Abstract
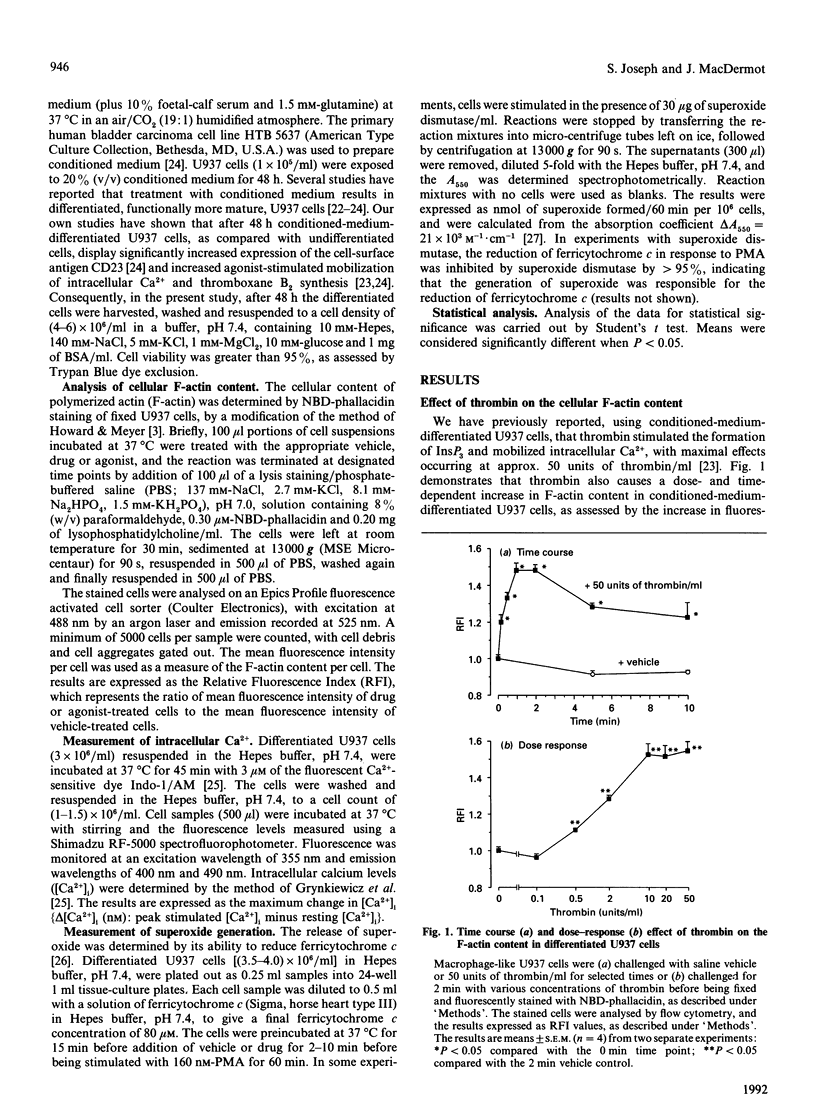
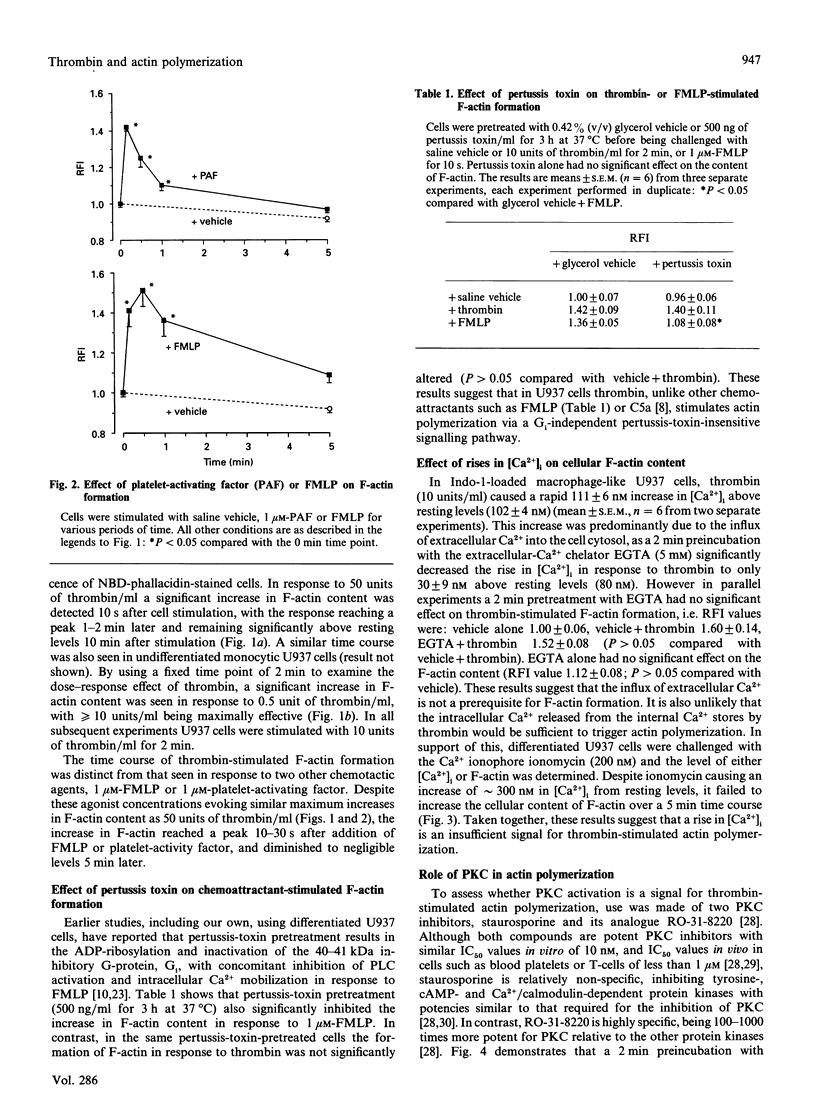
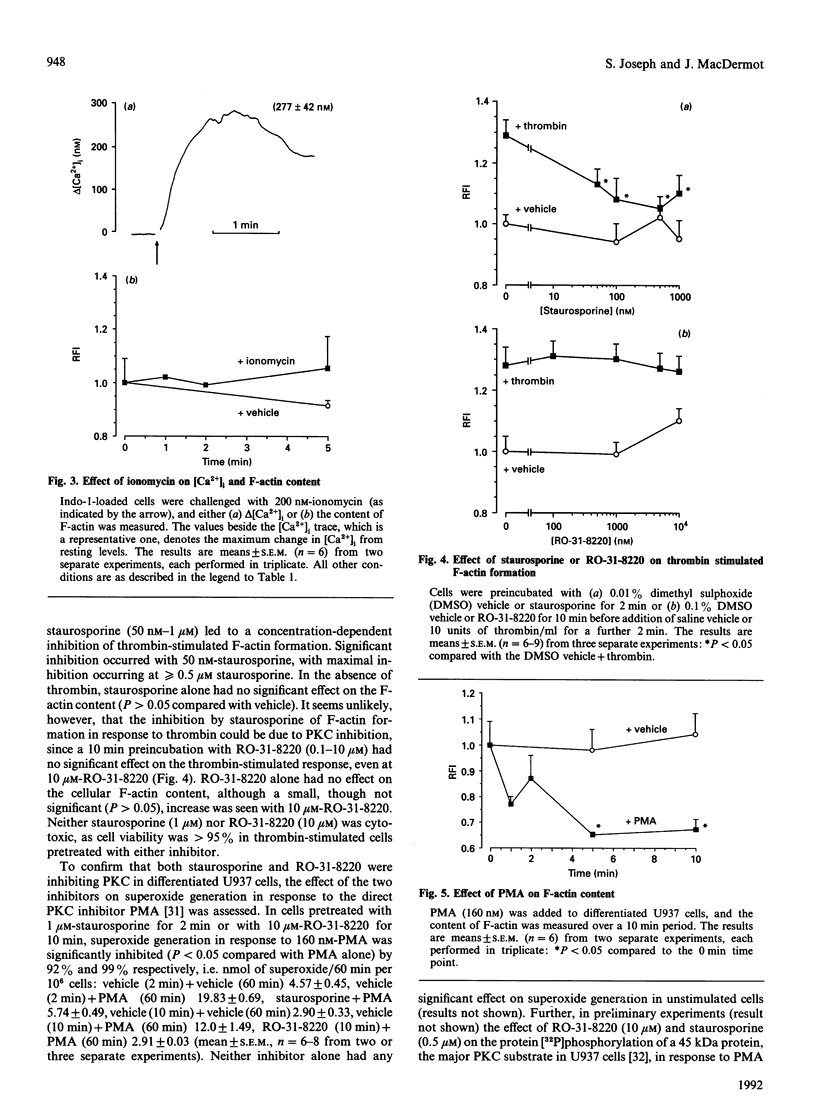
The U937 human monocyte-macrophage cell line was used to examine the effect of thrombin, an ill-defined chemoattractant, on the polymerization of actin, a process essential for cell motility. In differentiated macrophage-like U937 cells, thrombin (0.5-50 units/ml) caused a rapid dose-dependent increase in the formation of filamentous (F-) actin, detected by the staining of F-actin with the fluorescent toxin, 7-nitrobenz-2-oxa-1,3-diazole-phallacidin. In contrast with other chemoattractants such as N-formylmethionyl-leucylphenylalanine or C5a, actin polymerization in response to thrombin occurred via a pertussis-toxin-insensitive G1-(inhibitory G-protein) independent signalling pathway. Further, this response was not affected by the Ca2+ chelator EGTA or by the specific protein kinase C (PKC) inhibitor RO-31-8220. The response to thrombin was not mimicked by the Ca2+ ionophore ionomycin or by the direct PKC activator phorbol 12-myristate 13-acetate. The thrombin response was, however, inhibited by the non-specific protein kinase inhibitor staurosporine. The present results suggest that in U937 cells thrombin stimulates the formation of F-actin via a signalling pathway independent of (i) the activation of PKC, (ii) the mobilization of intracellular Ca2+ and (iii) the activation of Ca(2+)-dependent protein kinases, but dependent on the activation of an undefined staurosporine-sensitive protein kinase.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacon K. B., Camp R. D. Interleukin (IL)-8-induced in vitro human lymphocyte migration is inhibited by cholera and pertussis toxins and inhibitors of protein kinase C. Biochem Biophys Res Commun. 1990 Jun 29;169(3):1099–1104. doi: 10.1016/0006-291x(90)92008-n. [DOI] [PubMed] [Google Scholar]
- Banks P., Barker M. D., Burton D. R. Recruitment of actin to the cytoskeletons of human monocyte-like cells activated by complement fragment C5a. Is protein kinase C involved? Biochem J. 1988 Jun 15;252(3):765–769. doi: 10.1042/bj2520765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit R., Kahn A., Fenton J. W., 2nd, Wilner G. D. Chemotactic response of monocytes to thrombin. J Cell Biol. 1983 Jan;96(1):282–285. doi: 10.1083/jcb.96.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Shavit R., Kahn A., Fenton J. W., 2nd, Wilner G. D. Receptor-mediated chemotactic response of a macrophage cell line (J774) to thrombin. Lab Invest. 1983 Dec;49(6):702–707. [PubMed] [Google Scholar]
- Bartha K., Müller-Peddinghaus R., Van Rooijen L. A. Bradykinin and thrombin effects on polyphosphoinositide hydrolysis and prostacyclin production in endothelial cells. Biochem J. 1989 Oct 1;263(1):149–155. doi: 10.1042/bj2630149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belloc F., Vincendeau P., Freyburger G., Dumain P., Boisseau M. R. Flow cytometric study of the activation of polymorphonuclear cells. J Leukoc Biol. 1990 Oct;48(4):353–358. doi: 10.1002/jlb.48.4.353. [DOI] [PubMed] [Google Scholar]
- Bengtsson T., Särndahl E., Stendahl O., Andersson T. Involvement of GTP-binding proteins in actin polymerization in human neutrophils. Proc Natl Acad Sci U S A. 1990 Apr;87(8):2921–2925. doi: 10.1073/pnas.87.8.2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis P. D., Hill C. H., Keech E., Lawton G., Nixon J. S., Sedgwick A. D., Wadsworth J., Westmacott D., Wilkinson S. E. Potent selective inhibitors of protein kinase C. FEBS Lett. 1989 Dec 18;259(1):61–63. doi: 10.1016/0014-5793(89)81494-2. [DOI] [PubMed] [Google Scholar]
- Downey G. P., Chan C. K., Trudel S., Grinstein S. Actin assembly in electropermeabilized neutrophils: role of intracellular calcium. J Cell Biol. 1990 Jun;110(6):1975–1982. doi: 10.1083/jcb.110.6.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton J. W., 2nd Regulation of thrombin generation and functions. Semin Thromb Hemost. 1988 Jul;14(3):234–240. doi: 10.1055/s-2007-1002783. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Harris P., Ralph P. Human leukemic models of myelomonocytic development: a review of the HL-60 and U937 cell lines. J Leukoc Biol. 1985 Apr;37(4):407–422. doi: 10.1002/jlb.37.4.407. [DOI] [PubMed] [Google Scholar]
- Howard T. H., Meyer W. H. Chemotactic peptide modulation of actin assembly and locomotion in neutrophils. J Cell Biol. 1984 Apr;98(4):1265–1271. doi: 10.1083/jcb.98.4.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard T. H., Wang D. Calcium ionophore, phorbol ester, and chemotactic peptide-induced cytoskeleton reorganization in human neutrophils. J Clin Invest. 1987 May;79(5):1359–1364. doi: 10.1172/JCI112962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M. M., Bolen J. B., Barnwell J. W., Shattil S. J., Brugge J. S. Membrane glycoprotein IV (CD36) is physically associated with the Fyn, Lyn, and Yes protein-tyrosine kinases in human platelets. Proc Natl Acad Sci U S A. 1991 Sep 1;88(17):7844–7848. doi: 10.1073/pnas.88.17.7844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston R. B., Jr Measurement of O2- secreted by monocytes and macrophages. Methods Enzymol. 1984;105:365–369. doi: 10.1016/s0076-6879(84)05049-7. [DOI] [PubMed] [Google Scholar]
- Joseph S., MacDermot J. Thrombin signalling in U937 human monocytic cells is coupled to inositol phosphate formation but not to thromboxane B2 synthesis nor to inhibition of adenylate cyclase: distinct differences in thrombin signalling between U937 cells and platelets. Eur J Pharmacol. 1991 Oct 14;208(2):149–156. doi: 10.1016/0922-4106(91)90065-p. [DOI] [PubMed] [Google Scholar]
- MASSEY V. The microestimation of succinate and the extinction coefficient of cytochrome c. Biochim Biophys Acta. 1959 Jul;34:255–256. doi: 10.1016/0006-3002(59)90259-8. [DOI] [PubMed] [Google Scholar]
- Manning D. R., Brass L. F. The role of GTP-binding proteins in platelet activation. Thromb Haemost. 1991 Oct 1;66(4):393–399. [PubMed] [Google Scholar]
- Monk P. N., Banks P. Evidence for the involvement of multiple signalling pathways in C5a-induced actin polymerization and nucleation in human monocyte-like cells. J Mol Endocrinol. 1991 Jun;6(3):241–247. doi: 10.1677/jme.0.0060241. [DOI] [PubMed] [Google Scholar]
- Nakano H., Kobayashi E., Takahashi I., Tamaoki T., Kuzuu Y., Iba H. Staurosporine inhibits tyrosine-specific protein kinase activity of Rous sarcoma virus transforming protein p60. J Antibiot (Tokyo) 1987 May;40(5):706–708. doi: 10.7164/antibiotics.40.706. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The Albert Lasker Medical Awards. The family of protein kinase C for signal transduction. JAMA. 1989 Oct 6;262(13):1826–1833. [PubMed] [Google Scholar]
- Omann G. M., Allen R. A., Bokoch G. M., Painter R. G., Traynor A. E., Sklar L. A. Signal transduction and cytoskeletal activation in the neutrophil. Physiol Rev. 1987 Jan;67(1):285–322. doi: 10.1152/physrev.1987.67.1.285. [DOI] [PubMed] [Google Scholar]
- Omann G. M., Porasik-Lowes M. M. Graded G-protein uncoupling by pertussis toxin treatment of human polymorphonuclear leukocytes. J Immunol. 1991 Feb 15;146(4):1303–1308. [PubMed] [Google Scholar]
- Pollock K., Creba J., Mitchell F., Milligan G. Stimulus-response coupling in FMLP-stimulated U937 monocytes: effect of differentiation on Gi2 expression. Biochim Biophys Acta. 1990 Jan 23;1051(1):71–77. doi: 10.1016/0167-4889(90)90175-d. [DOI] [PubMed] [Google Scholar]
- Rana R. S., Hokin L. E. Role of phosphoinositides in transmembrane signaling. Physiol Rev. 1990 Jan;70(1):115–164. doi: 10.1152/physrev.1990.70.1.115. [DOI] [PubMed] [Google Scholar]
- Rossomando A. J., Payne D. M., Weber M. J., Sturgill T. W. Evidence that pp42, a major tyrosine kinase target protein, is a mitogen-activated serine/threonine protein kinase. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6940–6943. doi: 10.1073/pnas.86.18.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sha'afi R. I., Shefcyk J., Yassin R., Molski T. F., Volpi M., Naccache P. H., White J. R., Feinstein M. B., Becker E. L. Is a rise in intracellular concentration of free calcium necessary or sufficient for stimulated cytoskeletal-associated actin? J Cell Biol. 1986 Apr;102(4):1459–1463. doi: 10.1083/jcb.102.4.1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenker A., Goldsmith P., Unson C. G., Spiegel A. M. The G protein coupled to the thromboxane A2 receptor in human platelets is a member of the novel Gq family. J Biol Chem. 1991 May 15;266(14):9309–9313. [PubMed] [Google Scholar]
- Siess W. Molecular mechanisms of platelet activation. Physiol Rev. 1989 Jan;69(1):58–178. doi: 10.1152/physrev.1989.69.1.58. [DOI] [PubMed] [Google Scholar]
- Sklar L. A., Omann G. M., Painter R. G. Relationship of actin polymerization and depolymerization to light scattering in human neutrophils: dependence on receptor occupancy and intracellular Ca++. J Cell Biol. 1985 Sep;101(3):1161–1166. doi: 10.1083/jcb.101.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Storch J., Edwards R. J., MacDermot J. Thromboxane release by lymphokine-differentiated U937 human monocytic cells: response to platelet-activating factor (PAF) and chemotactic peptide (FMLP) but not to low affinity IGE-receptor (Fc epsilon RII/CD23) occupation. J Leukoc Biol. 1990 Sep;48(3):266–273. doi: 10.1002/jlb.48.3.266. [DOI] [PubMed] [Google Scholar]
- Stossel T. P. From signal to pseudopod. How cells control cytoplasmic actin assembly. J Biol Chem. 1989 Nov 5;264(31):18261–18264. [PubMed] [Google Scholar]
- Vu T. K., Hung D. T., Wheaton V. I., Coughlin S. R. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991 Mar 22;64(6):1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- Ways D. K., Dodd R. C., Bennett T. E., Gray T. K., Earp H. S. 1,25-Dihydroxyvitamin D3 enhances phorbol ester-stimulated differentiation and protein kinase C-dependent substrate phosphorylation activity in the U937 human monoblastoid cell. Endocrinology. 1987 Nov;121(5):1654–1661. doi: 10.1210/endo-121-5-1654. [DOI] [PubMed] [Google Scholar]
- Yin H. L., Hartwig J. H. The structure of the macrophage actin skeleton. J Cell Sci Suppl. 1988;9:169–184. doi: 10.1242/jcs.1988.supplement_9.9. [DOI] [PubMed] [Google Scholar]


