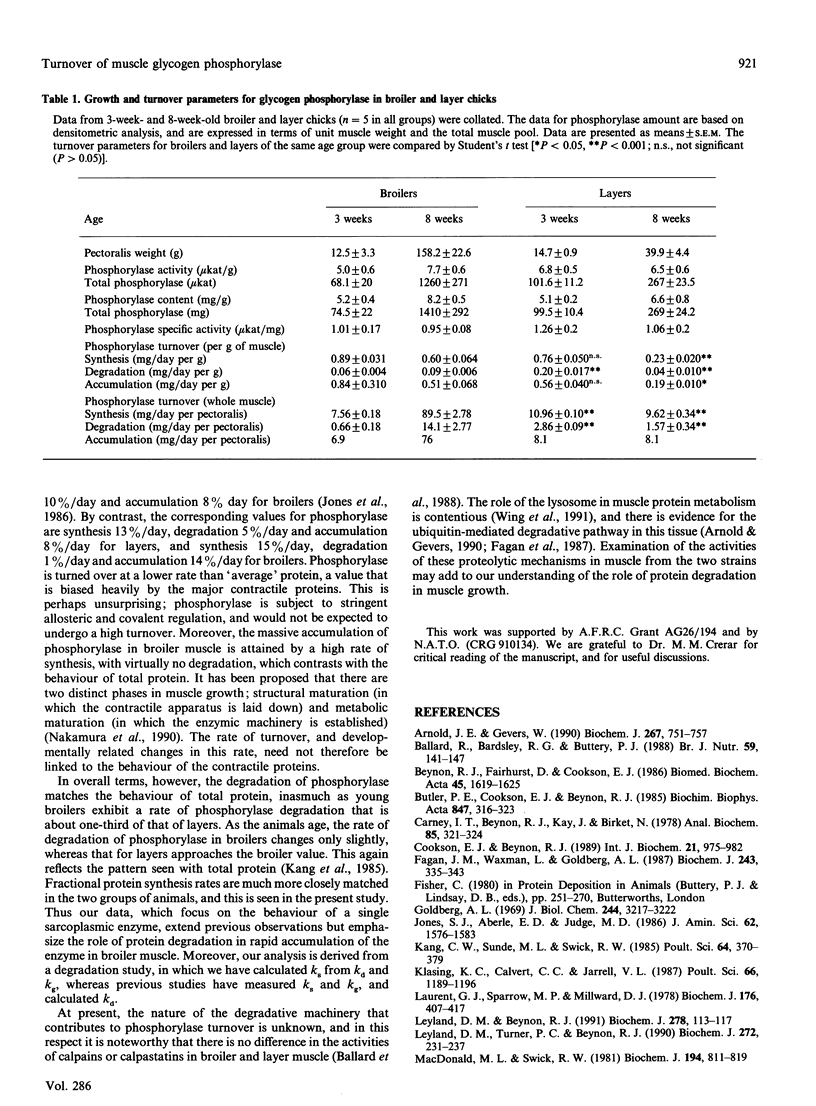
Abstract
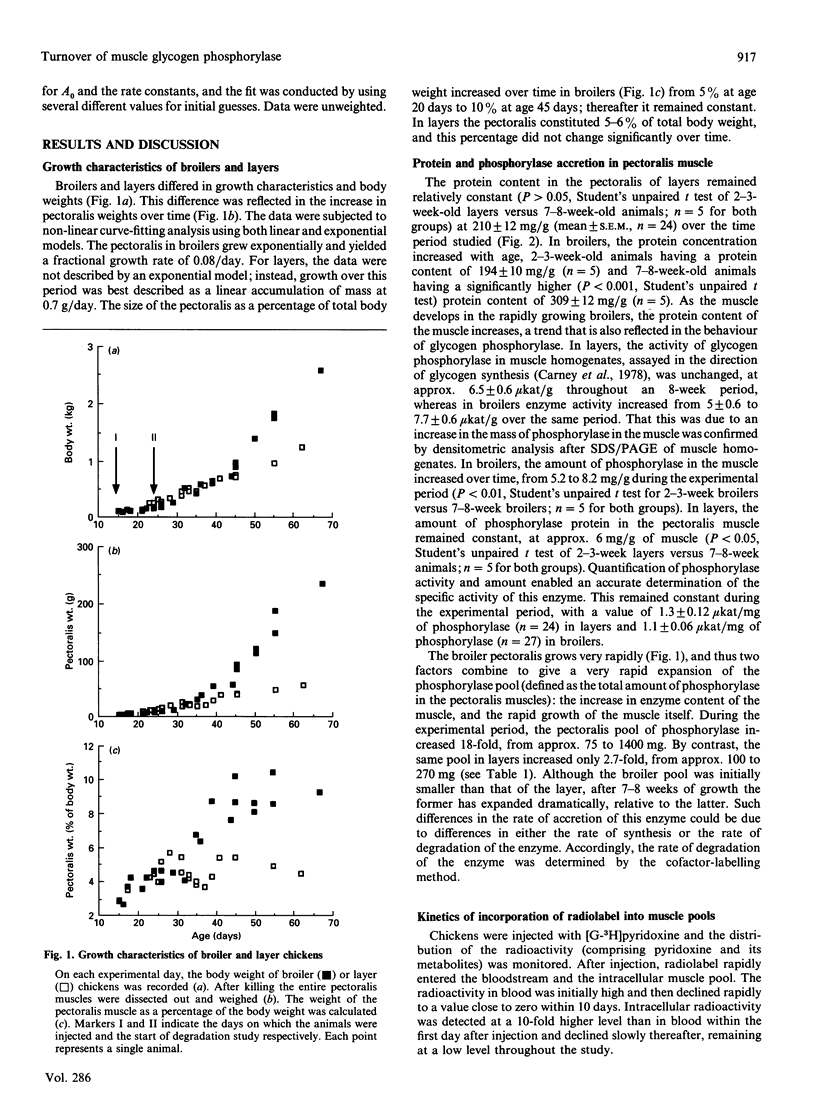
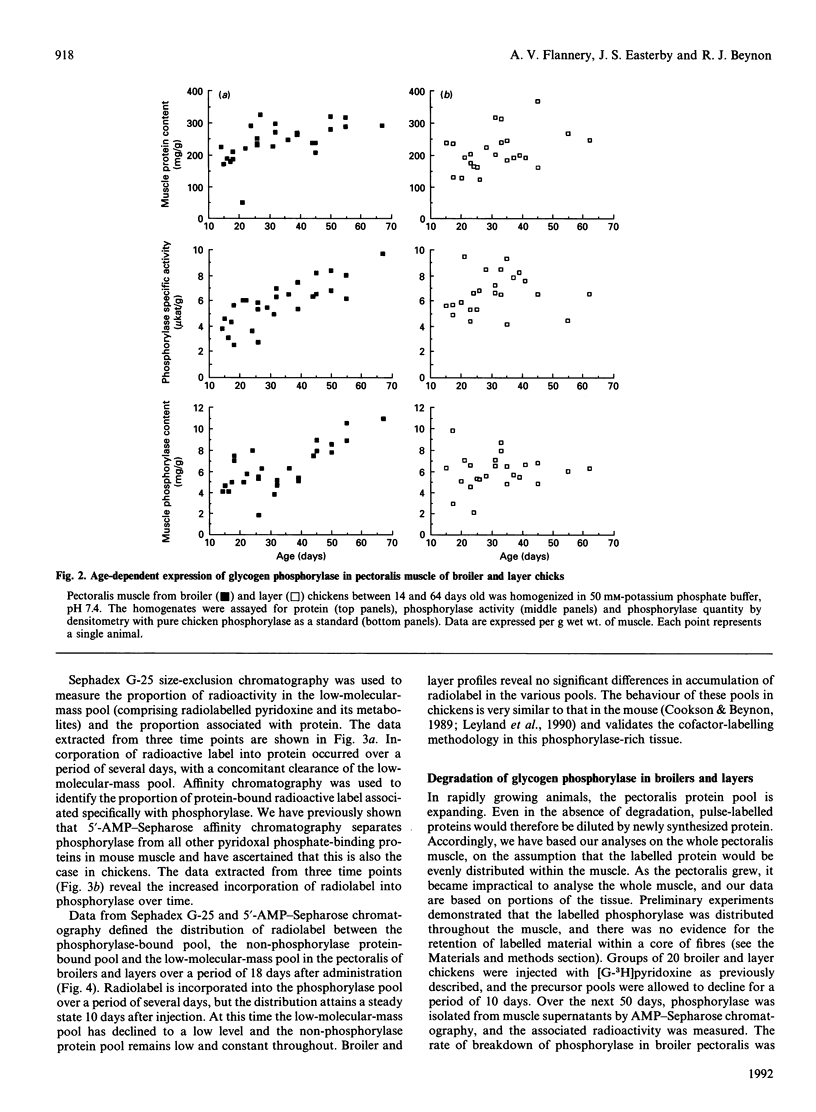
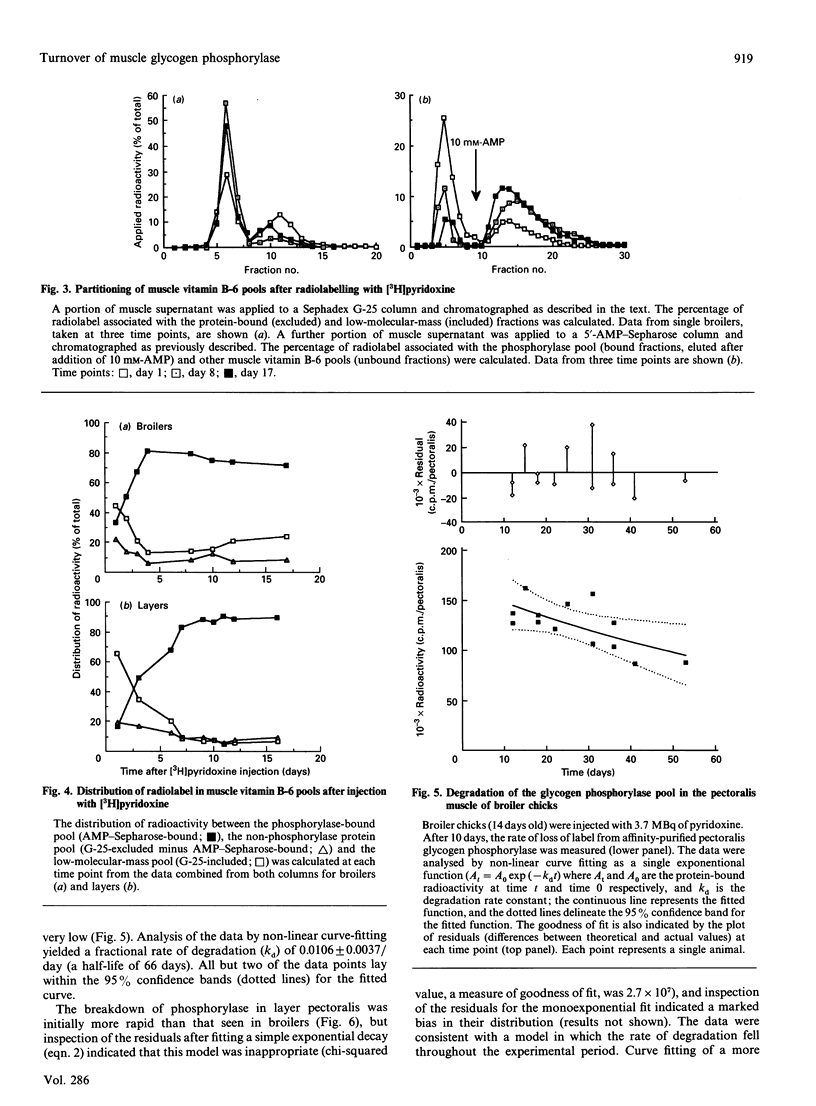
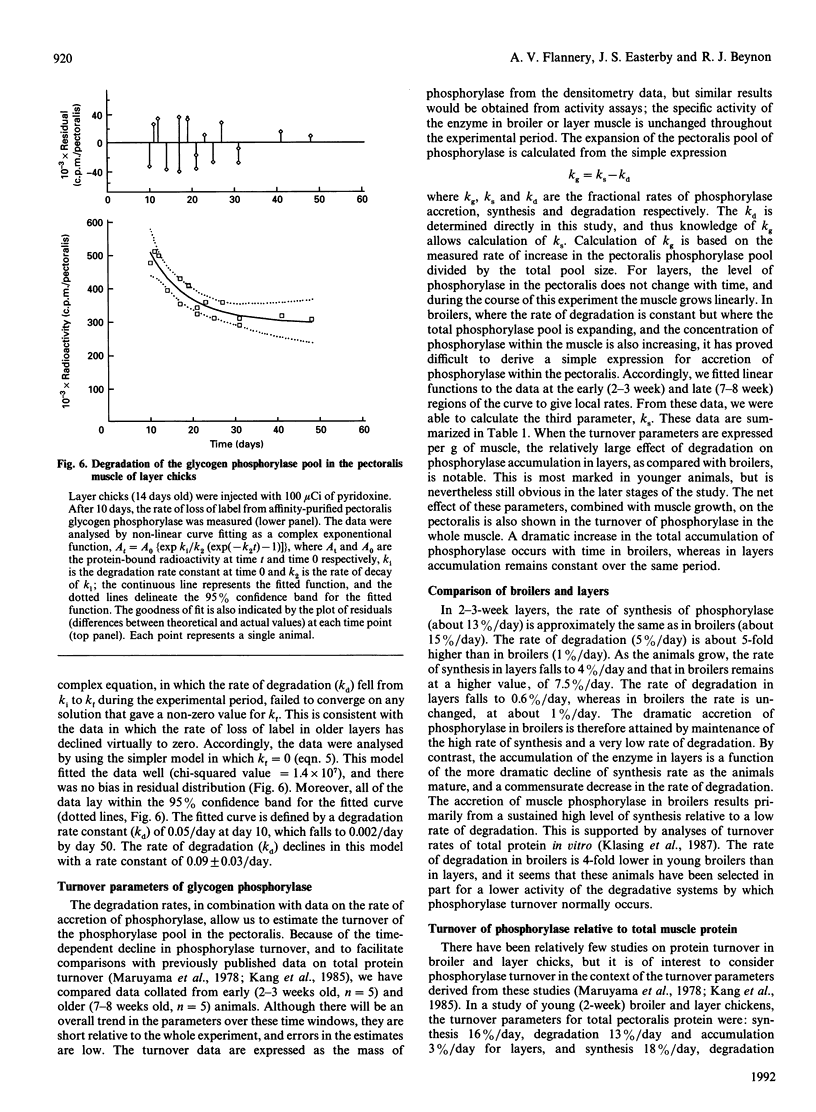
Glycogen phosphorylase is a major sarcoplasmic protein in chicken pectoralis muscle, constituting approx. 4% of the total protein complement. In slow-growing layer chicks phosphorylase accumulated in parallel with muscle accretion, but in fast-growing broiler chicks the concentration of phosphorylase in the muscle increased (from 5 to 8 mg/g wet wt.) with time. In a 5-week period, the total amount of phosphorylase in the pectoralis muscles increased 18-fold in broiler chicks (from approx. 75 to 1400 mg total), but only 3-fold (from approx. 100 to 270 mg total) in layers. Pyridoxal phosphate, the cofactor of the enzyme glycogen phosphorylase, was used as a specific label to measure the rate of degradation of the enzyme in the pectoralis muscle of growing broiler and layer chickens in vivo. In young animals, the fractional rate of phosphorylase synthesis was similar in broiler and layer chickens (approx. 15%/day), but the rate of degradation in layers (5%/day) was 5-fold higher than in broilers (1%/day). As the animals aged, the rate of synthesis decreased, but more so in layers than in broilers. The rate of degradation of phosphorylase also decreased in layers, but in broilers it remained at the low level seen in young animals. The dramatically higher rate of phosphorylase accretion in the pectoralis muscles of the broilers is therefore achieved by an initial lower rate of degradation combined with a sustained difference between rates of synthesis and degradation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnold J. E., Gevers W. Auto-ubiquitination of ubiquitin-activating enzymes from chicken breast muscle. Biochem J. 1990 May 1;267(3):751–757. doi: 10.1042/bj2670751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard R., Bardsley R. G., Buttery P. J. Changes in the activity of skeletal muscle calcium-activated neutral proteinase (EC 3.4.22.17) and its specific inhibitor in chickens grown at different rates in response to graded levels of dietary protein. Br J Nutr. 1988 Jan;59(1):141–147. doi: 10.1079/bjn19880017. [DOI] [PubMed] [Google Scholar]
- Beynon R. J., Fairhurst D., Cookson E. J. Turnover of skeletal muscle glycogen phosphorylase. Biomed Biochim Acta. 1986;45(11-12):1619–1625. [PubMed] [Google Scholar]
- Butler P. E., Cookson E. J., Beynon R. J. The turnover of skeletal muscle glycogen phosphorylase studied using the cofactor, pyridoxal phosphate, as a specific label. Biochim Biophys Acta. 1985 Dec 12;847(3):316–323. doi: 10.1016/0167-4889(85)90037-0. [DOI] [PubMed] [Google Scholar]
- Carney I. T., Beynon R. J., Kay J., Birket N. A semicontinuous assay for glycogen phosphorylase. Anal Biochem. 1978 Mar;85(1):321–324. doi: 10.1016/0003-2697(78)90309-3. [DOI] [PubMed] [Google Scholar]
- Cookson E. J., Beynon R. J. Further evaluation of cofactor as a turnover label for glycogen phosphorylase. Int J Biochem. 1989;21(9):975–982. doi: 10.1016/0020-711x(89)90229-2. [DOI] [PubMed] [Google Scholar]
- Fagan J. M., Waxman L., Goldberg A. L. Skeletal muscle and liver contain a soluble ATP + ubiquitin-dependent proteolytic system. Biochem J. 1987 Apr 15;243(2):335–343. doi: 10.1042/bj2430335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L. Protein turnover in skeletal muscle. I. Protein catabolism during work-induced hypertrophy and growth induced with growth hormone. J Biol Chem. 1969 Jun 25;244(12):3217–3222. [PubMed] [Google Scholar]
- Jones S. J., Aberle E. D., Judge M. D. Skeletal muscle protein turnover in broiler and layer chicks. J Anim Sci. 1986 Jun;62(6):1576–1583. doi: 10.2527/jas1986.6261576x. [DOI] [PubMed] [Google Scholar]
- Kang C. W., Sunde M. L., Swick R. W. Growth and protein turnover in the skeletal muscles of broiler chicks. Poult Sci. 1985 Feb;64(2):370–379. doi: 10.3382/ps.0640370. [DOI] [PubMed] [Google Scholar]
- Klasing K. C., Calvert C. C., Jarrell V. L. Growth characteristics, protein synthesis, and protein degradation in muscles from fast and slow-growing chickens. Poult Sci. 1987 Jul;66(7):1189–1196. doi: 10.3382/ps.0661189. [DOI] [PubMed] [Google Scholar]
- Laurent G. J., Sparrow M. P., Millward D. J. Turnover of muscle protein in the fowl. Changes in rates of protein synthesis and breakdown during hypertrophy of the anterior and posterior latissimus dorsi muscles. Biochem J. 1978 Nov 15;176(2):407–417. doi: 10.1042/bj1760407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyland D. M., Beynon R. J. The expression of glycogen phosphorylase in normal and dystrophic muscle. Biochem J. 1991 Aug 15;278(Pt 1):113–117. doi: 10.1042/bj2780113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyland D. M., Turner P. C., Beynon R. J. Effect of denervation on the expression of glycogen phosphorylase in mouse skeletal muscle. Biochem J. 1990 Nov 15;272(1):231–237. doi: 10.1042/bj2720231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald M. L., Swick R. W. The effect of protein depletion and repletion on muscle-protein turnover in the chick. Biochem J. 1981 Mar 15;194(3):811–819. doi: 10.1042/bj1940811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacNaughtan A. F. An ultrastructural and histochemical study of fibre types in the pectoralis thoracica and iliotibialis muscles of the fowl (Gallus domesticus). J Anat. 1974 Sep;118(Pt 1):171–186. [PMC free article] [PubMed] [Google Scholar]
- Maruyama K., Sunde M. L., Swick R. W. Growth and muscle protein turnover in the chick. Biochem J. 1978 Nov 15;176(2):573–582. doi: 10.1042/bj1760573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millward D. J., Garlick P. J., Stewart R. J., Nnanyelugo D. O., Waterlow J. C. Skeletal-muscle growth and protein turnover. Biochem J. 1975 Aug;150(2):235–243. doi: 10.1042/bj1500235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura M., Yao Y., Hirabayashi T. Coordinate and discoordinate accumulation of protein constituents in chicken breast muscle. Cell Differ Dev. 1990 Oct;32(1):61–69. doi: 10.1016/0922-3371(90)90099-i. [DOI] [PubMed] [Google Scholar]
- Wing S. S., Chiang H. L., Goldberg A. L., Dice J. F. Proteins containing peptide sequences related to Lys-Phe-Glu-Arg-Gln are selectively depleted in liver and heart, but not skeletal muscle, of fasted rats. Biochem J. 1991 Apr 1;275(Pt 1):165–169. doi: 10.1042/bj2750165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young V. R., Stothers S. C., Vilaire G. Synthesis and degradation of mixed proteins, and composition changes in skeletal muscle of malnourished and refed rats. J Nutr. 1971 Oct;101(10):1379–1390. doi: 10.1093/jn/101.10.1379. [DOI] [PubMed] [Google Scholar]


