Abstract
The major milk whey protein of sheep, beta-lactoglobulin (BLG), is expressed specifically in the mammary gland in a developmentally regulated pattern. To identify the cis-acting DNA regions involved in the regulation of BLG expression, resected gene constructs were analysed in transgenic mice. BLG transgenes which contain at least the proximal 406 bp of the 5' flanking region were expressed in all mice analysed, at levels related to transgene copy number, and thus were expressed in a position-independent manner. Expression was restricted to the mammary gland, except in a few lines where low-level expression was also detected in the salivary gland. In these mice, BLG transgenes were expressed during pregnancy and lactation in the appropriate temporal pattern. Further resection of the 5' proximal region to -146 bp resulted in a dramatically reduced frequency of expression, without affecting tissue specificity, while a construct which retained only 79 bp of 5' flanking region was not expressed. Chromatin analysis of isolated sheep nuclei showed that the promoter resides within a DNAaseI-hypersensitive region in the mammary gland but not in the liver. A BLG transgene displayed a similar tissue-specific pattern of DNAaseI hypersensitivity in mice. These data demonstrate an essential role of the proximal DNAaseI-hypersensitive sequences for position-independent expression of the BLG gene.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbey L. M., Witorsch R. J. Prolactin binding in normal human minor salivary gland tissue: an immunohistochemical study. Oral Surg Oral Med Oral Pathol. 1984 Dec;58(6):682–687. doi: 10.1016/0030-4220(84)90034-3. [DOI] [PubMed] [Google Scholar]
- Ali S., Clark A. J. Characterization of the gene encoding ovine beta-lactoglobulin. Similarity to the genes for retinol binding protein and other secretory proteins. J Mol Biol. 1988 Feb 5;199(3):415–426. doi: 10.1016/0022-2836(88)90614-6. [DOI] [PubMed] [Google Scholar]
- Ali S., McClenaghan M., Simons J. P., Clark A. J. Characterisation of the alleles encoding ovine beta-lactoglobulins A and B. Gene. 1990 Jul 16;91(2):201–207. doi: 10.1016/0378-1119(90)90089-a. [DOI] [PubMed] [Google Scholar]
- Allen N. D., Norris M. L., Surani M. A. Epigenetic control of transgene expression and imprinting by genotype-specific modifiers. Cell. 1990 Jun 1;61(5):853–861. doi: 10.1016/0092-8674(90)90195-k. [DOI] [PubMed] [Google Scholar]
- Archibald A. L., McClenaghan M., Hornsey V., Simons J. P., Clark A. J. High-level expression of biologically active human alpha 1-antitrypsin in the milk of transgenic mice. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5178–5182. doi: 10.1073/pnas.87.13.5178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball R. K., Friis R. R., Schoenenberger C. A., Doppler W., Groner B. Prolactin regulation of beta-casein gene expression and of a cytosolic 120-kd protein in a cloned mouse mammary epithelial cell line. EMBO J. 1988 Jul;7(7):2089–2095. doi: 10.1002/j.1460-2075.1988.tb03048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayna E. M., Rosen J. M. Tissue-specific, high level expression of the rat whey acidic protein gene in transgenic mice. Nucleic Acids Res. 1990 May 25;18(10):2977–2985. doi: 10.1093/nar/18.10.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifer C., Vidal M., Grosveld F., Sippel A. E. Tissue specific and position independent expression of the complete gene domain for chicken lysozyme in transgenic mice. EMBO J. 1990 Sep;9(9):2843–2848. doi: 10.1002/j.1460-2075.1990.tb07473.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnerot C., Grimber G., Briand P., Nicolas J. F. Patterns of expression of position-dependent integrated transgenes in mouse embryo. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6331–6335. doi: 10.1073/pnas.87.16.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinster R. L., Allen J. M., Behringer R. R., Gelinas R. E., Palmiter R. D. Introns increase transcriptional efficiency in transgenic mice. Proc Natl Acad Sci U S A. 1988 Feb;85(3):836–840. doi: 10.1073/pnas.85.3.836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bühler T. A., Bruyère T., Went D. F., Stranzinger G., Bürki K. Rabbit beta-casein promoter directs secretion of human interleukin-2 into the milk of transgenic rabbits. Biotechnology (N Y) 1990 Feb;8(2):140–143. doi: 10.1038/nbt0290-140. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Church G. M., Gilbert W. Genomic sequencing. Proc Natl Acad Sci U S A. 1984 Apr;81(7):1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman J., Hayes M., O'Day C., Edmunds T., Bartlett C., Hirani S., Ebert K. M., Gordon K., McPherson J. M. Transgenic expression of a variant of human tissue-type plasminogen activator in goat milk: purification and characterization of the recombinant enzyme. Biotechnology (N Y) 1991 Sep;9(9):839–843. doi: 10.1038/nbt0991-839. [DOI] [PubMed] [Google Scholar]
- Doppler W., Groner B., Ball R. K. Prolactin and glucocorticoid hormones synergistically induce expression of transfected rat beta-casein gene promoter constructs in a mammary epithelial cell line. Proc Natl Acad Sci U S A. 1989 Jan;86(1):104–108. doi: 10.1073/pnas.86.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenstein R. S., Rosen J. M. Both cell substratum regulation and hormonal regulation of milk protein gene expression are exerted primarily at the posttranscriptional level. Mol Cell Biol. 1988 Aug;8(8):3183–3190. doi: 10.1128/mcb.8.8.3183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgin S. C. The formation and function of DNase I hypersensitive sites in the process of gene activation. J Biol Chem. 1988 Dec 25;263(36):19259–19262. [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. "A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity". Addendum. Anal Biochem. 1984 Feb;137(1):266–267. doi: 10.1016/0003-2697(84)90381-6. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Forsyth I. A. Variation among species in the endocrine control of mammary growth and function: the roles of prolactin, growth hormone, and placental lactogen. J Dairy Sci. 1986 Mar;69(3):886–903. doi: 10.3168/jds.S0022-0302(86)80479-9. [DOI] [PubMed] [Google Scholar]
- Gaye P., Hue-Delahaie D., Mercier J. C., Soulier S., Vilotte J. L., Furet J. P. Ovine beta-lactoglobulin messenger RNA: nucleotide sequence and mRNA levels during functional differentiation of the mammary gland. Biochimie. 1986 Sep;68(9):1097–1107. doi: 10.1016/s0300-9084(86)80184-5. [DOI] [PubMed] [Google Scholar]
- Greaves D. R., Wilson F. D., Lang G., Kioussis D. Human CD2 3'-flanking sequences confer high-level, T cell-specific, position-independent gene expression in transgenic mice. Cell. 1989 Mar 24;56(6):979–986. doi: 10.1016/0092-8674(89)90631-4. [DOI] [PubMed] [Google Scholar]
- Greer P., Maltby V., Rossant J., Bernstein A., Pawson T. Myeloid expression of the human c-fps/fes proto-oncogene in transgenic mice. Mol Cell Biol. 1990 Jun;10(6):2521–2527. doi: 10.1128/mcb.10.6.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosveld F., van Assendelft G. B., Greaves D. R., Kollias G. Position-independent, high-level expression of the human beta-globin gene in transgenic mice. Cell. 1987 Dec 24;51(6):975–985. doi: 10.1016/0092-8674(87)90584-8. [DOI] [PubMed] [Google Scholar]
- Gupta P., Rosen J. M., D'Eustachio P., Ruddle F. H. Localization of the casein gene family to a single mouse chromosome. J Cell Biol. 1982 Apr;93(1):199–204. doi: 10.1083/jcb.93.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Günzburg W. H., Salmons B., Zimmermann B., Müller M., Erfle V., Brem G. A mammary-specific promoter directs expression of growth hormone not only to the mammary gland, but also to Bergman glia cells in transgenic mice. Mol Endocrinol. 1991 Jan;5(1):123–133. doi: 10.1210/mend-5-1-123. [DOI] [PubMed] [Google Scholar]
- Hall L., Emery D. C., Davies M. S., Parker D., Craig R. K. Organization and sequence of the human alpha-lactalbumin gene. Biochem J. 1987 Mar 15;242(3):735–742. doi: 10.1042/bj2420735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris S., Ali S., Anderson S., Archibald A. L., Clark A. J. Complete nucleotide sequence of the genomic ovine beta-lactoglobulin gene. Nucleic Acids Res. 1988 Nov 11;16(21):10379–10380. doi: 10.1093/nar/16.21.10379. [DOI] [PMC free article] [PubMed] [Google Scholar]
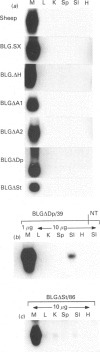
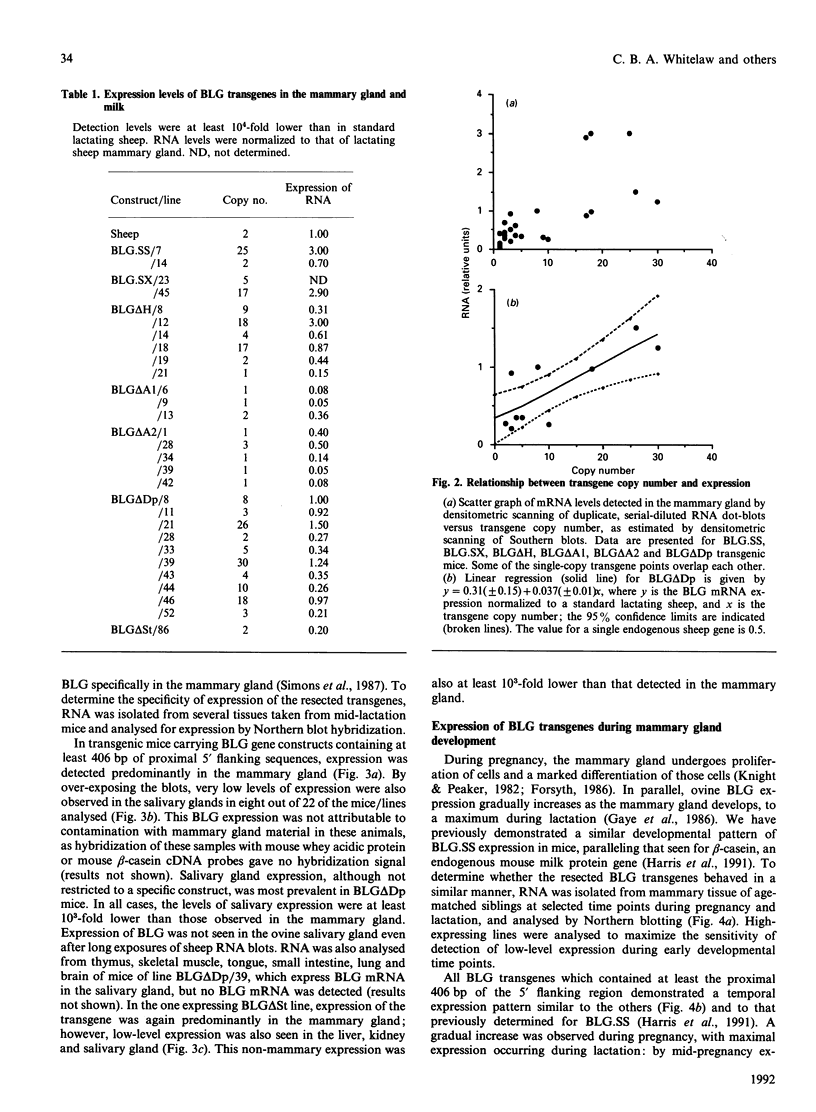
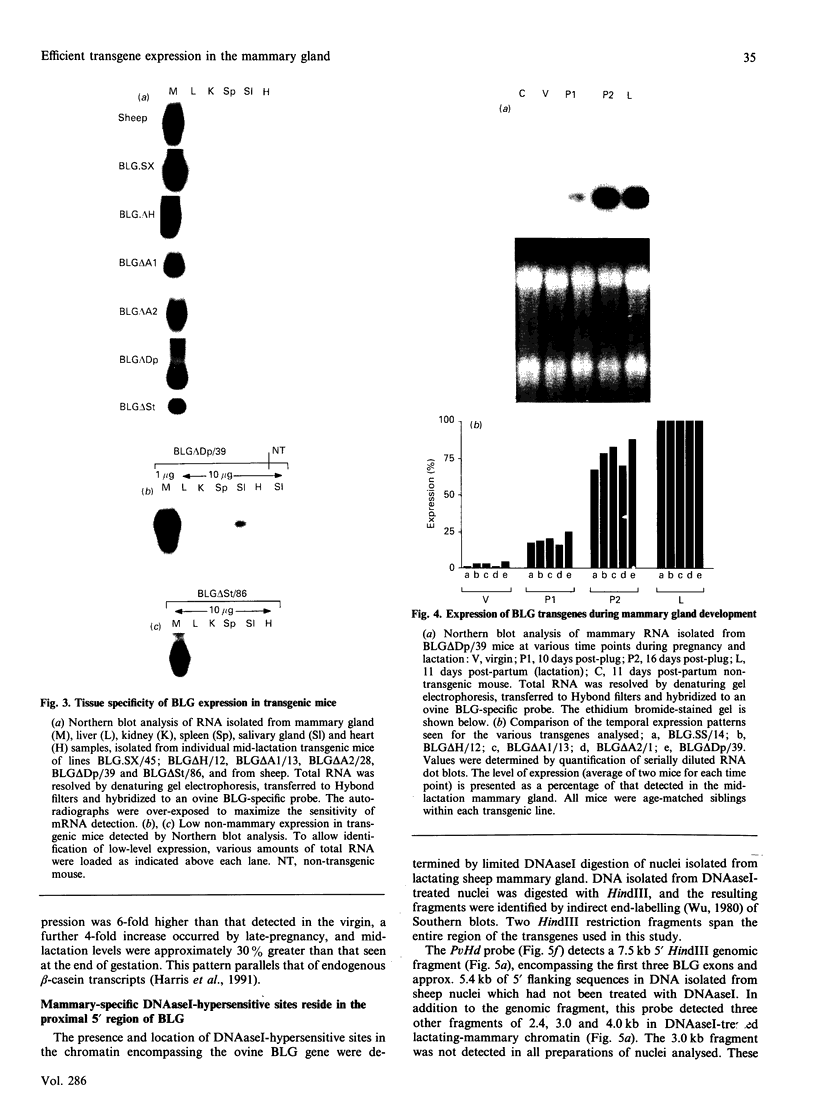
- Harris S., McClenaghan M., Simons J. P., Ali S., Clark A. J. Developmental regulation of the sheep beta-lactoglobulin gene in the mammary gland of transgenic mice. Dev Genet. 1991;12(4):299–307. doi: 10.1002/dvg.1020120407. [DOI] [PubMed] [Google Scholar]
- Hatakeyama S., Sashima M., Shirasuna K., Satoh M., Suzuki A. Glucocorticoid-induced growth inhibition with enhanced expression of ductal epithelium of human salivary gland adenocarcinoma cells transplanted into athymic nude mice. Cancer. 1988 Aug 15;62(4):716–722. doi: 10.1002/1097-0142(19880815)62:4<716::aid-cncr2820620412>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- Higgs D. R., Wood W. G., Jarman A. P., Sharpe J., Lida J., Pretorius I. M., Ayyub H. A major positive regulatory region located far upstream of the human alpha-globin gene locus. Genes Dev. 1990 Sep;4(9):1588–1601. doi: 10.1101/gad.4.9.1588. [DOI] [PubMed] [Google Scholar]
- Jantzen K., Fritton H. P., Igo-Kemenes T. The DNase I sensitive domain of the chicken lysozyme gene spans 24 kb. Nucleic Acids Res. 1986 Aug 11;14(15):6085–6099. doi: 10.1093/nar/14.15.6085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight C. H., Peaker M. Development of the mammary gland. J Reprod Fertil. 1982 Jul;65(2):521–536. doi: 10.1530/jrf.0.0650521. [DOI] [PubMed] [Google Scholar]
- Lang G., Wotton D., Owen M. J., Sewell W. A., Brown M. H., Mason D. Y., Crumpton M. J., Kioussis D. The structure of the human CD2 gene and its expression in transgenic mice. EMBO J. 1988 Jun;7(6):1675–1682. doi: 10.1002/j.1460-2075.1988.tb02995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathe R., Vilotte J. L., Clark A. J. Plasmid and bacteriophage vectors for excision of intact inserts. Gene. 1987;57(2-3):193–201. doi: 10.1016/0378-1119(87)90122-3. [DOI] [PubMed] [Google Scholar]
- Lee K. F., DeMayo F. J., Atiee S. H., Rosen J. M. Tissue-specific expression of the rat beta-casein gene in transgenic mice. Nucleic Acids Res. 1988 Feb 11;16(3):1027–1041. doi: 10.1093/nar/16.3.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine J. F., Stockdale F. E. Cell-cell interactions promote mammary epithelial cell differentiation. J Cell Biol. 1985 May;100(5):1415–1422. doi: 10.1083/jcb.100.5.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M. L., Aggeler J., Farson D. A., Hatier C., Hassell J., Bissell M. J. Influence of a reconstituted basement membrane and its components on casein gene expression and secretion in mouse mammary epithelial cells. Proc Natl Acad Sci U S A. 1987 Jan;84(1):136–140. doi: 10.1073/pnas.84.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubon H., Hennighausen L. Conserved region of the rat alpha-lactalbumin promoter is a target site for protein binding in vitro. Biochem J. 1988 Dec 1;256(2):391–396. doi: 10.1042/bj2560391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubon H., Hennighausen L. Nuclear proteins from lactating mammary glands bind to the promoter of a milk protein gene. Nucleic Acids Res. 1987 Mar 11;15(5):2103–2121. doi: 10.1093/nar/15.5.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade H., Gates L., Lacy E., Lonberg N. Bovine alpha S1-casein gene sequences direct high level expression of active human urokinase in mouse milk. Biotechnology (N Y) 1990 May;8(5):443–446. doi: 10.1038/nbt0590-443. [DOI] [PubMed] [Google Scholar]
- Orkin S. H. Globin gene regulation and switching: circa 1990. Cell. 1990 Nov 16;63(4):665–672. doi: 10.1016/0092-8674(90)90133-y. [DOI] [PubMed] [Google Scholar]
- Ott M. O., Sperling L., Herbomel P., Yaniv M., Weiss M. C. Tissue-specific expression is conferred by a sequence from the 5' end of the rat albumin gene. EMBO J. 1984 Nov;3(11):2505–2510. doi: 10.1002/j.1460-2075.1984.tb02164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinkert C. A., Ornitz D. M., Brinster R. L., Palmiter R. D. An albumin enhancer located 10 kb upstream functions along with its promoter to direct efficient, liver-specific expression in transgenic mice. Genes Dev. 1987 May;1(3):268–276. doi: 10.1101/gad.1.3.268. [DOI] [PubMed] [Google Scholar]
- Pittius C. W., Hennighausen L., Lee E., Westphal H., Nicols E., Vitale J., Gordon K. A milk protein gene promoter directs the expression of human tissue plasminogen activator cDNA to the mammary gland in transgenic mice. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5874–5878. doi: 10.1073/pnas.85.16.5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan T. M., Behringer R. R., Townes T. M., Palmiter R. D., Brinster R. L. High-level erythroid expression of human alpha-globin genes in transgenic mice. Proc Natl Acad Sci U S A. 1989 Jan;86(1):37–41. doi: 10.1073/pnas.86.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons J. P., McClenaghan M., Clark A. J. Alteration of the quality of milk by expression of sheep beta-lactoglobulin in transgenic mice. Nature. 1987 Aug 6;328(6130):530–532. doi: 10.1038/328530a0. [DOI] [PubMed] [Google Scholar]
- Sollner-Webb B., Reeder R. H. The nucleotide sequence of the initiation and termination sites for ribosomal RNA transcription in X. laevis. Cell. 1979 Oct;18(2):485–499. doi: 10.1016/0092-8674(79)90066-7. [DOI] [PubMed] [Google Scholar]
- Topper Y. J., Freeman C. S. Multiple hormone interactions in the developmental biology of the mammary gland. Physiol Rev. 1980 Oct;60(4):1049–1106. doi: 10.1152/physrev.1980.60.4.1049. [DOI] [PubMed] [Google Scholar]
- Vilotte J. L., Soulier S., Stinnakre M. G., Massoud M., Mercier J. C. Efficient tissue-specific expression of bovine alpha-lactalbumin in transgenic mice. Eur J Biochem. 1989 Dec 8;186(1-2):43–48. doi: 10.1111/j.1432-1033.1989.tb15175.x. [DOI] [PubMed] [Google Scholar]
- Watson C. J., Gordon K. E., Robertson M., Clark A. J. Interaction of DNA-binding proteins with a milk protein gene promoter in vitro: identification of a mammary gland-specific factor. Nucleic Acids Res. 1991 Dec 11;19(23):6603–6610. doi: 10.1093/nar/19.23.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H. Assembly and propagation of repressed and depressed chromosomal states. Cell. 1985 Oct;42(3):705–711. doi: 10.1016/0092-8674(85)90267-3. [DOI] [PubMed] [Google Scholar]
- Whitelaw C. B., Archibald A. L., Harris S., McClenaghan M., Simons J. P., Clark A. J. Targeting expression to the mammary gland: intronic sequences can enhance the efficiency of gene expression in transgenic mice. Transgenic Res. 1991 Dec;1(1):3–13. doi: 10.1007/BF02512991. [DOI] [PubMed] [Google Scholar]
- Wilkie T. M., Brinster R. L., Palmiter R. D. Germline and somatic mosaicism in transgenic mice. Dev Biol. 1986 Nov;118(1):9–18. doi: 10.1016/0012-1606(86)90068-0. [DOI] [PubMed] [Google Scholar]
- Wilmut I., Archibald A. L., Harris S., McClenaghan M., Simons J. P., Whitelaw C. B., Clark A. J. Modification of milk composition. J Reprod Fertil Suppl. 1990;41:135–146. [PubMed] [Google Scholar]
- Wright G., Carver A., Cottom D., Reeves D., Scott A., Simons P., Wilmut I., Garner I., Colman A. High level expression of active human alpha-1-antitrypsin in the milk of transgenic sheep. Biotechnology (N Y) 1991 Sep;9(9):830–834. doi: 10.1038/nbt0991-830. [DOI] [PubMed] [Google Scholar]
- Wu C. The 5' ends of Drosophila heat shock genes in chromatin are hypersensitive to DNase I. Nature. 1980 Aug 28;286(5776):854–860. doi: 10.1038/286854a0. [DOI] [PubMed] [Google Scholar]
- Yu S. H., Deen K. C., Lee E., Hennighausen L., Sweet R. W., Rosenberg M., Westphal H. Functional human CD4 protein produced in milk of transgenic mice. Mol Biol Med. 1989 Aug;6(4):255–261. [PubMed] [Google Scholar]
- al-Shawi R., Kinnaird J., Burke J., Bishop J. O. Expression of a foreign gene in a line of transgenic mice is modulated by a chromosomal position effect. Mol Cell Biol. 1990 Mar;10(3):1192–1198. doi: 10.1128/mcb.10.3.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]